Acute lymphoblastic leukemia (ALL) is a hematological disease characterized by the dysfunction of the hematopoietic system that leads to arrest at a specific stage of stem cells development, suppressing the average production of cellular hematologic components. BCP (B-cell progenitor)-ALL is a neoplasm of the B-cell lineage progenitor. BCP-ALL is caused and perpetuated by several mechanisms that provide the disease with its tumor potential and genetic and cytological characteristics. These pathological features are used for diagnosis and the prognostication of BCP-ALL. The BCP-ALL diagnostic protocol is well established. Firstly, it is necessary to demonstrate ≥ 20% lymphoblasts in bone marrow (BM) based on a BPM. Second, a hematopathological review is performed; it comprises a morphological assessment, and flow cytometric and genetic characterization.
- biomarkers
- circulating
- acute lymphoblastic leukemia
- BCP-ALL
1. Introduction
2. Diagnostic Biomarkers for BCP-ALL
The BCP-ALL diagnostic protocol is well established. Firstly, it is necessary to demonstrate ≥ 20% lymphoblasts in BM based on a BPM. Second, a hematopathological review is performed; it comprises a morphological assessment, and flow cytometric and genetic characterization. Once these studies are completed, a diagnosis according to the WHO classification can be made [12]. The BCP-ALL classification integrates morphology, immunophenotyping, and genetics/cytogenetics. There are no morphological features to distinguish between the BCP-ALL and TCP-ALL. Nevertheless, some lymphoblast characteristics are relevant: scant cytoplasm, size, and shape, wide relatively dispersed chromatin, and nuclear, and nucleolar peculiarities [59][34]. The immunophenotyping in BCP-ALL show some markers as almost always positive, namely CD19, cCD79a, cCD22, CD22, CD24, PAX5, and TdT; CD20, CD34, CD13, and CD33 expression is variable. Finally, among the genetic abnormalities BCR—ABL1, KMT2A-rearranged, ETV6-RUNX1, hyperdiploidy, hypodiploidy, IGH/IL3, TCF3-PBX1, BCR-ABL1-like, and iAMP21 can be mentioned [5]. The following circulating biomarkers have shown promise for either the diagnosis or prognosis of the illness.2.1. Proteins Type BCP-ALL Biomarkers
Proteins are helpful biomarkers because they have multiple crucial functions, making them central in biological systems [48,60][35][36]. In an individual manner, tumor necrosis factor α (TNF-α) is among the most studied biomarkers for ALL. TNF-α is a cytokine that induces apoptosis by inhibiting the activity of caspases [61][37]. Ahmed et al. evaluated its levels in serum from adult patients with ALL, comparing patients who received chemotherapy to those who did not (Table 1). Based on receiver operating characteristic (ROC) curve analysis, TNF-α showed good ALL diagnostic utility, an area under the ROC curve (AUC) of 0.94, a sensitivity of 91.7%, and a specificity of 100% [3]. These reports are consistent with the findings reported by Aref et al., who found an increase in TNF-α levels among patients newly diagnosed and in remission compared with controls [62][38]. Ahmed et al., was able to confirm this using an ROC curve, even though Aref’s cohort of patients had both BCP-ALL and BCP-CLL. These findings indicate that TNF-α can be a valuable tool in diagnosing ALL [3,62,63][3][38][39]. Table 1 summarizes the markers whose diagnostic values for ALL and/or BCP-ALL have been determined.| Biomarker | Blood Sample | Leukemia Type | Area under the ROC Curve | Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Reference |
|---|---|---|---|---|---|---|---|---|
| Smad 7 | Serum | ALL | 0.81 | 63 | 100 | 100 | 73 | |
| TGF-β1 | Serum | ALL | 0.79 | 57 | 93 | 89.5 | 68 | [16] |
| Smad 7 TGF-β1 miR-181a |
Serum | ALL | - | 100 | 93 | 93.7 | 100 | |
| IGF-I | Serum | ALL | - | 60.6 | 73.3 | - | - | [64][40] |
| IGF-II | Serum | ALL | - | 72.2 | 73.3 | - | - | |
| IGFBP-2 | Serum | ALL | - | 72.2 | 86.7 | - | - | |
| IGFBP-3 | Serum | ALL | - | 93.9 | 93.9 | - | - | |
| Anti-9-0AcSGs | Serum | ALL-GI | - | 98.9 | 92.1 | 96.8 | 97.2 | [65][41] |
| Anti-9-0AcSGs | Serum | ALL-GI | - | 96.8 | 95.9 | 96.8 | 95.9 | |
| PF4 CTAP-II |
Serum | ALL | - | 91.8 | 90 | - | - | [7] |
| C3f | Serum | AL | 0.99 | 97 | 100 | - | - | [45][42] |
| TNF-α | Serum | ALL | 0.94 | 91.7 | 100 | - | - | [3] |
| Survivin | Serum | ALL | 0.98 | 90 | 80 | - | - | |
| p53 | Serum | AL | 0.8 | 52 | 100 | - | - | [66][43] |
| EGFR | Serum | AL | 0.93 | 73.9 | 95.8 | - | - | |
| Pseudouridine | Serum | ALL | - | 90 | 97.5 | - | - | [67][44] |
| ADAM 17 | Plasma | BCP-ALL | 0.98 | 100 | 100 | - | - | [68][45] |
| ATG3 | Plasma | BCP-ALL | 0.95 | 100 | 100 | - | - | |
| AC133 * | Whole blood | ALL | - | 100 | 100 | 100 | 100 | [69][46] |
| miR-181a | Serum | ALL | 0.93 | 86.5 | 93.3 | 92.8 | 87.5 | [16] |
| miR-146a | Plasma | BCP-ALL | 1 | 100 | 100 | - | - | [9] |
| mRNA Survivin | Whole blood | BCP-ALL | 0.85 | 95 | 95 | - | - | [70][47] |
| mRNA HLA-G | PBMC | ALL | - | 74 | 100 | - | - | [71][48] |
| miR-125b-1 | Serum | ALL | 0.85 | 83.7 | 100 | - | - | [72][49] |
| miR-203 | Serum | ALL | 0.87 | 97.7 | 87 | - | - | |
| miR-100 | PBMC | ALL | 0.87 | 82.7 | 100 | - | - | [15] |
| miR-196a | PBMC | ALL | 0.537 | 46.6 | 100 | - | - | |
| miR-146a | PBMC | ALL | 1 | 100 | 100 | - | - | |
| miR-511 | Plasma | BCP-ALL | 1 | 100 | 100 | 1 | 1 | [73][50] |
| miR-34a | Plasma | BCP-ALL | 0.98 | 92 | 100 | 1 | 0.70 | |
| miR-22 | Plasma | BCP-ALL | 0.91 | 79 | 100 | 1 | 0.54 | |
| miR-26a | Plasma | BCP-ALL | 0.91 | 79 | 100 | 1 | 0.47 | |
| miR-221 | Plasma | BCP-ALL | 0.92 | 83 | 100 | 1 | 0.54 | |
| miR-223 | Plasma | BCP-ALL | 0.93 | 89 | 100 | 1 | 0.64 | |
| miR-21 | PBMC | ALL | 0.565 | 44 | 55 | - | - | [74][51] |
| miR-26 | PBMC | ALL | 0.464 | 54 | 50 | - | - | |
| miR-148a | PBMC | ALL | 0.719 | 74 | 79 | - | - | |
| miR-133b | PBMC | ALL | 0.669 | 70 | 60 | - | - | |
| miR-24 | PBMC | ALL | 0.785 | 72 | 81 | - | - | |
| miR-92a | PBMC and plasma | ALL | 0.99 | - | - | - | - | [75][52] |
| miR-92a | Plasma | ALL | 0.755 | 41.5 | 100 | 100 | 36.7 | [6] |
| miR-638 | Plasma | ALL | 0.86 | 54.7 | 100 | 100 | 42.9 | |
| miR-125b | PBMC | ALL | 0.99 | 98 | 96.7 | - | - | [76][53] |
| mRNA-Bcl-2 | PBMC | ALL | 0.9 | 96.7 | 70 | - | - | |
| miR-128b | PBMC | ALL | - | 75 | 87.5 | - | - | [77][54] |
| cf-DNA levels | Plasma | ALL, AML | 0.79 | 65 | 100 | - | - | [50][55] |
| cf-DNA integrity | Plasma | ALL, AML | 88 | 78 | 90 | - | - |
2.2. RNA Type BCP-ALL Biomarkers
RNA can generate numerous proteins through protein-coding regions. Most genomic sequences can be transcribed into protein-coding RNA. In contrast, the non-coding parts are transcribed to produce non-coding RNAs (ncRNAs) [84,85][62][63]. Based on their size, location, and interacting partners, ncRNAs are classified into several types: transfer RNAs (tRNAs), ribosome RNAs (rRNAs), small nucleolar RNAs (snoRNAs), small nuclear RNAs (snRNAs), microRNAs (miRNAs), small interfering RNAs (siRNAs), long non-coding RNAs (lncRNAs), circular RNA (circRNAs), Piwi-interacting RNAs (piRNAs), and enhancer RNAs (eRNAs) [84][62]. It has been discovered that numerous microRNAs are essential in the initiation, progression, and metastasis of cancer [86][64]. miRNAs are stable in serum and display distinct expression patterns in healthy individuals and those with cancer. Hence, they are suitable biomarkers for cancer detection and prognosis [32]. Furthermore, one miRNA can modulate the expression of several genes, and there are many miRNA expression patterns in patients with ALL [87][65]. In a study carried out in Mexico, the authors evaluated the diagnostic usefulness of the circulating miRNA expression profile in plasma samples from patients with BCP-ALL. miR-511 showed the highest mean overexpression (FC: 159.5, p = 0.002), while miR-199a-3p was the most under-expressed (FC: −13.48, p < 0.001). ROC curves analysis provided good values for miR-511 (cut-off = 9.458, specificity 1, sensitivity 1, AUC = 1), miR-34a (cut-off = 7.179, specificity 1, sensitivity 0.92, AUC = 0.98), miR-222 (cut-off = −0.1325, AUC = 0.91), miR-26a (cut-off = 2.073, AUC = 0.91), miR-221 (cut-off = −0.1861, AUC = 0.92), and miR-223 (cut-off −4.309, specificity 1, sensitivity 0.89, AUC = 0.93). Moreover, pathway analysis revealed that mainly Wnt, MAPK, TGF-beta, p53, Jak-STAT, NOTCH, and B- and T-cell receptor signaling pathways are activated by the evaluated miRNAs (miR-511, miR-19b, miR-195, miR-565, miR-34a, miR-222, miR-363, miR-181a, miR-181c, miR-199a-3p, miR-340, miR-335, miR-99b, miR-221, miR-744, miR-223, miR-26a, miR-224, and miR-151-3p). These miRNAs have value as markers for diagnosis and to identify therapeutic targets for BCP-ALL [73][50]. CircRNAs are a type of non-coding RNA and represent a recent research hotspot in the field of RNA. circRNAs form covalently closed loop structures with neither 5′–3′ polarities nor polyadenylated tails and lengths between 100 to thousands of nucleotides [88,89][66][67]. circRNAs can modulate miRNA-target expression, acting like miRNA axes. Moreover, they can interact with RNA-binding proteins and regulate cellular processes [90][68]. Circular RNA (circRNA) expression in PBMCs of pediatric patients with BCP-ALL has also been evaluated [90][68]. After the comparison of circRNA expression profiles in B- and T-cells, and monocyte populations, which was validated by reverse transcriptase quantitative polymerase chain reaction (RT-qPCR), four circRNAs were more highly expressed in healthy donors than in patients with BCP-ALL: circIL4R (p < 0.0001), circZCCH7 (p = 0.0307), and circX (intergenic) (p = 0.076). Furthermore, circPVT1 (p = 0.0002), circHIPK3 (p < 0.0001), circPAX5 (p < 0.0001), and circAFF3 (p = 0.0115) were overexpressed in patients with BCP-ALL. The expression of the target set of circRNAs according to BCP-ALL cytogenetic subtype showed circAFF2 was highly expressed in TCF3-PBX1 (p = 0.0252), BCP-ALL, and to a lesser extent in ETV6- RUNX1 BCP-ALL (p = 0.021). CircBCL2 (intronic) was upregulated in ETV6-RUNX1 fusions (p = 0.0166), circSETBP1 and circX (intergenic) were significantly reduced in MLL rearranged samples (p = 0.0274 and p = 0.0472, respectively), and circIKZF1 was lower in BCP-ABL and hyperdiploid leukemias than in the ETV6-RUNX1 subtype (p = 0.0154), in which the expression was conserved at levels comparable to B-cells. Overall, circRNAs have the potential to regulate specific cell functions, cell differentiation, maturation stages, and the growth of leukemic cells. As circRNA deregulation has been observed in patients with BCP-ALL, based on their cytogenetic subtype and previous cancers, circRNAs could potentially serve as markers for BCP-ALL. However, further research is necessary to determine the exact role that circRNAs play in leukemogenesis and their potential as markers for the disease [90,91][68][69]. Figure 1 shows a summary of the biomarkers associated with BCP-ALL grouped as protein markers (Figure 1A), nucleic acid molecules (Figure 1B,C), metabolites (Figure 1D), and according to the different stages of disease.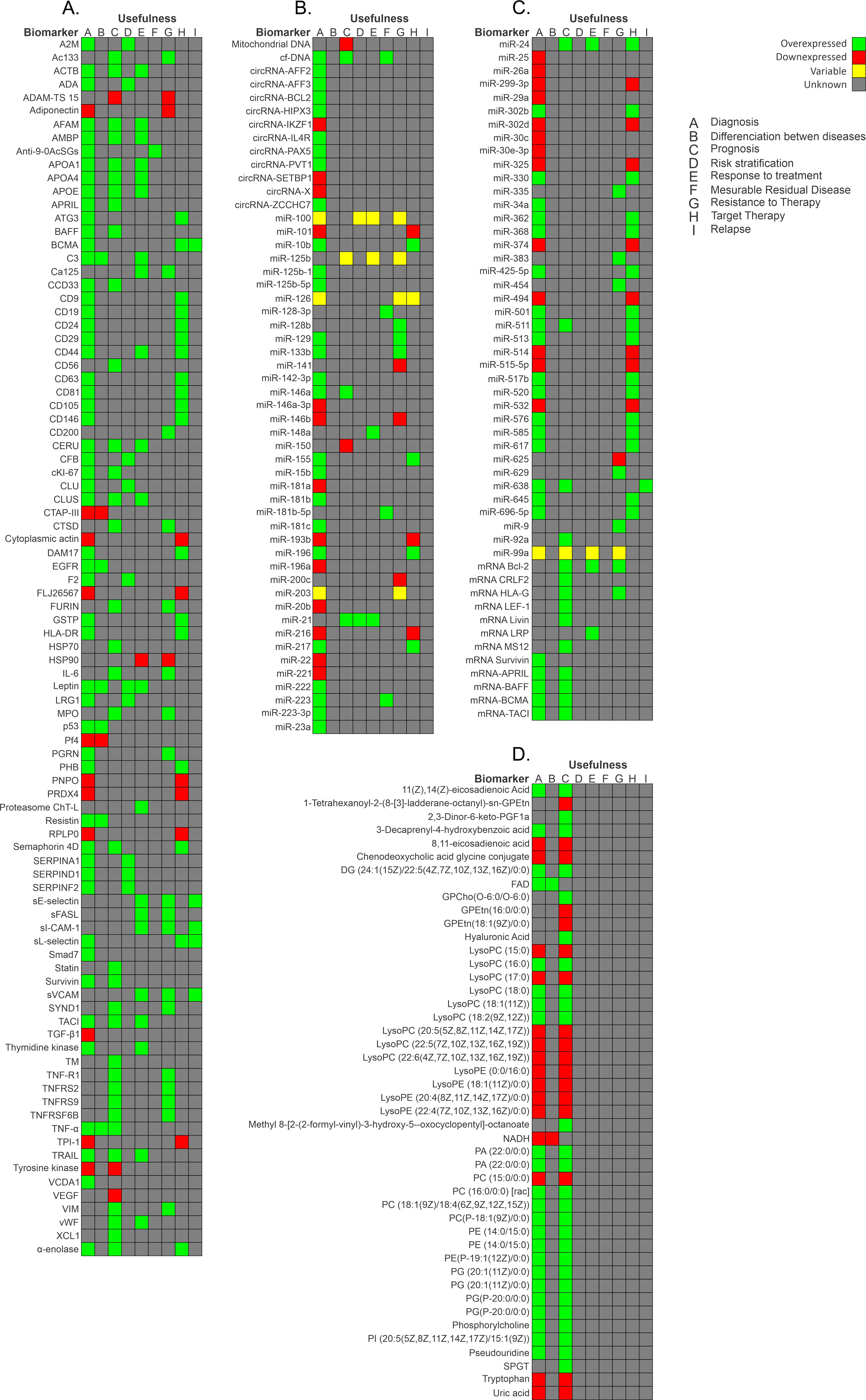
2.3. Metabolites Type BCP-ALL Biomarkers
Metabolites are the final products of gene expression and the direct outcome of enzymatic and protein activity. Metabolite profiles provide information about tumor microenvironments for ALL [30,92][30][70]. These metabolite profiles can be studied by metabolomics. “Metabolomics” involves the quantitative measurement of time of the metabolic responses of multicellular systems to pathophysiological stimuli or genetic modification [17]. Perturbation in the metabolism of patients at different disease stages could provide a unique metabolic signature to monitor treatment outcomes and disease progression [93][71]. Metabolism in cancer is a major research area in cancer biology. It examines how metabolic activities are changed in cancer cells compared to normal cells [30]. As shown in Table 1 and Figure 1D, researchers have reported metabolic differences in patients with ALL compared to healthy controls. Musharraf et al. [92][70] compared the metabolite profiles in the serum of 96 patients with ALL, AML, and APA, obtained by using the nuclear magnetic resonance spectroscopy technique. The authors reported high lactate levels and low levels of alanine, glutamine, histidine, lysine, valine, and proline. They theorized that patients adopt a secondary metabolic pathway to generate glucose because cancer cells require more glucose than can be provided by glycolysis. These findings are in contrast with those reported by Morad et al. [30], who evaluated the plasma of patients with ALL (n = 14), AML (n = 16), and breast cancer (n = 25). They reported high threonine, proline, glycine, alanine and lysine levels and low lactate levels in the ALL group. These changes could be due to tumor tissue competing for nitrogen compounds found in the amino acid structure [30,92][30][70]. Another group found that fatty acids were elevated as reservoirs, corresponding with an accumulation of carnitine, which plays a role in fatty acid metabolism [92][70]. Upon closer analysis, there seem to be notable variations in the approaches employed, which could explain the discrepancies in the outcomes. A crucial factor to consider is that the patient selection was comprised of diverse cancer types, like breast cancer and aplastic anemia, which may have influenced the results. Furthermore, though both studies utilized nuclear magnetic resonance spectroscopy, the validation techniques differed. One study used variable importance in projection values, while the other used principal component analysis. To gain a comprehensive understanding of the implications of these disparities, providing further context and specific examples would be highly beneficial. Overall, with a more thoughtful and informed examination, people can better appreciate the nuances of these findings. In a recent study focused on developing a novel photo-diagnostic strategy using fluorescence emission spectra, the authors used plasma or red blood cell extract from 45 patients with AL. They found four metabolites that were altered and correlated: nicotinamide adenine dinucleotide (NAD) + hydrogen (H) (NADH), FAD, tyrosine kinase, and tryptophan (p < 0.05). NADH and tryptophan were decreased in patients with ALL, and there was a connection between tyrosine kinase and tryptophan. However, FAD was increased in patients with AL. Thus, the AL diagnosis can be made based on the FAD, NADH, tyrosine kinase, and tryptophan levels, but the ratios can help to discern between AML and ALL [8].References
- Fujita, T.C.; Sousa-Pereira, N.; Amarante, M.K.; Watanabe, M.A.E. Acute lymphoid leukemia etiopathogenesis. Mol. Biol. Rep. 2021, 48, 817–822.
- Kaplan, J.A. Leukemia in Children. Pediatr. Rev. 2019, 40, 319–331.
- Ahmed, M.B.; Shehata, H.H.; Moussa, M.; Ibrahim, T.M. Prognostic significance of survivin and tumor necrosis factor-alpha in adult acute lymphoblastic leukemia. Clin. Biochem. 2012, 45, 112–116.
- Wang, D.; Lv, Y.Q.; Liu, Y.F.; Du, X.J.; Li, B. Differential protein analysis of lymphocytes between children with acute lymphoblastic leukemia and healthy children. Leuk. Lymphoma 2013, 54, 381–386.
- Swerdlow, S. WHO Classification of Haematopoietic and Lymphoid Tissues; International Agency for Research on Cancer: Lyon, France, 2017.
- Fayed, D.; Donia, T.; El-Shanshory, M.; Ali, E.M.M.; Mohamed, T.M. Evaluation of MicroRNA92, MicroRNA638 in Acute Lymphoblastic Leukemia of Egyptian Children. Asian Pac. J. Cancer Prev. 2021, 22, 1567–1572.
- Shi, L.; Zhang, J.; Wu, P.; Feng, K.; Li, J.; Xie, Z.; Xue, P.; Cai, T.; Cui, Z.; Chen, X.; et al. Discovery and identification of potential biomarkers of pediatric acute lymphoblastic leukemia. Proteome Sci. 2009, 7, 7.
- Masilamani, V.; Devanesan, S.; AlSalhi, M.S.; AlQahtany, F.S.; Farhat, K.H. Fluorescence spectral detection of acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML): A novel photodiagnosis strategy. Photodiag. Photodyn. Ther. 2020, 29, 101634.
- Shahid, S.; Shahid, W.; Shaheen, J.; Akhtar, M.W.; Sadaf, S. Circulating miR-146a expression as a non-invasive predictive biomarker for acute lymphoblastic leukemia. Sci. Rep. 2021, 11, 22783.
- Hassan, N.M.; Refaat, L.A.; Ismail, G.N.; Abdellateif, M.; Fadel, S.A.; AbdelAziz, R.S. Diagnostic, prognostic and predictive values of miR-100 and miR-210 in pediatric acute lymphoblastic Leukemia. Hematology 2020, 25, 405–413.
- Brown, P.; Inaba, H.; Annesley, C.; Beck, J.; Colace, S.; Dallas, M.; DeSantes, K.; Kelly, K.; Kitko, C.; Lacayo, N.; et al. Pediatric Acute Lymphoblastic Leukemia, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2020, 18, 81–112.
- Brown, P.A.; Shah, B.; Advani, A.; Aoun, P.; Boyer, M.W.; Burke, P.W.; DeAngelo, D.J.; Dinner, S.; Fathi, A.T.; Gauthier, J.; et al. Acute Lymphoblastic Leukemia, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1079–1109.
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748.
- Yu, R.; Yang, S.; Liu, Y.; Zhu, Z. Identification and validation of serum autoantibodies in children with B-cell acute lymphoblastic leukemia by serological proteome analysis. Proteome Sci. 2022, 20, 3.
- Swellam, M.; El-Khazragy, N. Clinical impact of circulating microRNAs as blood-based marker in childhood acute lymphoblastic leukemia. Tumour Biol. 2016, 37, 10571–10576.
- Nabhan, M.; Louka, M.L.; Khairy, E.; Tash, F.; Ali-Labib, R.; El-Habashy, S. MicroRNA-181a and its target Smad 7 as potential biomarkers for tracking child acute lymphoblastic leukemia. Gene 2017, 628, 253–258.
- Bai, Y.; Zhang, H.; Sun, X.; Sun, C.; Ren, L. Biomarker identification and pathway analysis by serum metabolomics of childhood acute lymphoblastic leukemia. Clin. Chim. Acta 2014, 436, 207–216.
- Carceles-Alvarez, A.; Ortega-Garcia, J.A.; Lopez-Hernandez, F.A.; Fuster-Soler, J.L.; Ramis, R.; Kloosterman, N.; Castillo, L.; Sanchez-Solis, M.; Claudio, L.; Ferris-Tortajada, J. Secondhand smoke: A new and modifiable prognostic factor in childhood acute lymphoblastic leukemias. Environ. Res. 2019, 178, 108689.
- Vrooman, L.M.; Silverman, L.B. Treatment of Childhood Acute Lymphoblastic Leukemia: Prognostic Factors and Clinical Advances. Curr. Hematol. Malig. Rep. 2016, 11, 385–394.
- Aly, R.M.; Yousef, A.B. Prognostic significance of lymphoid enhancer-binding factor-1 expression in egyptian adult B-acute lymphocytic leukemia patients. Turk. J. Haematol. 2015, 32, 15–20.
- Gutierrez-Aguirre, C.H.; Flores-Jimenez, J.A.; Alatorre-Ricardo, J.; Cantu-Rodriguez, O.G.; Rosas-Taraco, A.; Salazar-Riojas, R.; Jaime-Perez, J.C.; Sanchez-Cardenas, M.; Lopez-Silva, L.; Martinez-Castilla, A.M.; et al. The prognostic significance of serum XCL1 concentration in patients with acute lymphoblastic leukemia: A pilot study. Ann. Hematol. 2017, 96, 2015–2024.
- Kruse, A.; Abdel-Azim, N.; Kim, H.N.; Ruan, Y.; Phan, V.; Ogana, H.; Wang, W.; Lee, R.; Gang, E.J.; Khazal, S.; et al. Minimal Residual Disease Detection in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2020, 21, 1054.
- Zhang, Q.; Shi, C.; Han, L.; Jain, N.; Roberts, K.G.; Ma, H.; Cai, T.; Cavazos, A.; Tabe, Y.; Jacamo, R.O.; et al. Inhibition of mTORC1/C2 signaling improves anti-leukemia efficacy of JAK/STAT blockade in CRLF2 rearranged and/or JAK driven Philadelphia chromosome-like acute B-cell lymphoblastic leukemia. Oncotarget 2018, 9, 8027–8041.
- Cante-Barrett, K.; Spijkers-Hagelstein, J.A.; Buijs-Gladdines, J.G.; Uitdehaag, J.C.; Smits, W.K.; van der Zwet, J.; Buijsman, R.C.; Zaman, G.J.; Pieters, R.; Meijerink, J.P. MEK and PI3K-AKT inhibitors synergistically block activated IL7 receptor signaling in T-cell acute lymphoblastic leukemia. Leukemia 2016, 30, 1832–1843.
- Tovar, C.F.; Zeron, H.M.; Romero, M.D.; Sanchez, Y.V.; Romero, I.T. Glycogen Synthase Kinase-3beta (GSK-3beta) and Nuclear Factor Kappa-B (NFKB) in Childhood Acute Lymphoblastic Leukemia. Adv. Clin. Exp. Med. 2016, 25, 1139–1147.
- Vrooman, L.M.; Silverman, L.B. Childhood acute lymphoblastic leukemia: Update on prognostic factors. Curr. Opin. Pediatr. 2009, 21, 1–8.
- Aly, R.M.; Ghazy, H.F. Prognostic significance of MSI2 predicts unfavorable outcome in adult B-acute lymphoblastic leukemia. Int. J. Lab. Hematol. 2015, 37, 272–278.
- Aguirre-Guillen, W.A.; Angeles-Floriano, T.; Lopez-Martinez, B.; Reyes-Morales, H.; Zlotnik, A.; Valle-Rios, R. Omics techniques and biobanks to find new biomarkers for the early detection of acute lymphoblastic leukemia in middle-income countries: A perspective from Mexico. Bol. Med. Hosp. Infant. Mex. 2017, 74, 227–232.
- Yan, M.; Liu, H.; Xu, J.; Cen, X.; Wang, Q.; Xu, W.; Wang, W.; Qiu, Z.; Ou, J.; Dong, Y.; et al. Expression of human Krüppel-like factor 3 in peripheral blood as a promising biomarker for acute leukemia. Cancer Med. 2020, 9, 2803–2811.
- Morad, H.M.; Abou-Elzahab, M.M.; Aref, S.; El-Sokkary, A.M.A. Diagnostic Value of (1)H NMR-Based Metabolomics in Acute Lymphoblastic Leukemia, Acute Myeloid Leukemia, and Breast Cancer. ACS Omega 2022, 7, 8128–8140.
- Hussan, S.S.; Maqsood, N.; Wang, Q.; Tao, S.; Sadaf, S. A panel of epigenetically dysregulated Wnt signaling pathway genes for non-invasive diagnosis of pediatric acute lymphoblastic leukemia. Cancer Biomark. 2021, 32, 459–470.
- Li, G.; Hu, J.; Hu, G. Biomarker Studies in Early Detection and Prognosis of Breast Cancer. Adv. Exp. Med. Biol. 2017, 1026, 27–39.
- Damanti, C.C.; Gaffo, E.; Lovisa, F.; Garbin, A.; Di Battista, P.; Gallingani, I.; Tosato, A.; Pillon, M.; Carraro, E.; Mascarin, M.; et al. MiR-26a-5p as a Reference to Normalize MicroRNA qRT-PCR Levels in Plasma Exosomes of Pediatric Hematological Malignancies. Cells 2021, 10, 101.
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; International Agency for Research on Cancer: Lyon, France, 2008; Volume 2.
- Cristea, I.M.; Gaskell, S.J.; Whetton, A.D. Proteomics techniques and their application to hematology. Blood 2004, 103, 3624–3634.
- Cui, J.W.; Wang, J.; He, K.; Jin, B.F.; Wang, H.X.; Li, W.; Kang, L.H.; Hu, M.R.; Li, H.Y.; Yu, M.; et al. Proteomic analysis of human acute leukemia cells: Insight into their classification. Clin. Cancer Res. 2004, 10, 6887–6896.
- Malcles, M.H.; Wang, H.W.; Koumi, A.; Tsai, Y.H.; Yu, M.; Godfrey, A.; Boshoff, C. Characterisation of the anti-apoptotic function of survivin-DeltaEx3 during TNFalpha-mediated cell death. Br. J. Cancer 2007, 96, 1659–1666.
- Aref, S.; Salama, O.; Shamaa, S.; El-Refaie, M.; Mourkos, H. Angiogenesis factor pattern differs in acute lymphoblastic leukemia and chronic lymphocytic leukemia. Hematology 2007, 12, 319–324.
- Potapnev, M.P.; Petyovka, N.V.; Belevtsev, M.V.; Savitskiy, V.P.; Migal, N.V. Plasma level of tumor necrosis factor-alpha (TNF-alpha) correlates with leukocytosis and biological features of leukemic cells, but not treatment response of children with acute lymphoblastic leukemia. Leuk. Lymphoma 2003, 44, 1077–1079.
- Zakhary, N.I.; Boshra, S.A.; El-Sawalhi, M.M.; Fahim, A.T.; Ebeid, E.N. Insulin-like growth factor system in Egyptian children with acute lymphoblastic leukemia. Genet. Test. Mol. Biomark. 2012, 16, 1067–1072.
- Pal, S.; Bandyopadhyay, S.; Chatterjee, M.; Bhattacharya, D.K.; Minto, L.; Hall, A.G.; Mandal, C. Antibodies against 9-O-acetylated sialoglycans: A potent marker to monitor clinical status in childhood acute lymphoblastic leukemia. Clin. Biochem. 2004, 37, 395–403.
- Liang, T.; Wang, N.; Li, W.; Li, A.; Wang, J.; Cui, J.; Liu, N.; Li, Y.; Li, L.; Yang, G.; et al. Identification of complement C3f-desArg and its derivative for acute leukemia diagnosis and minimal residual disease assessment. Proteomics 2010, 10, 90–98.
- Abdel-Aziz, M.M. Clinical significance of serum p53 and epidermal growth factor receptor in patients with acute leukemia. Asian Pac. J. Cancer Prev. 2013, 14, 4295–4299.
- Pane, F.; Savoia, M.; Fortunato, G.; Camera, A.; Rotoli, B.; Salvatore, F.; Sacchetti, L. Serum pseudouridine in the diagnosis of acute leukaemias and as a novel prognostic indicator in acute lymphoblastic leukaemia. Clin. Biochem. 1993, 26, 513–520.
- Zhu, S.; Xing, C.; Li, R.; Cheng, Z.; Deng, M.; Luo, Y.; Li, H.; Zhang, G.; Sheng, Y.; Peng, H.; et al. Proteomic profiling of plasma exosomes from patients with B-cell acute lymphoblastic leukemia. Sci. Rep. 2022, 12, 11975.
- Elgendi, H.M.; Mekawy, M.A.; Abdel Wahab, S.E.; Tawfik, L.M.; Ismail, E.A.; Adly, A.A. AC133 expression in egyptian children with acute leukemia: Impact on treatment response and disease outcome. J. Pediatr. Hematol. Oncol. 2010, 32, 286–293.
- Mohammadi, M.; Amirmahani, F.; Goharrizi, K.J.; Pakzad, R.; Dolat, H. Evaluating the expression level of Survivin gene in different groups of B-cell acute lymphoblastic leukemia patients of Iran. Mol. Biol. Rep. 2019, 46, 2679–2684.
- Alkhouly, N.; Shehata, I.; Ahmed, M.B.; Shehata, H.; Hassan, S.; Ibrahim, T. HLA-G expression in acute lymphoblastic leukemia: A significant prognostic tumor biomarker. Med. Oncol. 2013, 30, 460.
- Swellam, M.; Hashim, M.; Mahmoud, M.S.; Ramadan, A.; Hassan, N.M. Aberrant Expression of Some Circulating miRNAs in Childhood Acute Lymphoblastic Leukemia. Biochem. Genet. 2018, 56, 283–294.
- Luna-Aguirre, C.M.; de la Luz Martinez-Fierro, M.; Mar-Aguilar, F.; Garza-Veloz, I.; Trevino-Alvarado, V.; Rojas-Martinez, A.; Jaime-Perez, J.C.; Malagon-Santiago, G.I.; Gutierrez-Aguirre, C.H.; Gonzalez-Llano, O.; et al. Circulating microRNA expression profile in B-cell acute lymphoblastic leukemia. Cancer Biomark. 2015, 15, 299–310.
- El-maadawy, E.A.; Bakry, R.M.; Moussa, M.M.; El-Naby, S.; Talaat, R.M.J.C. Alteration in miRNAs expression in paediatric acute lymphocyticleukaemia: Insight into patients’ therapeutic response. Pharmacol. Pharm. 2021, 48, 35–43.
- Ohyashiki, J.H.; Umezu, T.; Kobayashi, C.; Hamamura, R.S.; Tanaka, M.; Kuroda, M.; Ohyashiki, K. Impact on cell to plasma ratio of miR-92a in patients with acute leukemia: In vivo assessment of cell to plasma ratio of miR-92a. BMC Res. Notes 2010, 3, 347.
- El-Khazragy, N.; Elshimy, A.A.; Hassan, S.S.; Matbouly, S.; Safwat, G.; Zannoun, M.; Riad, R.A. Dysregulation of miR-125b predicts poor response to therapy in pediatric acute lymphoblastic leukemia. J. Cell Biochem. 2018, 120, 7428–7438.
- Nemes, K.; Csoka, M.; Nagy, N.; Mark, A.; Varadi, Z.; Danko, T.; Kovacs, G.; Kopper, L.; Sebestyen, A. Expression of certain leukemia/lymphoma related microRNAs and its correlation with prognosis in childhood acute lymphoblastic leukemia. Pathol. Oncol. Res. 2015, 21, 597–604.
- Gao, Y.J.; He, Y.J.; Yang, Z.L.; Shao, H.Y.; Zuo, Y.; Bai, Y.; Chen, H.; Chen, X.C.; Qin, F.X.; Tan, S.; et al. Increased integrity of circulating cell-free DNA in plasma of patients with acute leukemia. Clin. Chem. Lab. Med. 2010, 48, 1651–1656.
- Abu Sabaa, A.; Shen, Q.; Lennmyr, E.B.; Enblad, A.P.; Gammelgard, G.; Molin, D.; Hein, A.; Freyhult, E.; Kamali-Moghaddam, M.; Hoglund, M.; et al. Plasma protein biomarker profiling reveals major differences between acute leukaemia, lymphoma patients and controls. N Biotechnol. 2022, 71, 21–29.
- Wik, L.; Nordberg, N.; Broberg, J.; Bjorkesten, J.; Assarsson, E.; Henriksson, S.; Grundberg, I.; Pettersson, E.; Westerberg, C.; Liljeroth, E.; et al. Proximity Extension Assay in Combination with Next-Generation Sequencing for High-throughput Proteome-wide Analysis. Mol. Cell. Proteom. MCP 2021, 20, 100168.
- Limijadi, E.K.S.; Budiwijono, I.; Samsuria, I.K.; Adhipireno, P.; Devi, W.R. Coagulation and Fibrinolysis Profiles of Acute Myeloblastic Leukemia: Preliminary Assessment of Hypercoagulability. Eur. J. Mol. Clin. Med. 2021, 8, 607–615.
- Seftalioglu, A.; Karakus, S. Syndecan-1/CD138 expression in normal myeloid, acute lymphoblastic and myeloblastic leukemia cells. Acta Histochem. 2003, 105, 213–221.
- Lenting, P.J.; Christophe, O.D.; Denis, C.V. von Willebrand factor biosynthesis, secretion, and clearance: Connecting the far ends. Blood 2015, 125, 2019–2028.
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756.
- Maimaitiyiming, Y.; Ye, L.; Yang, T.; Yu, W.; Naranmandura, H. Linear and Circular Long Non-Coding RNAs in Acute Lymphoblastic Leukemia: From Pathogenesis to Classification and Treatment. Int. J. Mol. Sci. 2022, 23, 4442.
- Li, Y.; Xu, J.; Shao, T.; Zhang, Y.; Chen, H.; Li, X. RNA Function Prediction. Methods Mol. Biol. 2017, 1654, 17–28.
- He, L.; He, X.; Lim, L.P.; de Stanchina, E.; Xuan, Z.; Liang, Y.; Xue, W.; Zender, L.; Magnus, J.; Ridzon, D.; et al. A microRNA component of the p53 tumour suppressor network. Nature 2007, 447, 1130–1134.
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269.
- Qu, S.; Yang, X.; Li, X.; Wang, J.; Gao, Y.; Shang, R.; Sun, W.; Dou, K.; Li, H. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015, 365, 141–148.
- Perez de Acha, O.; Rossi, M.; Gorospe, M. Circular RNAs in Blood Malignancies. Front. Mol. Biosci. 2020, 7, 109.
- Gaffo, E.; Boldrin, E.; Dal Molin, A.; Bresolin, S.; Bonizzato, A.; Trentin, L.; Frasson, C.; Debatin, K.-M.; Meyer, L.H.; te Kronnie, G.; et al. Circular RNA differential expression in blood cell populations and exploration of circRNA deregulation in pediatric acute lymphoblastic leukemia. Sci. Rep. 2019, 9, 14670.
- Chen, J.; Li, Y.; Zheng, Q.; Bao, C.; He, J.; Chen, B.; Lyu, D.; Zheng, B.; Xu, Y.; Long, Z.; et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. 2017, 388, 208–219.
- Musharraf, S.G.; Siddiqui, A.J.; Shamsi, T.; Choudhary, M.I.; Rahman, A.U. Serum metabonomics of acute leukemia using nuclear magnetic resonance spectroscopy. Sci. Rep. 2016, 6, 30693.
- Bannur, Z.; Teh, L.K.; Hennesy, T.; Rosli, W.R.; Mohamad, N.; Nasir, A.; Ankathil, R.; Zakaria, Z.A.; Baba, A.; Salleh, M.Z. The differential metabolite profiles of acute lymphoblastic leukaemic patients treated with 6-mercaptopurine using untargeted metabolomics approach. Clin. Biochem. 2014, 47, 427–431.
