Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Laura Veschetti | -- | 3846 | 2023-08-29 10:45:54 | | | |
| 2 | Lindsay Dong | Meta information modification | 3846 | 2023-08-30 02:35:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Veschetti, L.; Treccani, M.; De Tomi, E.; Malerba, G. Genomic Instability Evolutionary Footprints on Human Health. Encyclopedia. Available online: https://encyclopedia.pub/entry/48581 (accessed on 08 March 2026).
Veschetti L, Treccani M, De Tomi E, Malerba G. Genomic Instability Evolutionary Footprints on Human Health. Encyclopedia. Available at: https://encyclopedia.pub/entry/48581. Accessed March 08, 2026.
Veschetti, Laura, Mirko Treccani, Elisa De Tomi, Giovanni Malerba. "Genomic Instability Evolutionary Footprints on Human Health" Encyclopedia, https://encyclopedia.pub/entry/48581 (accessed March 08, 2026).
Veschetti, L., Treccani, M., De Tomi, E., & Malerba, G. (2023, August 29). Genomic Instability Evolutionary Footprints on Human Health. In Encyclopedia. https://encyclopedia.pub/entry/48581
Veschetti, Laura, et al. "Genomic Instability Evolutionary Footprints on Human Health." Encyclopedia. Web. 29 August, 2023.
Copy Citation
Genomic instability comprises not only the accumulation of mutations but also telomeric shortening, epigenetic alterations and other mechanisms that could contribute to genomic information conservation or corruption.
genomic instability
DNA repair
human complex disorder
evolutionary genetics
ncRNA
1. Introduction
A struggle of the collective drive toward complexity, auto-organization and genomic diversification against the “self” need to protect one’s own genomic information has been taking place since the beginning of life. On one plate of the scale, there is the dynamism of mechanisms involving genomic variability: gene transfer, duplication, rearrangements, recombination and exchange of mobile genetic elements, which are the events that most likely lead the drive to biological complexification [1][2]. On the other plate, there is the reliability of DNA conservation: a plethora of repair systems adapted and evolved together with organisms’ genomes to preserve them from corruption during individuals’ and cells’ lifespans and reproduction [3].
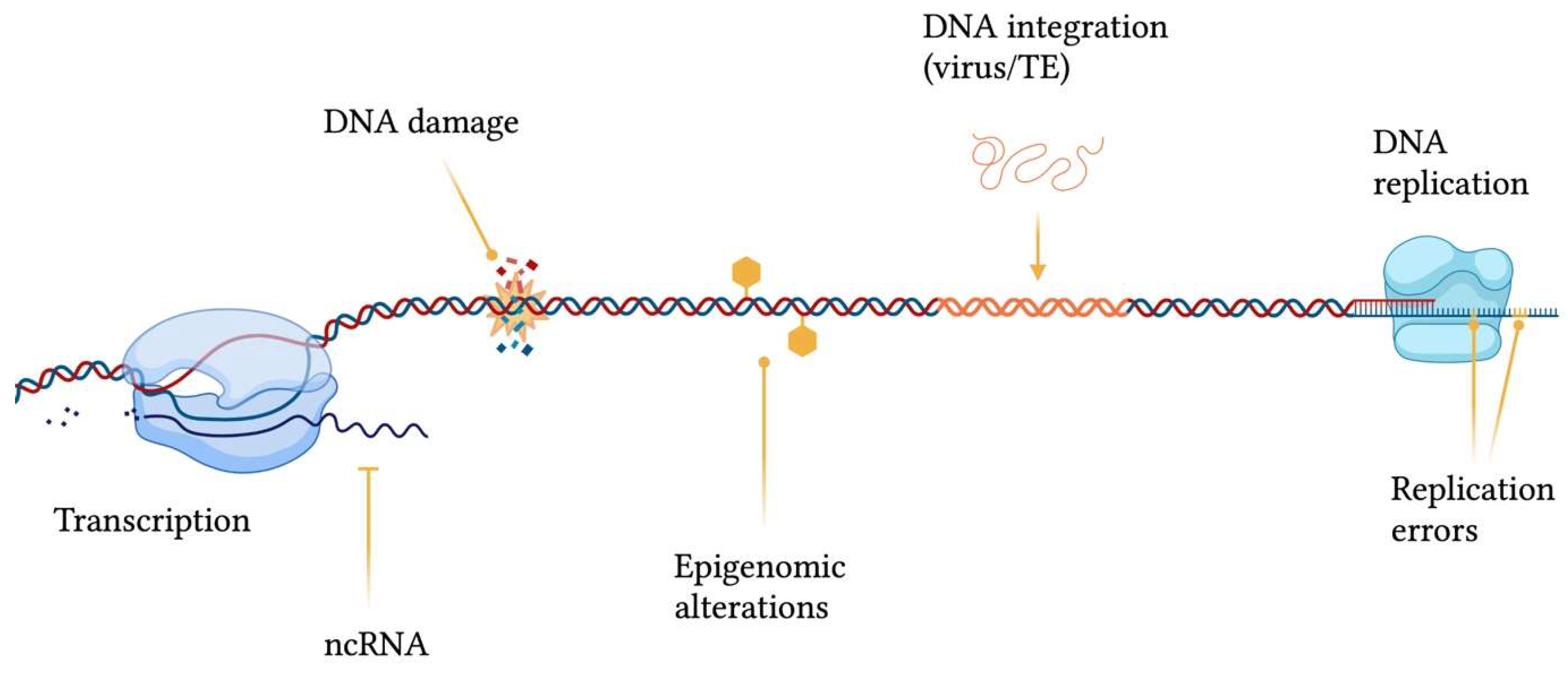
2. The Balance between Variability and Conservation
Genomic structural integrity and functional stability are constantly threatened by DNA and chromatin damaging agents ranging from exogenous sources, such as environmental toxins (e.g., polycyclic aromatic hydrocarbons), ultraviolet light (UV), ionizing radiation and mutagenic chemicals, to endogenous processes, including DNA replication and repair errors, epigenetic dysregulation, telomere shortening, spontaneous decay of DNA, transposable elements (TEs) insertions and oxidative stress (e.g., generation of reactive oxygen species) [4] (Figure 1). The consequences of damaging events are displayed as a wide variety of genomic wounds which comprehend base mismatches, single-strand breaks (SSB), double-strand breaks (DSB), inter-strand crosslinks, intra-strand crosslinks, bulky adducts and genomic rearrangements [5].

Figure 1. Sources contributing to genomic instability. ncRNA = non-coding RNA; TE = transposable element. This figure was generated using BioRender.
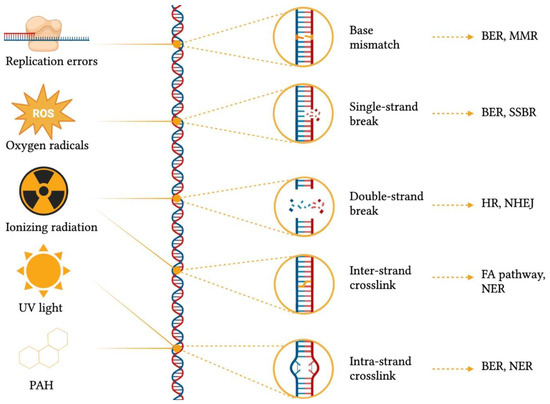
In order to remediate DNA damage, a plethora of DNA repair mechanisms (Figure 2) have emerged across the tree of life, and their importance is evidenced by the presence of redundant, complementary and conserved repair systems. Indeed, such systems are so important that in the debated research for a minimal genome, researchers found that up to 5% of the required genes have to be committed to DNA repair mechanisms [6][7]. The rationale behind having such a wealth of repair systems lies in the fact that each mechanism is able to recognize and fix specific damage substrates [8]. For example, base-excision repair (BER) can resolve base mismatches, single-strand breaks, and intra-strand crosslinks by generating an apurinic/apyrimidinic site (AP site), which is then cleaved by an AP endonuclease, thus creating a single-strand break that is then closed by nucleotide synthesis [9]. In addition, base mismatches can also be detected and corrected by mismatch repair (MMR) together with replication slippages [10], whereas single-strand breaks are also repaired by SSB repair (SSBR) [11]. Conversely, DSB—the most deleterious form of DNA damage—can be either resolved by homologous recombination (HR), which uses a homologous DNA template for repair [12], or by nonhomologous end-joining (NHEJ) through DNA ends ligation [13]. Finally, inter-strand crosslinks can be processed via the Fanconi anaemia (FA) pathway and nucleotide excision repair (NER), which removes bulky, helix-distorting lesions, including intra-strand crosslinks [14].

Figure 2. Common DNA damaging agents, types of genomic scars caused by different damage sources, and damage repair mechanisms. BER = base-excision repair; FA = Fanconi anaemia; HR = homologous recombination; MMR = mismatch repair; NER = nucleotide excision repair; NHEJ = nonhomologous end-joining; PAH = polycyclic aromatic hydrocarbons, ROS = reactive oxygen species; SSBR = single-strand break repair; UV = ultra-violet. This figure was generated using BioRender.
Both DNA damage and repair systems leave genomic scars typical of the mechanisms involved, thus generating genomic diversity. Such scars can either be driving forces to physiological processes—as adaptive immune response and meiosis (e.g., DSB-mediated recombination) [15][16]—or side effects of a defective restoration of information. For example, MMR deficiency may induce single nucleotide substitution and variation in the length of short repetitive DNA sequences (e.g., microsatellites) [17], whereas HR defects typically lead to loss-of-heterozygosity, allelic imbalances extending to the telomeres and large-scale rearrangements [18].
DNA damage and repair, change and conservation of information, and adaptation and selection are factors whose weights reside on opposite plates of the scale, and the balance between them is ruled by laws that still need to be fully understood. In this context, the environment emerges as a not-so-hidden judge favouring one plate over the other. A notable example is the onset of “hypermutator” phenotypes in response to environmental factors [19][20]. This phenomenon implies increased mutation rates and can be observed in several events, such as microbial adaptation and cancer evolution [21][22][23]. Many theories have been proposed to explain genomic instability in terms of fitness in certain environments. Focusing on microbial adaptation, the prevailing hypothesis states that genomic instability is a profitable event because it increases the microbial population’s overall chance of survival [24][25][26]. Breivik and colleagues proposed a shift of perspective from colony to single-cell level by focusing on the biological cost of DNA repair: genomic instability arises because DNA repair may cost more than the errors it prevents in mutagenic environments [27]. Even though the debate on the possible advantages brought by genomic instability is still open, unstable genomes seem to be transiently favoured in stressful environments, whereas stable ones adapt more successfully in the long run [28][29].
3. The Guardians of Genomic Stability across the Tree of Life
One of the first attempts to study the evolution of repair systems was carried out by Aravind, Walker and Koonin in 1999 [8]. The scientists searched for homologues of the repair proteins sequences of model organisms Escherichia coli and Saccharomyces cerevisiae in many bacteria, archaea and eukaryotes genomes. The authors reported a considerable heterogeneity in repair systems across the tree of life: they found that proteins involved in DNA repair seem to follow the “domain Lego” principle, according to which proteins are generated by copying, shuffling and recombining a limited number of conserved domains. Moreover, horizontal gene transfer—between bacteria and archaea, as well as between organellar and eukaryotic genomes—was proposed as a key mechanism contributing to the richness of repair systems.
The last universal common ancestor is supposed to have evolved in a high temperature and anoxic environment when the Earth magnetic field was still weak and the planet vulnerable to ionizing radiation. Since that time, the environment and conditions in which life develops and thrives have dramatically transformed and the revolutionary change has been the increase in oxygenation levels, determined by the evolving organisms themselves [30]. Indeed, before the Great Oxidation Event (around 2.4–2.0 giga-annum ago), the predominant threats to genomic integrity were base loss, cytosine deamination, and damages induced by UV light, ionizing radiation, and alkylation, whereas in today’s world oxidative DNA damage is a substantially bigger concern [31].
3.1. Nucleotide Excision Repair Pathway
Modern NER evolved only after the separation of bacteria and eukaryote domains and introduced the ability to repair a wide range of bulky helix-distorting DNA adducts by damage recognition, lesion excision and DNA synthesis [32]. In particular, NER is present in bacteria in the form of the widely conserved UvrABC protein complex: UvrA is involved in damage recognition, UvrB is a helicase that opens the dsDNA and UvrC a nuclease that operates cuts on both sides of the damage [33]. In eukaryotes, an analogous and more complex NER pathway can be found: XPC-hr23b performs damage recognition, transcription factor IIH opens the dsDNA and binds to XPA and RPA proteins, and XPF-ERCC1 and XPG nucleases are finally recruited to perform lesion excision [33].
3.2. Mismatch Repair System
Another universal repair pathway is MMR which has as key players MutS and MutL proteins in bacterial species (except for Actinobacteria) and their respective homologues in eukaryotes [34]. Interestingly, most archaea lack MutS and MutL genes homologues and the few groups that harbour them tend to be temperature mesophiles such as halophiles and methanogens, which most likely acquired these genes via the horizontal transfer. In the great majority of archaeal organisms an alternative pathway, named endonuclease mismatch specific (EndoMS), that detects and corrects mismatches can be found [35]. Curiously, EndoMS is also present in bacterial genomes belonging to the Actinobacteria phylum, where MutS and MutL are absent [36]. MMR deficiency in humans is associated with microsatellite instability (MSI) across different cancer types as colorectal and endometrial carcinomas [37].
3.3. Double-Strand Break Repair
Both HR and NHEJ systems are involved in the repair of DSBs. HR is one of the universal DNA repair systems and is implicated in the restart of DNA replication at stalled forks [38]. It is also involved in promoting genetic diversity via DNA transfer [39][40]. However, this is an energetically demanding and complex process, and, for this reason, simpler but less accurate pathways, such as NHEJ, operate alongside HR. In eukaryotic cells, NHEJ is commonly used in the G1 phase of the cell cycle since it does not depend on the presence of a homologous DNA duplex [33]. The NHEJ pathway has also been reported in bacteria by Weller and colleagues, who underlined the need of a DNA end-binding bacterial Ku protein for the correct operation of this system [41].
3.4. DNA Repair: Going Viral
At the end of this excursus through the branches of the tree of life, it is necessary to include a reflection on viruses. Indeed, DNA damage response can be activated by incoming viral DNA, during the integration of retroviruses, in response to aberrant DNA structures generated upon active viral DNA synthesis, or during persistence of extrachromosomal viral genomes [42][43].
4. Genomic (in)Stability in Homo sapiens: Just a Matter of Luck?
4.1. Nuclear and Mitochondrial DNA
Nuclear DNA and mitochondrial (mtDNA) are two separated genomes, indeed they present structurally different DNA molecules: the diploid linear nuclear genome and the multi-copy haploid circular mitochondrial genome. In recent decades, several studies pointed out their interconnection relatively to the handling of DNA damage [44][45][46]. For example, Baulch reported how genomic instability induced by radiation may alter cellular epigenetic mechanisms and can reduce mitochondrial functions; at the same time, mitochondrial dysfunction hampers the cell epigenetic profiles [47].
Several DNA repair mechanisms emerged throughout evolution, and more than 125 genes involved in such systems have been identified in humans [48][49]. It has been observed that the mutation rate is higher in mitochondria than in the nuclear DNA [50][51]. Specifically, mtDNA is more prone to oxidative damage due to the presence of a higher concentration of ROS—which account for approximately 10,000 daily DNA lesions per cell [52]—and the lack of chromatin protection [53]. The accumulation of mtDNA damage can lead to mitochondrial dysfunction and has been linked to age-related diseases, such as Parkinson’s disease [54] or Werner syndrome [55], and to different types of cancer [56].
4.2. Evolutionary Insights
Genomic instability is gradually acquiring a central role in our knowledge on human evolution, providing novel insights on our past as humankind, as well as new perspectives on future therapeutical targets. Breivik and Gaudernack [27] were probably the first to hypothesize the thin trade-off intrinsic to genomic instability: the loss of genomic stability might give to evolutionary mechanisms the opportunity to take action and to explore possibilities for fitness advancement and novel adaptation [57], but, at the same time, it might be indirectly associated with an increased risk of several late-onset diseases [58], which from an evolutionary perspective are unfavourable explorations. From a similar perspective, Little gave insight on the concept of randomness, profoundly inborn in evolution and, hence, in the evolutionary trade-off concept [59]: not only does evolution take place in random ways but also DNA damage and genomic instability happen randomly either in time (in terms of lifetime as well as triggering causes) or in space (in terms of genomic location or cellular localisation).
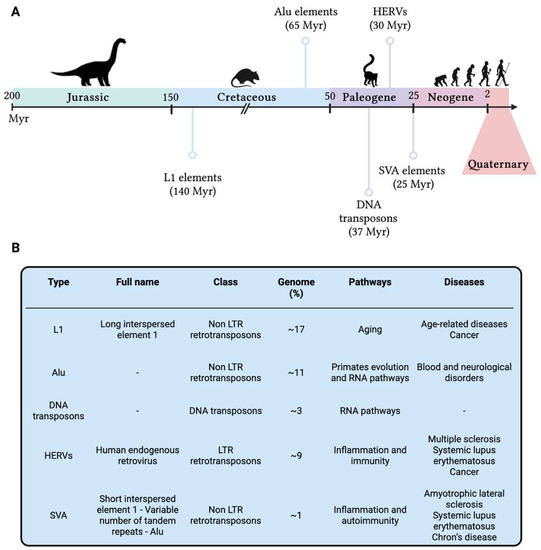
When it comes to humans, this novel perspective amplifies the research focus not only on present-day biological processes but also on our genomic history as human beings: all our genetic changes are written in our DNA, hence investigating DNA lesions scarred in our genomes might provide novel insights on host-pathogen co-evolution, genomic instability, and disease onset and/or severity. To better understand genomic (in)stability across the human evolution, Cordaux and Batzer [60] focused on the analysis of transposable elements (TEs) as major players still influencing the human genome functionality. TEs are DNA sequences that can move within the genome, originally identified in maize [61] and subsequently confirmed in humans [62]. Nearly half of the human genome seems to be composed of TEs, and this could be an underestimation due to the presence of extremely ancient TEs which are no longer recognisable [63]. In human, several TEs have been identified such as DNA transposons, long terminal repeats (LTRs) retrotransposons as the human endogenous retroviruses (HERVs), non-LTR retrotransposons as long interspersed element 1 (LINE-1 or L1), Alu and SINE-R-VNTR-Alu (SVA—composed of short interspersed nuclear element of retroviral origin [SINE-R], variable number of tandem repeats [VNTR], and Alu), and other minor elements [64]. All these elements are densely distributed along the genome and have a strong impact in shaping human genomic structural and functional features, carrying information of past adaptation as well as the seeds of evolution, either in terms of fitness improvement and genomic innovation [65], or as causes of genomic instability and genetic disorders [66]. However, TEs are currently not mobile in the human genome and their last activity is definitely far from present days. TEs were active at different ages along the evolutionary history of mammalian organisms, and their origin is dated several millions of years (Myr) ago (Figure 3).

Figure 3. (A) Timeline of the activities of the primary transposable elements which had a fundamental role in the evolution of present-day humans. (B) Main transposable elements in the human genome reported in chronological order of activity. Genome = the percentage of human genome identified as TE type; Pathways = pathways in which TEs have been shown to play a role; Diseases = diseases in which TEs have been reported to be implicated. HERVs = Human Endogenous Retroviruses; L1 = Long Interspersed Nuclear Elements 1; LTR = Long Terminal Repeats; Myr = millions of years; SVA = SINE-R-VNTR-Alu. This figure was generated using BioRender.
SVA elements [67] are not so present in the human genome, accounting for roughly 3000 copies [68], probably due to their nonautonomous nature and their LINE-1-related origin [69]; however, like most of the previous reported TEs, SVA seems to be associated with inflammatory conditions and autoimmune diseases, such as amyotrophic lateral sclerosis [70], systemic lupus erythematosus and Chron’s disease [71]. TEs showed to be fundamental for hominoid and human evolution, shaping their development and genomic advancement for several hundreds of millions of years and resulting in an increase in size of the human genome and a significant inter-individual variability in TEs content [72], turning out to be a highly informative vault for human evolutionary history. Moreover, their presence in present-day humans is a strong signal of evolutionary advantage, since they have been maintained in our genome for several millennia: some examples are TEs contribution to genetic innovation, such as the introduction of new genes in the whole known lifespan of humankind [60] and their implication in some regulatory networks [73], including immunity [74] and embryonic development [75].
5. Genomic Instability, Aging and Late Onset Complex Diseases
5.1. Telomeric Instability
Human telomeres are composed of a long stretch (up to tens of kilo-base pairs) of TTAGGG nucleotide repeats located at the end of each chromosome to protect them from degradation and ensure their stability [76][77]. Indeed, cells carry a variety of mechanisms and proteins—including the shelterin complex and telomerase—responsible for the maintenance of telomeres length [77]. However, the mitotic process determines a shortening of telomeres in daughter cells compared to the parent cell, thus telomeres have been proposed as “molecular clocks” for aging [78]. Moreover, shortened telomeres trigger replicative senescence and impair the regenerative capacity of tissues, which is undesirable in the case of pluripotent stem cells and adult stem cell compartments [79].
Impairment of telomeric maintenance and accelerated telomere shortening have been found to be associated with some of the leading causes of disease and death; among them: central obesity [80][81], lifetime accumulation of stress [82][83], increased risk of cardiovascular events [84][85], and reduced immune response to influenza vaccination [86]. In particular, somatic mutations in genes involved in telomeres maintenance have been linked to the functional decline of B lymphocytes, skeletal muscle cells, and neurons [78].
5.2. Microsatellites Structural Maintenance
Microsatellites are short tandemly repeated sequence motifs consisting of 1–6 bp that are typically repeated up to 50 times in millions of locations across the genome [87]. At least two main mechanisms can play a role in the failure of microsatellite structure maintenance: (1) DNA replication errors (e.g., due to polymerase slippage) that impact the length of microsatellites, and (2) defects in DNA repair mechanisms that determine an accumulation of errors leading to the generation of shorter/longer novel fragments (also known as MSI).
In the first case, the number of repeat units changes from one generation to the next due to replication slippage. In particular, alleles with a higher repeat number appear to be less stable than those with a lower number of repeats, which explains why a highly significant excess (compared to the expectation under the assumption of random effect) of long microsatellites has been observed in humans and across different species [88]. This type of instability can affect different genomic locations with a varying magnitude, which is reflected both in repeat expansion disorder onset timeframe (i.e., the greater the damage, the earlier the onset age, also known as anticipation) and phenotypic severity (i.e., ranging from mild to severe phenotypes).
5.3. Mitochondrial Dysfunction
Mitochondria are additional key players involved in aging and CD development. Actually, the role of mitochondria in aging is so determining that a “mitochondrial theory of aging” has been proposed [89]: with age, mitochondria accumulate ROS-induced damage and become dysfunctional, and the function of cells declines causing aging. It is apparent that mitochondrial dysfunction particularly affects organs that require high levels of energy such as the heart, skeletal muscles and brain [90].
5.4. Human Networking: The Systemic Complexity of Life
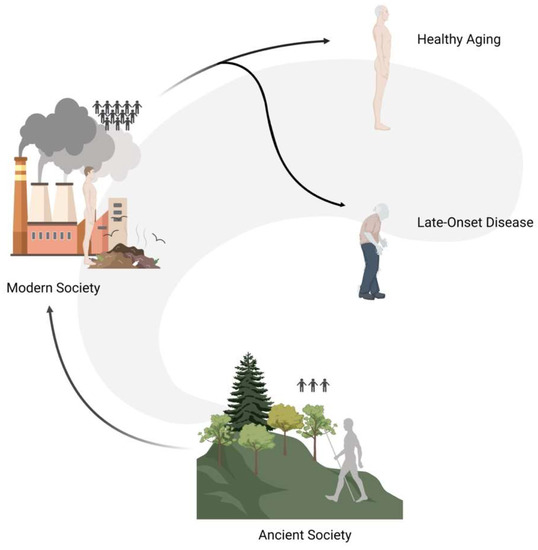
As humans, we do not live as single entities but we are a part of complex systems and communities (Figure 4). In the past, the great majority of people lived in isolated groups composed of a small number of individuals (i.e., 50–100 people), and only recently—for evolutionary times—have people started gathering in larger cities. Such transition went hand in hand with a radical change in social dynamics that had repercussions on the population genetic scale: in the past, the genetic variation pool of individual communities was very limited and selective pressures (e.g., disease, famine, etc.) were particularly high, whereas today humans make up a very extensive community with an overall rich pool of genetic variation.

Figure 4. Graphical representation of social dynamics changes that had repercussions on the populations genetic level. In the past, people lived in isolated groups composed of a small number of individuals (ancient society), and only recently have people started gathering in larger cities (modern society). The modern ability to prevent the impact of selective pressures determined the maintenance of variants that in a natural setup would have been filtered out, thus possibly causing late-onset diseases.
Up to the XX century infectious diseases were the main cause of mortality, whereas, in recent decades, this role has been taken up by diseases such as cancers, cardiovascular diseases, and metabolic disorders [91]. This might indicate that the attenuation of selective pressures acting on human beings might allow variants with mild effects to play a detectable role in the long term, either through damage accumulation (e.g., threshold effect) or simply because they have the chance to manifest their effects with the progression of aging.
6. The Epigenome: Shedding Light on the Dark Side of the Genome
As emerges from this broad overview, the long-term survival of a species is naturally linked to adaptation and depends on a thin balance between genome stability and its intrinsic tendency to corrupt and change. Over the past decade, numerous studies have tried to identify classes of molecular mechanisms related to aging and disease. López-Otín and colleagues proposed a total of nine hallmarks, including the epigenome, that has emerged as an important player in the decline of cell function observed both in aging and late-onset CDs [92][93]. The epigenome consists of chemical alterations to the DNA and histone proteins that results in changes to the structure of chromatin and function of the genome that can be inherited from parent to offspring [94].
Functional studies in humans and model organisms have shown that epigenetic modifications are crucial at all stages of development because of their ability to regulate genes transcriptionally. Particularly, multiple epigenetic events were found altered across different species during aging: accumulation of histone variants, changes in chromatin accessibility, loss of histones and heterochromatin, histone modifications, and deregulated expression/activity of microRNAs (miRNAs) [95][96]. Over the years, aging has been associated with increased transcriptional noise characterized by aberrant production and maturation of both many mRNAs and ncRNAs [97][98]. With the advent of new sequencing technologies, several tissue- and organism-specific transcriptional signatures of aging have been identified [99][100][101]. Barth and colleagues have identified conserved aging-related transcriptional signatures that characterize all tissues of long-lived individuals [102][103]. These transcriptional signatures involve the downregulation of a specific class of miRNAs associated with aging, called geromiR, which can influence lifespan by negatively controlling the gene expression of target components that are part of longevity networks [92].
6.1. The Non-Coding Impact on Coding
MicroRNAs are involved in the regulation of almost all cellular processes through specific downregulation of gene expression at the post-transcriptional level. Indeed, they can influence the translation of more than 60%
of the protein-coding genes [104]. In addition to their intracellular functions, miRNAs can act as active messengers that trigger a systemic response. Among these, the group called inflamma-miRs can affect inflammatory pathways [105]. An excess of inflammatory activation has been associated with the development of major age-related diseases, such as cardiovascular disease, Alzheimer’s disease, rheumatoid arthritis, type 2 diabetes mellitus and cancers [106]. The dysregulation of most circulating inflamma-miRs may contribute to the development and progression of these diseases by cooperatively regulating a given biological process [107].
Although miRNAs have been well studied in humans, they are just the tip of the iceberg. A series of ncRNAs can play significant roles, among them: small nuclear RNAs (snoRNAs), circular RNAs (circRNAs), PIWI-interacting RNAs (piRNAs), and a large group of long non-coding RNAs (lncRNAs), including non-coding transcripts from intergenic regions (lincRNAs). These ncRNAs function as part of a complex network that intervenes in many processes, including aging and senescence, through the modulation of gene expression, genomic imprinting and nuclear organization [93][108][109]. Moreover, several studies have shown that ncRNAs play a crucial role in regulating genes involved in DNA damage repair mechanisms, and in maintaining genomic stability through the activation of cell cycle checkpoints and induction of apoptosis when the damage is irreparable [110]. In response to damage, the action of ncRNAs functions as a key node connecting the rapid DR-mediated protein modifications and the late response mediated by transcriptional regulation [111]. However, at the same time, DNA damage can alter ncRNA expression at multiple levels, including transcriptional and post-transcriptional regulation and degradation [107][112][113]. Alterations of their regulatory functions are particularly relevant in the context of aging.
6.2. The Diamond in the “Junk”
Unlike DNA mutations, epigenetic alterations and deregulations of ncRNAs—which were once considered “junk”—are theoretically reversible, offering opportunities for the development of new perspectives and insights on possible new therapeutic interventions [92][114]. In recent years, there has been growing interest in using ncRNAs as therapeutic agents for a wide range of pathologies. However, there are several challenges in designing effective therapies that exploit the effects of ncRNAs because multiple molecular mechanisms are involved in different pathologies.
References
- Igamberdiev, A.U. The Drawbridge of Nature: Evolutionary Complexification as a Generation and Novel Interpretation of Coding Systems. Biosystems 2021, 207, 104454.
- Pross, A. On the Emergence of Biological Complexity: Life as a Kinetic State of Matter. Orig. Life Evol. Biosph. 2005, 35, 151–166.
- Voskarides, K.; Dweep, H.; Chrysostomou, C. Evidence That DNA Repair Genes, a Family of Tumor Suppressor Genes, Are Associated with Evolution Rate and Size of Genomes. Hum. Genom. 2019, 13, 26.
- Basu, A.K.; Essigmann, J.M. Establishing Linkages Among DNA Damage, Mutagenesis, and Genetic Diseases. Chem. Res. Toxicol. 2022, 35, 1655–1675.
- Clarke, T.L.; Mostoslavsky, R. DNA Repair as a Shared Hallmark in Cancer and Ageing. Mol. Oncol. 2022, 16, 3352–3379.
- Gibson, D.G.; Glass, J.I.; Lartigue, C.; Noskov, V.N.; Chuang, R.-Y.; Algire, M.A.; Benders, G.A.; Montague, M.G.; Ma, L.; Moodie, M.M.; et al. Creation of a Bacterial Cell Controlled by a Chemically Synthesized Genome. Science 2010, 329, 52–56.
- Callaway, E. ‘Minimal’ Cell Raises Stakes in Race to Harness Synthetic Life. Nature 2016, 531, 557–558.
- Aravind, L.; Walker, D.R.; Koonin, E.V. Conserved Domains in DNA Repair Proteins and Evolution of Repair Systems. Nucleic Acids Res. 1999, 27, 1223–1242.
- Krokan, H.E.; Bjørås, M. Base Excision Repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012583.
- Kunkel, T.A.; Erie, D.A. Eukaryotic Mismatch Repair in Relation to DNA Replication. Annu. Rev. Genet. 2015, 49, 291–313.
- Caldecott, K.W. Single-Strand Break Repair and Genetic Disease. Nat. Rev. Genet. 2008, 9, 619–631.
- Kowalczykowski, S.C. An Overview of the Molecular Mechanisms of Recombinational DNA Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a016410.
- Waters, C.A.; Strande, N.T.; Wyatt, D.W.; Pryor, J.M.; Ramsden, D.A. Nonhomologous End Joining: A Good Solution for Bad Ends. DNA Repair. 2014, 17, 39–51.
- Hashimoto, S.; Anai, H.; Hanada, K. Mechanisms of Interstrand DNA Crosslink Repair and Human Disorders. Genes Environ. 2016, 38, 9.
- Christie, S.M.; Fijen, C.; Rothenberg, E. V(D)J Recombination: Recent Insights in Formation of the Recombinase Complex and Recruitment of DNA Repair Machinery. Front. Cell Dev. Biol. 2022, 10, 886718.
- Hanscom, T.; McVey, M. Regulation of Error-Prone DNA Double-Strand Break Repair and Its Impact on Genome Evolution. Cells 2020, 9, 1657.
- Olave, M.C.; Graham, R.P. Mismatch Repair Deficiency: The What, How and Why It Is Important. Genes Chromosomes Cancer 2022, 61, 314–321.
- Gonzalez, D.; Stenzinger, A. Homologous Recombination Repair Deficiency (HRD): From Biology to Clinical Exploitation. Genes Chromosomes Cancer 2021, 60, 299–302.
- Shiomi, T.; Hieda-Shiomi, N.; Sato, K.; Tsuji, H.; Takahashi, E.-I.; Tobari, I. A Mouse-Cell Mutant Sensitive to Ionizing Radiation Is Hypermutable by Low Doses of γ-Radiation. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1981, 83, 107–116.
- Todd, P.A.; Brouwer, J.; Glickman, B.W. Influence of DNA-Repair Deficiencies on MMS- and EMS-Induced Mutagenesis in Escherichia Coli K-12. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1981, 82, 239–250.
- Veschetti, L.; Sandri, A.; Krogh Johansen, H.; Lleò, M.M.; Malerba, G. Hypermutation as an Evolutionary Mechanism for Achromobacter Xylosoxidans in Cystic Fibrosis Lung Infection. Pathogens 2020, 9, 72.
- Campbell, B.B.; Light, N.; Fabrizio, D.; Zatzman, M.; Fuligni, F.; de Borja, R.; Davidson, S.; Edwards, M.; Elvin, J.A.; Hodel, K.P.; et al. Comprehensive Analysis of Hypermutation in Human Cancer. Cell 2017, 171, 1042–1056.e10.
- Grote, A.; Earl, A.M. Within-Host Evolution of Bacterial Pathogens during Persistent Infection of Humans. Curr. Opin. Microbiol. 2022, 70, 102197.
- Tenaillon, O.; Le Nagard, H.; Godelle, B.; Taddei, F. Mutators and Sex in Bacteria: Conflict between Adaptive Strategies. Proc. Natl. Acad. Sci. USA 2000, 97, 10465–10470.
- Janin, N. A Simple Model for Carcinogenesis of Colorectal Cancers with Microsatellite Instability. Adv. Cancer Res. 2000, 77, 189–221.
- Shaver, A.C.; Dombrowski, P.G.; Sweeney, J.Y.; Treis, T.; Zappala, R.M.; Sniegowski, P.D. Fitness Evolution and the Rise of Mutator Alleles in Experimental Escherichia Coli Populations. Genetics 2002, 162, 557–566.
- Breivik, J.; Gaudernack, G. Resolving the Evolutionary Paradox of Genetic Instability: A Cost-Benefit Analysis of DNA Repair in Changing Environments. FEBS Lett. 2004, 563, 7–12.
- Scheuerl, T.; Hopkins, M.; Nowell, R.W.; Rivett, D.W.; Barraclough, T.G.; Bell, T. Bacterial Adaptation Is Constrained in Complex Communities. Nat. Commun. 2020, 11, 754.
- Veschetti, L.; Boaretti, M.; Saitta, G.M.; Passarelli Mantovani, R.; Lleò, M.M.; Sandri, A.; Malerba, G. Achromobacter Spp. Prevalence and Adaptation in Cystic Fibrosis Lung Infection. Microbiol. Res. 2022, 263, 127140.
- Lyons, T.W.; Reinhard, C.T.; Planavsky, N.J. The Rise of Oxygen in Earth’s Early Ocean and Atmosphere. Nature 2014, 506, 307–315.
- Warke, M.R.; Di Rocco, T.; Zerkle, A.L.; Lepland, A.; Prave, A.R.; Martin, A.P.; Ueno, Y.; Condon, D.J.; Claire, M.W. The Great Oxidation Event Preceded a Paleoproterozoic “Snowball Earth”. Proc. Natl. Acad. Sci. USA 2020, 117, 13314–13320.
- Goosen, N.; Moolenaar, G.F. Repair of UV Damage in Bacteria. DNA Repair. 2008, 7, 353–379.
- Brunette, G.J.; Jamalruddin, M.A.; Baldock, R.A.; Clark, N.L.; Bernstein, K.A. Evolution-Based Screening Enables Genome-Wide Prioritization and Discovery of DNA Repair Genes. Proc. Natl. Acad. Sci. USA 2019, 116, 19593–19599.
- Kelman, Z.; White, M.F. Archaeal DNA Replication and Repair. Curr. Opin. Microbiol. 2005, 8, 669–676.
- Ishino, S.; Nishi, Y.; Oda, S.; Uemori, T.; Sagara, T.; Takatsu, N.; Yamagami, T.; Shirai, T.; Ishino, Y. Identification of a Mismatch-Specific Endonuclease in Hyperthermophilic Archaea. Nucleic Acids Res. 2016, 44, 2977–2986.
- Castañeda-García, A.; Prieto, A.I.; Rodríguez-Beltrán, J.; Alonso, N.; Cantillon, D.; Costas, C.; Pérez-Lago, L.; Zegeye, E.D.; Herranz, M.; Plociński, P.; et al. A Non-Canonical Mismatch Repair Pathway in Prokaryotes. Nat. Commun. 2017, 8, 14246.
- Schöniger, S.; Rüschoff, J. Mismatch Repair Deficiency and Microsatellite Instability. Encyclopedia 2022, 2, 1559–1576.
- White, M.F. Homologous Recombination in the Archaea: The Means Justify the Ends. Biochem. Soc. Trans. 2011, 39, 15–19.
- van Wolferen, M.; Wagner, A.; van der Does, C.; Albers, S.-V. The Archaeal Ced System Imports DNA. Proc. Natl. Acad. Sci. USA 2016, 113, 2496–2501.
- Naor, A.; Altman-Price, N.; Soucy, S.M.; Green, A.G.; Mitiagin, Y.; Turgeman-Grott, I.; Davidovich, N.; Gogarten, J.P.; Gophna, U. Impact of a Homing Intein on Recombination Frequency and Organismal Fitness. Proc. Natl. Acad. Sci. USA 2016, 113, E4654–E4661.
- Weller, G.R.; Kysela, B.; Roy, R.; Tonkin, L.M.; Scanlan, E.; Della, M.; Devine, S.K.; Day, J.P.; Wilkinson, A.; di Fagagna, F. d’Adda; et al. Identification of a DNA Nonhomologous End-Joining Complex in Bacteria. Science 2002, 297, 1686–1689.
- Luftig, M.A. Viruses and the DNA Damage Response: Activation and Antagonism. Annu. Rev. Virol. 2014, 1, 605–625.
- Weitzman, M.D.; Fradet-Turcotte, A. Virus DNA Replication and the Host DNA Damage Response. Annu. Rev. Virol. 2018, 5, 141–164.
- Rusecka, J.; Kaliszewska, M.; Bartnik, E.; Tońska, K. Nuclear Genes Involved in Mitochondrial Diseases Caused by Instability of Mitochondrial DNA. J. Appl. Genet. 2018, 59, 43–57.
- Shimura, T.; Kunugita, N. Mitochondrial Reactive Oxygen Species-Mediated Genomic Instability in Low-Dose Irradiated Human Cells through Nuclear Retention of Cyclin D1. Cell Cycle 2016, 15, 1410–1414.
- Tsai, M.-H.; Fang, W.-H.; Lin, S.-W.; Yen, S.-J.; Chou, S.-J.; Yang, Y.-C. Mitochondrial Genomic Instability in Colorectal Cancer: No Correlation to Nuclear Microsatellite Instability and Allelic Deletion of HMSH2, HMLH1, and P53 Genes, but Prediction of Better Survival for Dukes’ Stage C Disease. Ann. Surg. Oncol. 2009, 16, 2918–2925.
- Baulch, J.E. Radiation-Induced Genomic Instability, Epigenetic Mechanisms and the Mitochondria: A Dysfunctional Ménage a Trois? Int. J. Radiat. Biol. 2019, 95, 516–525.
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA Damage, Repair, and Mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263.
- Ronen, A.; Glickman, B.W. Human DNA Repair Genes. Environ. Mol. Mutagen. 2001, 37, 241–283.
- Alexeyev, M.; Shokolenko, I.; Wilson, G.; LeDoux, S. The Maintenance of Mitochondrial DNA Integrity—Critical Analysis and Update. Cold Spring Harb. Perspect. Biol. 2013, 5, a012641.
- Zinovkina, L.A. Mechanisms of Mitochondrial DNA Repair in Mammals. Biochem. Mosc. 2018, 83, 233–249.
- Martin, L.J.; Liu, Z. DNA Damage Profiling in Motor Neurons: A Single-Cell Analysis by Comet Assay. Neurochem. Res. 2002, 27, 1093–1104.
- Rong, Z.; Tu, P.; Xu, P.; Sun, Y.; Yu, F.; Tu, N.; Guo, L.; Yang, Y. The Mitochondrial Response to DNA Damage. Front. Cell Dev. Biol. 2021, 9, 669379.
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Coordination of Mitophagy and Mitochondrial Biogenesis during Ageing in C. Elegans. Nature 2015, 521, 525–528.
- Fang, E.F.; Hou, Y.; Lautrup, S.; Jensen, M.B.; Yang, B.; SenGupta, T.; Caponio, D.; Khezri, R.; Demarest, T.G.; Aman, Y.; et al. NAD+ Augmentation Restores Mitophagy and Limits Accelerated Aging in Werner Syndrome. Nat. Commun. 2019, 10, 5284.
- Liu, H.; Xu, J.; Dai, X.; Shi, J.-B.; Xu, S.; Gao, J.; Yao, Q.; Liu, F. Both DNA Damage and Mitochondrial Dysfunction Are Involved in Novel OxadiazoloPyrimidine Nucleoside Derivatives-Induced Cancer Cell Death. J. Appl. Toxicol. 2009, 29, 489–495.
- McKenzie, G.J.; Rosenberg, S.M. Adaptive Mutations, Mutator DNA Polymerases and Genetic Change Strategies of Pathogens. Curr. Opin. Microbiol. 2001, 4, 586–594.
- Sieber, O.M.; Heinimann, K.; Tomlinson, I.P.M. Genomic Instability—The Engine of Tumorigenesis? Nat. Rev. Cancer 2003, 3, 701–708.
- Little, M.P. Cancer Models, Genomic Instability and Somatic Cellular Darwinian Evolution. Biol. Direct 2010, 5, 19.
- Cordaux, R.; Batzer, M.A. The Impact of Retrotransposons on Human Genome Evolution. Nat. Rev. Genet. 2009, 10, 691–703.
- McClintock, B. Controlling Elements and the Gene. Cold Spring Harb. Symp. Quant. Biol. 1956, 21, 197–216.
- Williamson, A.R. The Biological Origin of Antibody Diversity. Annu. Rev. Biochem. 1976, 45, 467–500.
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial Sequencing and Analysis of the Human Genome. Nature 2001, 409, 860–921.
- Wells, J.N.; Feschotte, C. A Field Guide to Eukaryotic Transposable Elements. Annu. Rev. Genet. 2020, 54, 539–561.
- Qin, S.; Jin, P.; Zhou, X.; Chen, L.; Ma, F. The Role of Transposable Elements in the Origin and Evolution of MicroRNAs in Human. PLoS ONE 2015, 10, e0131365.
- Ayarpadikannan, S.; Kim, H.-S. The Impact of Transposable Elements in Genome Evolution and Genetic Instability and Their Implications in Various Diseases. Genom. Inf. 2014, 12, 98–104.
- Hancks, D.C.; Kazazian, H.H. SVA Retrotransposons: Evolution and Genetic Instability. Semin. Cancer Biol. 2010, 20, 234–245.
- Ostertag, E.M.; Goodier, J.L.; Zhang, Y.; Kazazian, H.H. SVA Elements Are Nonautonomous Retrotransposons That Cause Disease in Humans. Am. J. Hum. Genet. 2003, 73, 1444–1451.
- Raiz, J.; Damert, A.; Chira, S.; Held, U.; Klawitter, S.; Hamdorf, M.; Löwer, J.; Strätling, W.H.; Löwer, R.; Schumann, G.G. The Non-Autonomous Retrotransposon SVA Is Trans -Mobilized by the Human LINE-1 Protein Machinery. Nucleic Acids Res. 2012, 40, 1666–1683.
- Savage, A.L.; Wilm, T.P.; Khursheed, K.; Shatunov, A.; Morrison, K.E.; Shaw, P.J.; Shaw, C.E.; Smith, B.; Breen, G.; Al-Chalabi, A.; et al. An Evaluation of a SVA Retrotransposon in the FUS Promoter as a Transcriptional Regulator and Its Association to ALS. PLoS ONE 2014, 9, e90833.
- Lauc, G.; Huffman, J.E.; Pučić, M.; Zgaga, L.; Adamczyk, B.; Mužinić, A.; Novokmet, M.; Polašek, O.; Gornik, O.; Krištić, J.; et al. Loci Associated with N-Glycosylation of Human Immunoglobulin G Show Pleiotropy with Autoimmune Diseases and Haematological Cancers. PLoS Genet. 2013, 9, e1003225.
- Jurka, J.; Kapitonov, V.V.; Kohany, O.; Jurka, M.V. Repetitive Sequences in Complex Genomes: Structure and Evolution. Annu. Rev. Genom. Hum. Genet. 2007, 8, 241–259.
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory Activities of Transposable Elements: From Conflicts to Benefits. Nat. Rev. Genet. 2017, 18, 71–86.
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory Evolution of Innate Immunity through Co-Option of Endogenous Retroviruses. Science 2016, 351, 1083–1087.
- Gerdes, P.; Richardson, S.R.; Mager, D.L.; Faulkner, G.J. Transposable Elements in the Mammalian Embryo: Pioneers Surviving through Stealth and Service. Genome Biol. 2016, 17, 100.
- Blasco, M.A. Telomeres and Human Disease: Ageing, Cancer and Beyond. Nat. Rev. Genet. 2005, 6, 611–622.
- Palm, W.; de Lange, T. How Shelterin Protects Mammalian Telomeres. Annu. Rev. Genet. 2008, 42, 301–334.
- Ferrucci, L.; Gonzalez-Freire, M.; Fabbri, E.; Simonsick, E.; Tanaka, T.; Moore, Z.; Salimi, S.; Sierra, F.; de Cabo, R. Measuring Biological Aging in Humans: A Quest. Aging Cell 2020, 19, e13080.
- Marion, R.M.; Strati, K.; Li, H.; Tejera, A.; Schoeftner, S.; Ortega, S.; Serrano, M.; Blasco, M.A. Telomeres Acquire Embryonic Stem Cell Characteristics in Induced Pluripotent Stem Cells. Cell Stem Cell 2009, 4, 141–154.
- García-Calzón, S.; Gea, A.; Razquin, C.; Corella, D.; Lamuela-Raventós, R.M.; Martínez, J.A.; Martínez-González, M.A.; Zalba, G.; Marti, A. Longitudinal Association of Telomere Length and Obesity Indices in an Intervention Study with a Mediterranean Diet: The PREDIMED-NAVARRA Trial. Int. J. Obes. 2014, 38, 177–182.
- Mundstock, E.; Sarria, E.E.; Zatti, H.; Mattos Louzada, F.; Kich Grun, L.; Herbert Jones, M.; Guma, F.T.C.R.; Mazzola (in Memoriam), J.; Epifanio, M.; Stein, R.T.; et al. Effect of Obesity on Telomere Length: Systematic Review and Meta-Analysis. Obesity 2015, 23, 2165–2174.
- Osler, M.; Bendix, L.; Rask, L.; Rod, N.H. Stressful Life Events and Leucocyte Telomere Length: Do Lifestyle Factors, Somatic and Mental Health, or Low Grade Inflammation Mediate This Relationship? Results from a Cohort of Danish Men Born in 1953. Brain Behav. Immun. 2016, 58, 248–253.
- Puterman, E.; Gemmill, A.; Karasek, D.; Weir, D.; Adler, N.E.; Prather, A.A.; Epel, E.S. Lifespan Adversity and Later Adulthood Telomere Length in the Nationally Representative US Health and Retirement Study. Proc. Natl. Acad. Sci. USA 2016, 113, E6335–E6342.
- Baragetti, A.; Palmen, J.; Garlaschelli, K.; Grigore, L.; Humphries, S.; Catapano, A.L.; Talmud, P.J.; Giuseppe Danilo, N. Leukocyte Telomere Length, Genetically Determined, Is Causally Associated with the Progression of Carotid Intima-Media Thickness and Incidence of Cardiovascular Events. Atherosclerosis 2016, 252, e252.
- Hammadah, M.; Al Mheid, I.; Wilmot, K.; Ramadan, R.; Abdelhadi, N.; Alkhoder, A.; Obideen, M.; Pimple, P.M.; Levantsevych, O.; Kelli, H.M.; et al. Telomere Shortening, Regenerative Capacity, and Cardiovascular Outcomes. Circ. Res. 2017, 120, 1130–1138.
- Najarro, K.; Nguyen, H.; Chen, G.; Xu, M.; Alcorta, S.; Yao, X.; Zukley, L.; Metter, E.J.; Truong, T.; Lin, Y.; et al. Telomere Length as an Indicator of the Robustness of B- and T-Cell Response to Influenza in Older Adults. J. Infect. Dis. 2015, 212, 1261–1269.
- Halldorsson, B.V.; Eggertsson, H.P.; Moore, K.H.S.; Hauswedell, H.; Eiriksson, O.; Ulfarsson, M.O.; Palsson, G.; Hardarson, M.T.; Oddsson, A.; Jensson, B.O.; et al. The Sequences of 150,119 Genomes in the UK Biobank. Nature 2022, 607, 732–740.
- Dieringer, D.; Schlötterer, C. Two Distinct Modes of Microsatellite Mutation Processes: Evidence from the Complete Genomic Sequences of Nine Species. Genome Res. 2003, 13, 2242–2251.
- Harman, D. The Free Radical Theory of Aging. Antioxid. Redox Signal. 2003, 5, 557–561.
- Holloway, G.P.; Holwerda, A.M.; Miotto, P.M.; Dirks, M.L.; Verdijk, L.B.; van Loon, L.J.C. Age-Associated Impairments in Mitochondrial ADP Sensitivity Contribute to Redox Stress in Senescent Human Skeletal Muscle. Cell Rep. 2018, 22, 2837–2848.
- Mathers, C.D.; Boerma, T.; Ma Fat, D. Global and Regional Causes of Death. Br. Med. Bull. 2009, 92, 7–32.
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217.
- Pal, S.; Tyler, J.K. Epigenetics and Aging. Sci. Adv. 2016, 2, e1600584.
- Bernstein, B.E.; Meissner, A.; Lander, E.S. The Mammalian Epigenome. Cell 2007, 128, 669–681.
- Kane, A.E.; Sinclair, D.A. Epigenetic Changes during Aging and Their Reprogramming Potential. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 61–83.
- Saul, D.; Kosinsky, R.L. Epigenetics of Aging and Aging-Associated Diseases. Int. J. Mol. Sci. 2021, 22, 401.
- Bahar, R.; Hartmann, C.H.; Rodriguez, K.A.; Denny, A.D.; Busuttil, R.A.; Dollé, M.E.T.; Calder, R.B.; Chisholm, G.B.; Pollock, B.H.; Klein, C.A.; et al. Increased Cell-to-Cell Variation in Gene Expression in Ageing Mouse Heart. Nature 2006, 441, 1011–1014.
- Nicholas, A.; de Magalhaes, J.P.; Kraytsberg, Y.; Richfield, E.K.; Levanon, E.Y.; Khrapko, K. Age-Related Gene-Specific Changes of A-to-I MRNA Editing in the Human Brain. Mech. Ageing Dev. 2010, 131, 445–447.
- Zhang, S.; Duan, J.; Du, Y.; Xie, J.; Zhang, H.; Li, C.; Zhang, W. Long Non-Coding RNA Signatures Associated With Liver Aging in Senescence-Accelerated Mouse Prone 8 Model. Front. Cell Dev. Biol. 2021, 9, 698442.
- Stegeman, R.; Weake, V.M. Transcriptional Signatures of Aging. J. Mol. Biol. 2017, 429, 2427–2437.
- Costa, M.C.; Leitão, A.L.; Enguita, F.J. Noncoding Transcriptional Landscape in Human Aging. Curr. Top Microbiol. Immunol. 2016, 394, 177–202.
- Barth, E.; Srivastava, A.; Stojiljkovic, M.; Frahm, C.; Axer, H.; Witte, O.W.; Marz, M. Conserved Aging-Related Signatures of Senescence and Inflammation in Different Tissues and Species. Aging 2019, 11, 8556–8572.
- Frahm, C.; Srivastava, A.; Schmidt, S.; Mueller, J.; Groth, M.; Guenther, M.; Ji, Y.; Priebe, S.; Platzer, M.; Witte, O.W. Transcriptional Profiling Reveals Protective Mechanisms in Brains of Long-Lived Mice. Neurobiol. Aging 2017, 52, 23–31.
- Esteller, M. Non-Coding RNAs in Human Disease. Nat. Rev. Genet. 2011, 12, 861–874.
- Moskalev, A.; Aliper, A.; Smit-McBride, Z.; Buzdin, A.; Zhavoronkov, A. Genetics and Epigenetics of Aging and Longevity. Cell Cycle 2014, 13, 1063–1077.
- Olivieri, F.; Rippo, M.R.; Procopio, A.D.; Fazioli, F. Circulating Inflamma-MiRs in Aging and Age-Related Diseases. Front. Genet. 2013, 4, 121.
- Wang, L.-L.; Huang, Y.; Wang, G.; Chen, S.-D. The Potential Role of MicroRNA-146 in Alzheimer’s Disease: Biomarker or Therapeutic Target? Med. Hypotheses 2012, 78, 398–401.
- DiStefano, J.K. The Emerging Role of Long Noncoding RNAs in Human Disease. In Disease Gene Identification: Methods and Protocols; DiStefano, J.K., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; pp. 91–110. ISBN 978-1-4939-7471-9.
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391.
- Curtin, N.J. DNA Repair Dysregulation from Cancer Driver to Therapeutic Target. Nat. Rev. Cancer 2012, 12, 801–817.
- Zhang, C.; Peng, G. Non-Coding RNAs: An Emerging Player in DNA Damage Response. Mutat. Res./Rev. Mutat. Res. 2015, 763, 202–211.
- Wan, G.; Hu, X.; Liu, Y.; Han, C.; Sood, A.K.; Calin, G.A.; Zhang, X.; Lu, X. A Novel Non-Coding RNA LncRNA-JADE Connects DNA Damage Signalling to Histone H4 Acetylation. EMBO J. 2013, 32, 2833–2847.
- Wei, W.; Ba, Z.; Gao, M.; Wu, Y.; Ma, Y.; Amiard, S.; White, C.I.; Rendtlew Danielsen, J.M.; Yang, Y.-G.; Qi, Y. A Role for Small RNAs in DNA Double-Strand Break Repair. Cell 2012, 149, 101–112.
- Mattick, J.S.; Makunin, I.V. Non-Coding RNA. Hum. Mol. Genet. 2006, 15, R17–R29.
More
Information
Subjects:
Genetics & Heredity
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
645
Revisions:
2 times
(View History)
Update Date:
30 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





