You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrea Ehrmann | -- | 1737 | 2023-08-28 10:41:16 | | | |
| 2 | Catherine Yang | -12 word(s) | 1725 | 2023-08-28 10:54:41 | | | | |
| 3 | Catherine Yang | Meta information modification | 1725 | 2023-08-30 04:48:50 | | | | |
| 4 | Catherine Yang | Meta information modification | 1725 | 2023-08-30 04:49:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Blachowicz, T.; Ehrmann, A.; Wortmann, M. Exchange Bias in Nanostructures. Encyclopedia. Available online: https://encyclopedia.pub/entry/48524 (accessed on 01 January 2026).
Blachowicz T, Ehrmann A, Wortmann M. Exchange Bias in Nanostructures. Encyclopedia. Available at: https://encyclopedia.pub/entry/48524. Accessed January 01, 2026.
Blachowicz, Tomasz, Andrea Ehrmann, Martin Wortmann. "Exchange Bias in Nanostructures" Encyclopedia, https://encyclopedia.pub/entry/48524 (accessed January 01, 2026).
Blachowicz, T., Ehrmann, A., & Wortmann, M. (2023, August 28). Exchange Bias in Nanostructures. In Encyclopedia. https://encyclopedia.pub/entry/48524
Blachowicz, Tomasz, et al. "Exchange Bias in Nanostructures." Encyclopedia. Web. 28 August, 2023.
Copy Citation
Exchange bias (EB) is a unidirectional anisotropy occurring in exchange-coupled ferromagnetic/antiferromagnetic systems, such as thin films, core–shell particles, or nanostructures. In addition to a horizontal shift of the hysteresis loop, defining the exchange bias, asymmetric loops and even vertical shifts can often be found.
exchange bias (EB)
hysteresis loop shift
coercivity
ferromagnet
antiferromagnet
1. Introduction
The exchange bias (EB), a unidirectional magnetic anisotropy, was first reported by Meiklejohn and Bean for Co/CoO core–shell particles [1][2] and has since been extensively investigated. The main effect is a horizontal shift of the hysteresis loop in a system consisting of a ferromagnet (FM) exchange-coupled to an antiferromagnet (AFM), similar to a frozen internal magnetic field applied to the FM by the AFM’s fixed magnetic moments. Nevertheless, this simple explanation and corresponding naïve models cannot fully simulate the value of the horizontal shift, nor the other correlated changes in the hysteresis loop as compared to pure ferromagnets, i.e., an often-visible asymmetry of the loop as well as a potential vertical shift [3].
In addition to the first investigations of core–shell particles, usually with a ferromagnetic core and oxidized antiferromagnetic shell, experiments have evolved rapidly towards thin film systems [4][5] and further to nanostructured systems [6][7][8]. The materials under investigation are often ferromagnets coupled with antiferromagnets or ferrimagnets, such as Co/CoO [9], Fe/FeF2 [10], or Fe/MnF2 [11][12]. Recently, more sophisticated systems have become part of experimental and theoretical studies, e.g., Fe/LaAlO3 [13] or Pr0.67Sr0.33MnO3/SrTiO3 [14].
In addition to developing new materials for innovative EB systems with an enhanced effect size, more asymmetric loops, or other technically useful effects, e.g., applications for hard disk read/write heads and spintronics devices, the magnetic properties of well-known material systems can also be manipulated by creating nanostructures with different shapes and dimensions. In their comprehensive review from 2005, Nogués et al. described the effects in detail of the former state of research [6]. However, research activities dealing with exchange bias in general and exchange bias in nanostructures in particular have considerably increased since then, as depicted in Figure 1. It is intriguing that including the word “nano” in the search causes the bibliographic data to pass through a maximum value of around 2015. This indicates the achievement of some kind of technological excellence in the preparation of exchange-biased structures.
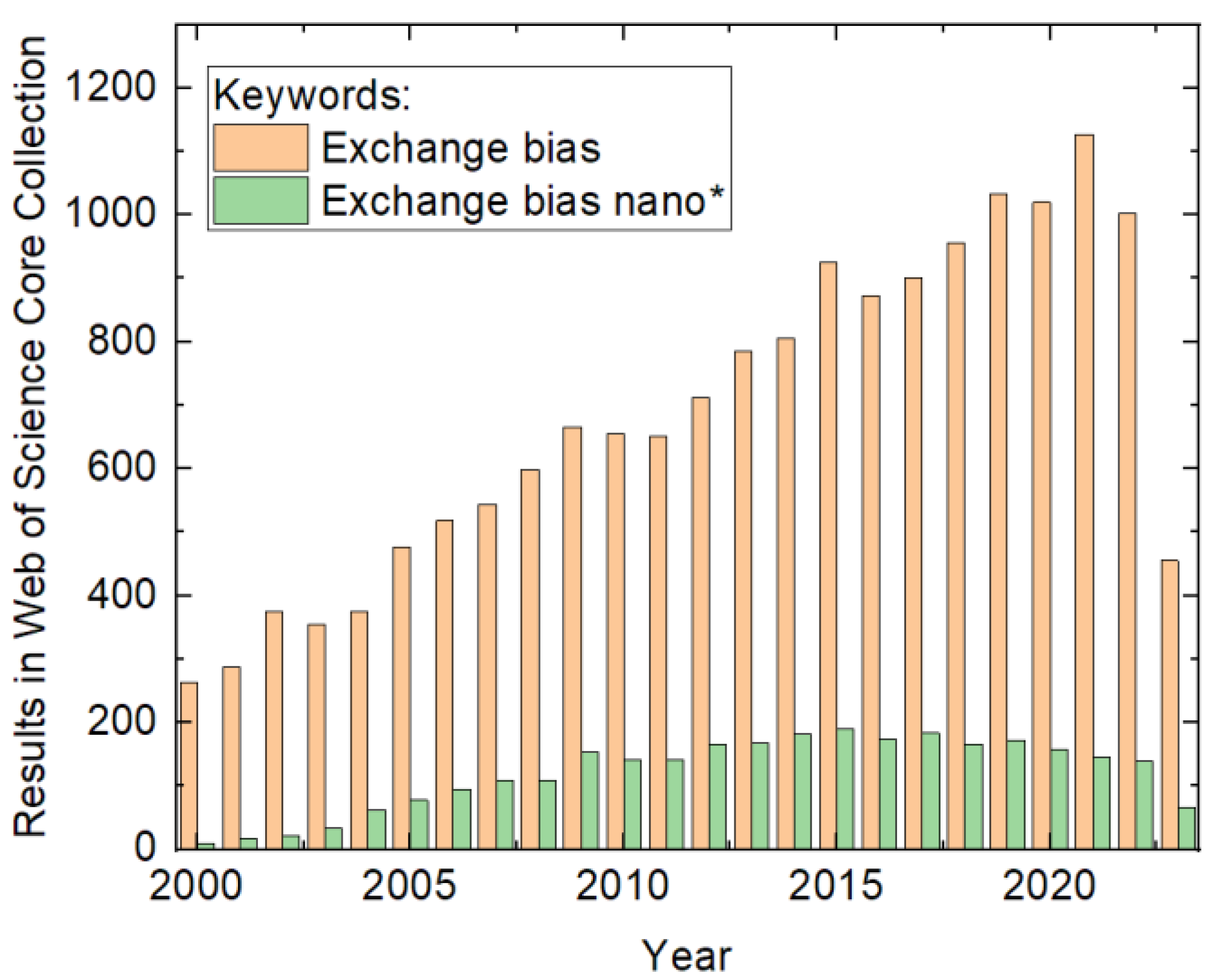
Figure 1. Numbers of results in the Web of Science Core Collection for the keywords provided in the inset, counted on 15 July 2023.
2. Properties of Exchange-Biased Nanostructures
Generally, exchange bias systems consisting of a ferromagnet and an antiferromagnet show a horizontal shift of their magnetization hysteresis loop when they are cooled through the Néel temperature of the AFM (Figure 2a). This is often accompanied by a broadening of the loop (Figure 2b), a vertical loop shift, or an asymmetry of the loop, which are attributed to unidirectional exchange bias anisotropy [6].
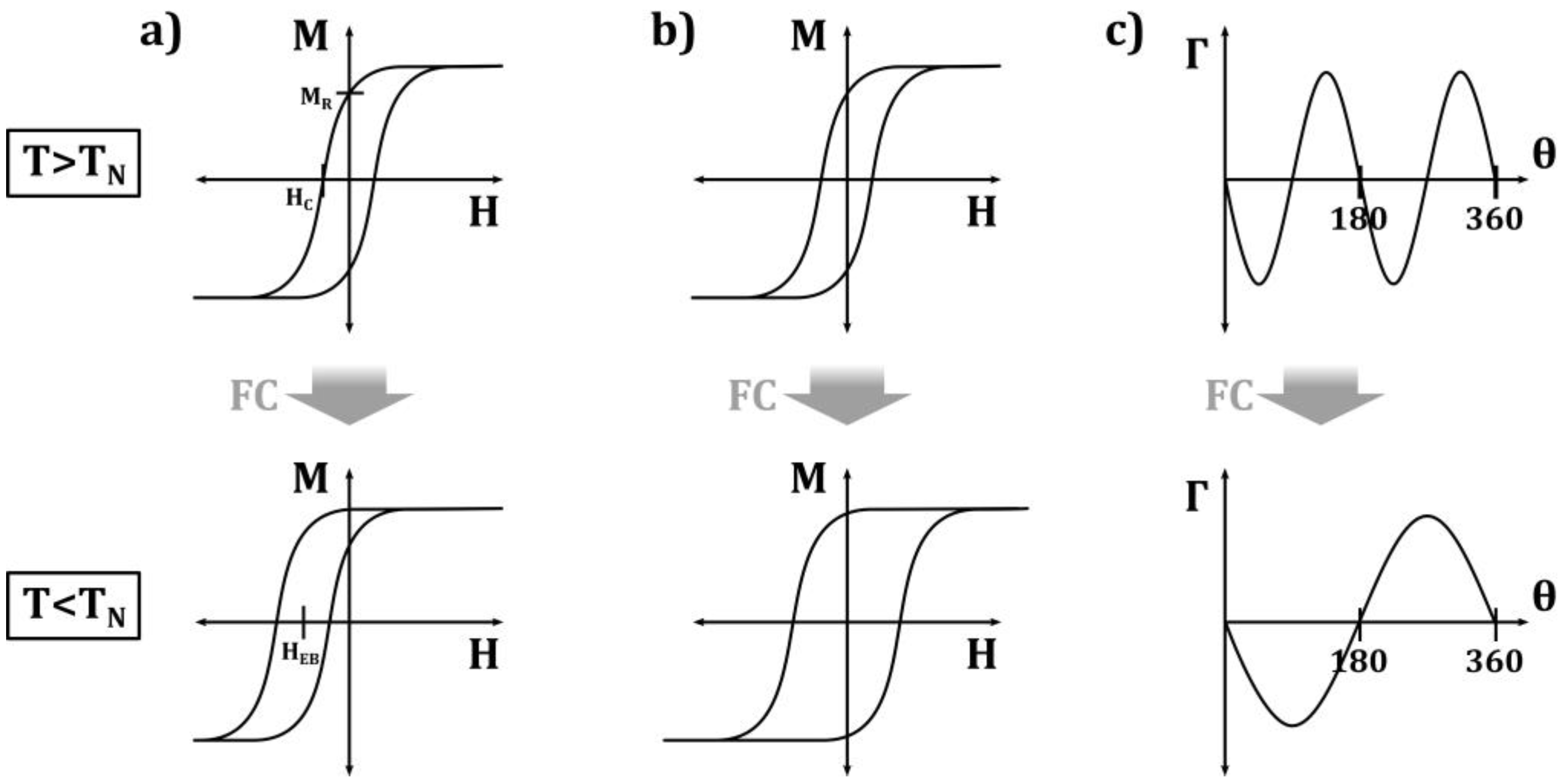
Figure 2. Schematic representation of the main effects induced by the FM–AFM exchange coupling causing (a) loop shift, (b) coercivity enhancement, and (c) unidirectional anisotropy. Redrawn illustration from [6].
All these effects are temperature-dependent, with a larger EB typically at lower temperatures, while a sign change in the loop shift near the Néel temperature is also possible [15][16]. The blocking temperature TB, above which no EB is visible, can be significantly lower than the Néel temperature.
Other parameters affecting the exchange bias are the thickness of the layers or shells [5], the cooling field [17], the roughness of the interface between the FM and AFM [18], as well as the AFM orientation and crystallinity [5][19].
Especially in nanostructured EB systems, other effects may occur. Generally, a size reduction in a single FM or AFM may lead to a change in magnetic properties, as compared to bulk or even thin film materials [20][21][22][23][24]. The superposition of the EB-induced anisotropy with the shape anisotropy, which becomes increasingly important at smaller scales, can lead to even more interesting and partly counterintuitive results [25][26][27].
As the reader can recognize, there are no clear dependencies of the exchange bias on the material, structural, or other parameters—a thicker AFM can increase or decrease the EB field, a larger cooling field can increase or decrease the EB or even switch its sign, another crystal orientation can completely change the temperature-dependent asymmetry of the hysteresis loop, etc.
3. Ni/NiO Nanostructures
Similar to Co/CoO, Ni/NiO can also be found in the form of core/shell particles. Querejeta-Fernández et al. described the preparation of such nanoparticles with an average diameter of 10 nm by the thermal decomposition of a medium containing a Ni2+ salt, followed by a reduction step to yield Ni crystallization and finally the oxidation of the shell [28]. They observed large EB shifts for small- and middle-core diameters and smaller EB fields for larger cores with thin NiO shells. Johnston-Peck et al. used solution chemistry with subsequent solution-phase oxidation instead of preparing Ni/NiO core–shell nanoparticles with shell thicknesses of 2–3 nm and core diameters of 8–24 nm [29]. While the temperature-dependence of the sample magnetization depended on the core and shell diameters, these samples generally showed no horizontal EB shifts, but small increases in coercivity indicating a weak EB. For core–shell particles prepared by a sol-gel route with diameters of 8–27 nm, Thakur et al. investigated the cooling field dependence and observed a slightly reduced EB for cooling fields larger than 20 kOe [30].
Rinaldi-Montes et al. prepared Ni/NiO core/shell nanoparticles by the pyrolysis of an inorganic precursor in the pores of an active carbon matrix, followed by oxidation in air [31]. These nanoparticles showed a shell thickness of 2 nm and varying core diameters, depending on the pyrolysis temperature. The authors reported that the shell froze into a spin glass state below approximately 40 K, correlated to an EB shift of the measured hysteresis loops below this temperature, which was far below the bulk Néel temperature of NiO of 523 K.
While most studies of Ni/NiO nanostructures are based on core/shell structures, a few other exchange-biased Ni/NiO nanostructures were investigated. Kremenovic et al. prepared nanocomposites of 62% NiO with crystallite sizes of about 11 nm and much larger crystallite sizes of 278 nm for Ni [32]. Using thermal annealing in air, the NiO content and crystallite sizes increased, while the Ni crystallite sizes decreased. However, high-energy ball milling resulted in a reduction in the NiO content and overall decreased the crystallite size. An EB was found in milled samples with particle sizes of 10 nm for NiO and 11 nm for Ni, while larger crystallites resulted in a reduced coupling area and correspondingly vanishing EB.
4. Other Exchange-Biased Nanostructures Containing Nickel Oxides
Similar to cobalt oxide nanostructures exchange-coupled to ferromagnets other than cobalt, there are also few reports about NiO combined with other ferro- or ferrimagnets. Tsopoe et al. prepared core–shell nanoparticles combining NiO with the ferrimagnet Fe3O4, testing the AFM as a core and shell, respectively [33]. They observed rod-shaped NiO nanoparticles, while pure Fe3O4 nanoparticles and both sorts of core–shell nanoparticles were spherical, as shown in Figure 3. All diameters were in the range of 30–50 nm. For both sorts of core–shell nanoparticles, the blocking temperature was around 200–250 K, with the highest EB of 330 Oe at 60 K observed for NiO@Fe3O4 core–shell nanoparticles. Interestingly, the authors observed an EB shift along the positive x-axis, i.e., opposite to the common direction, for Fe3O4@NiO core–shell particles, which they explained by more pinning of down-spins at the core–shell interface. The coercive fields of all nanoparticles, both pure and core–shell, decreased with the increasing temperature. Embedding NiFe2O4 ferrimagnetic nanoparticles in a NiO matrix, Tian et al. also observed a blocking temperature of approximately 250 K [34]. The authors explained the EB by the exchange interaction between the ferrimagnetic nanoparticles and the spin glass-like interface phase.

Figure 3. Scanning electron microscope images of (a) pure Fe3O4 and (b) NiO nanoparticles, as well as core–shell nanoparticles of (c) Fe3O4@NiO and (d) NiO@Fe3O4. Adapted from [33], originally published under a CC-BY license.
Such a spin glass state, formed below 10 K, was also mentioned by Rinaldi-Montes et al. who prepared NiO nanoparticles [35]. Similar to the aforementioned CoO or Co3O4 nanoparticles, they observed an EB for nanoparticles larger than a 2 nm diameter, which they attributed to the magnetic coupling between the AFM core and spin glass shell. Winkler et al. reported the spin glass state of 3 nm NiO nanoparticles to occur below 15 K [36]. Makhlouf et al. investigated the temperature dependence of the EB in NiO nanoparticles depending on the NP diameter and observed a lower blocking temperature and also smaller exchange bias shift for smaller nanoparticles, while the greatest EB was achieved for a nanoparticle diameter of 26 nm [37].
5. FeO-Based Exchange-Biased Nanostructures
While exchange-biased thin film systems with Fe as a ferromagnet often contain FeF2 or MnF2 as an antiferromagnet due to their interesting magnetic anisotropies [10][12], only very few nanostructures are based on these AFMs [38][39][40]. Most often, Fe/FeO and other nanostructures containing FeO are investigated instead.
Martínez-Boubeta et al. investigated naturally oxidized Fe nanoparticles with diameter of 5–13 nm, which were prepared by the thermal decomposition of iron pentacarbonyl, followed by oxidation in air [41]. They observed low blocking temperatures of only 19 K for the core–shell nanoparticles with a diameter of 5 nm, while the largest NPs showed a blocking temperature of 160 K and greater EB shifts for larger particles. This finding is similar to the results of Makhlouf et al. who also recognized lower blocking temperatures and a smaller EB for smaller NiO nanoparticles [37]. Similarly, Unni et al. prepared single-crystalline Fe nanoparticles, which showed an EB after oxidation, while the addition of oxygen during the thermal decomposition synthesis resulted in pure magnetite nanoparticles [42].
In addition to antiferromagnetic FeO, there are other common iron oxides, e.g., ferrimagnetic magnetite (Fe3O4), ferrimagnetic maghemite (γ-Fe2O3), and antiferromagnetic hematite (α-Fe2O3) [43]. Especially Fe3O4/FeO is often investigated. Sun et al. prepared FeO/Fe3O4 core/shell nanoparticles by oxidizing FeO nanoparticles at different temperatures and observed a large exchange bias shift with clear loop asymmetry, both of which depended on the relative dimensions of the core and shell [44]. Nanocomposite Fe3O4/FeO nanoparticles were prepared by pulsed laser irradiation in ethyl acetate and showed a positive correlation of the coercive field and EB with the relative fraction of FeO, as well as a blocking temperature close to the FeO Néel temperature of 198 K [45].
References
- Meiklejohn, W.H.; Bean, C.P. New magnetic anisotropy. Phys. Rev. 1956, 102, 1413.
- Meiklejohn, W.H.; Bean, C.P. New magnetic anisotropy. Phys. Rev. 1957, 105, 904.
- Nogués, J.; Moran, T.J.; Lederman, D.; Schuller, I.K.; Rao, K.V. Role of interfacial structure on exchange-biased FeF2–Fe. Phys. Rev. B 1999, 59, 6984–6993.
- Blachowicz, T.; Ehrmann, A. Exchange bias in thin films—An update. Coatings 2021, 11, 122.
- Nogués, J.; Schuller, I.K. Exchange bias. J. Magn. Magn. Mat. 1999, 192, 203–232.
- Nogués, J.; Sort, J.; Langlais, V.; Skumryev, V.; Surinach, S.; Munoz, J.S.; Baró, M.D. Exchange bias in nanostructures. Phys. Rep. 2005, 422, 65–117.
- Laureti, S.; Suck, S.Y.; Haas, H.; Prestat, E.; Bourgeouis, O.; Givord, D. Size dependence of exchange bias in Co/CoO nanostructures. Phys. Rev. Lett. 2012, 108, 077205.
- Javed, K.; Li, W.J.; Ali, S.S.; Shi, D.W.; Khan, U.; Riaz, S.; Han, X.F. Enhanced exchange bias and improved ferromagnetic properties in Permalloy–BiFe0.95Co0.05O3 core–shell nanostructures. Sci. Rep. 2016, 5, 18203.
- Schneider, V.; Reinholdt, A.; Kreibig, U.; Weirich, T.; Güntherodt, G.; Beschoten, B.; Tillmanns, A.; Krenn, H.; Rumpf, K.; Granitzer, P. Structural and Magnetic Properties of Ni/NiOxide and Co/CoOxide Core/Shell Nanoparticles and their possible Use for Ferrofluids. Z. Phys. Chem. 2006, 220, 173–187.
- Tillmanns, A.; Oertker, S.; Beschoten, B.; Güntherodt, G.; Eisenmenger, J.; Schuller, I.K. Angular dependence and origin of asymmetric magnetization reversal in exchange-biased Fe/FeF2(110). Phys. Rev. B 2008, 78, 012401.
- Macedo, W.A.A.; Sahoo, B.; Kuncser, V.; Eisenmenger, J.; Felner, I.; Nogués, J.; Liu, K.; Keune, W.; Schuller, I.K. Changes in ferromagnetic spin structure induced by exchange bias in Fe/MnF2 films. Phys. Rev. B 2004, 70, 224414.
- Tillmanns, A.; Oertker, S.; Beschoten, B.; Güntherodt, G.; Leighton, C.; Schuller, I.K.; Nogués, J. Magneto-optical study of magnetization reversal asymmetry in exchange bias. Appl. Phys. Lett. 2006, 89, 202512.
- Hussain, Z.; Bera, A.K.; Dev, A.S.; Kumar, D.; Reddy, V.R. Exchange bias effect in Fe/LaAlO3: An interface induced effect. J. Alloys Compd. 2020, 849, 156484.
- Zhang, B.M. Correlation of microstructure with magnetic properties in Pr0.67Sr0.33MnO3 thin films. J. Mater. Sci. Mater. Electron. 2020, 31, 19875–19882.
- Blachowicz, T.; Tillmanns, A.; Fraune, M.; Beschoten, B.; Güntherodt, G. Exchange-bias in (110)-oriented CoO/Co bilayers with different magnetocrystalline anisotropies. Phys. Rev. B 2007, 75, 054425.
- Ali, M.; Adie, P.; Marrows, C.H.; Greig, D.; Hickey, B.J.; Stamps, R.L. Exchange bias using a spin glass. Nat. Mater. 2007, 6, 70–75.
- Ambrose, T.; Chien, C.L. Dependence of exchange field and coercitivity on cooling field in NiFe/CoO bilayers. J. Appl. Phys. 1998, 83, 7222–7224.
- Leighton, C.; Nogués, J.; Suhl, H.; Schuller, I.K. Competing interfacial exchange and Zeeman energies in exchange biases bilayers. Phys. Rev. B 1999, 60, 12837.
- Moran, T.J.; Gallego, J.M.; Schuller, I.K. Increased exchange anisotropy due to disorder at permalloy/CoO interfaces. J. Appl. Phys. 1995, 78, 1887–1891.
- Demokritiv, S.O.; Hillebrands, B. Inelastic light scattering in magnetic dots and wires. J. Magn. Magn. Mater. 1999, 200, 706–719.
- McHenry, M.E.; Laughlin, E.E. Nano-scale materials development for future magnetic applications. Acta Mater. 2000, 48, 223–238.
- Bansmann, J.; Baker, S.H.; Binns, C.; Blackman, J.A.; Bucher, J.-P.; Dorantes-Dávila, J.; Dupuis, V.; Favre, L.; Kechrakos, D.; Kleibert, A.; et al. Magnetic and structural properties of isolated and assembled clusters. Surf. Sci. Rep. 2005, 56, 189–275.
- Cao, L.-F.; Xie, D.; Guo, M.-X.; Park, H.S.; Fujita, T. Size and shape effects on Curie temperature of ferromagnetic nanoparticles. Trans. Nonferrous Met. Soc. China 2007, 17, 1451–1455.
- Xue, D.S.; Chai, G.Z.; Li, X.L.; Fan, X.L. Effects of grain size distribution on coercivity and permeability of ferromagnets. J. Magn. Magn. Mater. 2008, 320, 1541–1543.
- Liedke, M.O.; Potzger, K.; Bothmer, A.H.; Fassbender, J.; Hillebrands, B.; Rickart, M.; Freitas, P.P. Domain structure during magnetization reversal of PtMn/CoFe exchange bias micropatterned lines. J. Appl. Phys. 2006, 100, 043918.
- Mendoza-Reséndez, R.; Luna, C. Shape Anisotropy and Exchange Bias in Magnetic Flattened Nanospindles with Metallic/Oxide Core/Shell Structures. J. Nanosci. Nanotechnol. 2012, 12, 7577–7581.
- Tomita, A.; Reginka, M.; Huhnstock, R.; Merkel, M.; Holzinger, D.; Ehresmann, A. Magnetic textures in hemispherical thin film caps with in-plane exchange bias. J. Appl. Phys. 2021, 129, 015305.
- Querejeta-Fernández, A.; Parras, M.; Varela, A.; del Monte, F.; García-Hernández, M.; González-Calbet, J.M. Urea-Melt Assisted Synthesis of Ni/NiO Nanoparticles Exhibiting Structural Disorder and Exchange Bias. Chem. Mater. 2010, 22, 6529–6541.
- Johnston-Peck, A.C.; Wang, J.W.; Tracy, J.B. Synthesis and Structural and Magnetic Characterization of Ni(Core)/NiO(Shell) Nanoparticles. ACS Nano 2009, 3, 1077–1084.
- Thakur, M.; Patra, M.; Majumdar, S.; Giri, S. Influence of cooling field on the magnetic properties of Ni/NiO nanostructure. J. Alloys Compd. 2009, 480, 193–197.
- Rinaldi-Montes, N.; Gorria, P.; Martínez-Blanco, D.; Amghouz, Z.; Fuertes, A.B.; Fernández Barquín, L.; de Pedro, I.; Olivi, L.; Blanco, J.A. Unravelling the onset of the exchange bias effect in Ni(core)@NiO(shell) nanoparticles embedded in a mesoporous carbon matrix. J. Mater. Chem. C 2015, 3, 5674–5682.
- Kremenovic, A.; Jancar, B.; Ristic, M.; Vucinic-Vasic, M.; Rogan, J.; Pacevski, A.; Antic, B. Exchange-Bias and Grain-Surface Relaxations in Nanostructured NiO/Ni Induced by a Particle Size Reduction. J. Phys. Chem. C 2012, 116, 4356–4364.
- Tsopoe, S.P.; Borgohain, C.; Fopase, R.; Pandey, L.M.; Borah, J.P. A comparative investigation of normal and inverted exchange bias effect for magnetic fluid hyperthermia applications. Sci. Rep. 2020, 10, 18666.
- Tian, Z.M.; Yuan, S.L.; Yin, S.Y.; Liu, L.; He, J.H.; Duan, H.N.; Li, P.; Wang, C.H. Exchange bias effect in a granular system of NiFe2O4 nanoparticles embedded in an antiferromagnetic NiO matrix. Appl. Phys. Lett. 2008, 93, 222505.
- Rinaldi-Montes, N.; Gorria, P.; Martínez-Blanco, D.; Fuertes, A.B.; Fernández Barquín, L.; Puente-Orench, I.; Blanco, J.A. Scrutinizing the role of size reduction on the exchange bias and dynamic magnetic behavior in NiO nanoparticles. Nanotechnology 2015, 26, 305705.
- Winkler, E.; Zysler, R.D.; Mansilla, M.V.; Fiorani, D. Surface anisotropy effects in NiO nanoparticles. Phys. Rev. B 2005, 72, 132409.
- Makhlouf, S.A.; Al-Attar, H.; Kodama, R.H. Particle size and temperature dependence of exchange bias in NiO nanoparticles. Solid State Commun. 2008, 145, 1–4.
- Eisenmenger, J.; Li, Z.-P.; Macedo, W.A.A.; Schuller, I.K. Exchange Bias and Asymmetric Reversal in Nanostructured Dot Arrays. Phys. Rev. Lett. 2005, 94, 057203.
- Dumas, R.K.; Li, C.-P.; Roshchin, I.V.; Schuller, I.K.; Liu, K. Deconvoluting reversal modes in exchange-biased nanodots. Phys. Rev. B 2012, 86, 144410.
- Reboucas, G.O.G.; Silva, A.S.W.T.; Dantas, A.L.; Camley, R.E.; Carrico, A.S. Magnetic hysteresis of interface-biased flat iron dots. Phys. Rev. B 2009, 79, 104402.
- Martínez-Boubeta, C.; Simeonidis, K.; Angelakeris, M.; Pazos-Pérez, N.; Giersig, M.; Delimitis, A.; Nalbandian, L.; Alexandrakis, V.; Niarchos, D. Critical radius for exchange bias in naturally oxidized Fe nanoparticles. Phys. Rev. B 2006, 74, 054430.
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal Decomposition Synthesis of Iron Oxide Nanoparticles with Diminished Magnetic Dead Layer by Controlled Addition of Oxygen. ACS Nano 2017, 11, 2284–2303.
- Santos, B.; Loginova, E.; Mascaraque, A.; Schmid, A.K.; McCarty, K.F.; de la Figuera, J. Structure and magnetism in ultrathin iron oxides characterized by low energy electron microscopy. J. Phys. Condens. Matter 2009, 21, 314011.
- Sun, X.L.; Frey Huls, N.; Sigdei, A.; Sun, S.H. Tuning Exchange Bias in Core/Shell FeO/Fe3O4 Nanoparticles. Nano Lett. 2012, 12, 246–251.
- Swiatkowska-Warkocka, Z.; Kawaguchi, K.; Wang, H.Q.; Katou, Y.; Koshizaki, N. Controlling exchange bias in Fe3O4/FeO composite particles prepared by pulsed laser irradiation. Nano Express 2011, 6, 226.
More
Information
Subjects:
Physics, Condensed Matter
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
4 times
(View History)
Update Date:
30 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




