Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zijian Zhu | -- | 2750 | 2023-08-26 02:19:17 | | | |
| 2 | Dean Liu | Meta information modification | 2750 | 2023-08-28 04:21:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhu, Z.; Jiang, L.; Ding, X. Genomic Profiling for Breast Cancer Heterogeneity Analysis. Encyclopedia. Available online: https://encyclopedia.pub/entry/48501 (accessed on 07 February 2026).
Zhu Z, Jiang L, Ding X. Genomic Profiling for Breast Cancer Heterogeneity Analysis. Encyclopedia. Available at: https://encyclopedia.pub/entry/48501. Accessed February 07, 2026.
Zhu, Zijian, Lai Jiang, Xianting Ding. "Genomic Profiling for Breast Cancer Heterogeneity Analysis" Encyclopedia, https://encyclopedia.pub/entry/48501 (accessed February 07, 2026).
Zhu, Z., Jiang, L., & Ding, X. (2023, August 26). Genomic Profiling for Breast Cancer Heterogeneity Analysis. In Encyclopedia. https://encyclopedia.pub/entry/48501
Zhu, Zijian, et al. "Genomic Profiling for Breast Cancer Heterogeneity Analysis." Encyclopedia. Web. 26 August, 2023.
Copy Citation
Breast cancer continues to pose a significant healthcare challenge worldwide for its inherent molecular heterogeneity.
breast cancer
heterogeneity
single-cell genome
1. Introduction
Breast cancer is one of the most prevalent cancers in women globally. In 2012, there were 464,000 diagnosed cases of breast cancer and 131,000 deaths among European women [1]. In 2020, breast cancer accounted for 2.26 million new cases globally, surpassing lung cancer (2.2 million cases) to become the most commonly diagnosed cancer worldwide (Figure 1). In the same year, the disease caused an estimated 685,000 deaths [1][2][3]. Projections suggest that by 2030, new cases will reach 3.9 million globally, with fatalities rising to 766,000 [4].
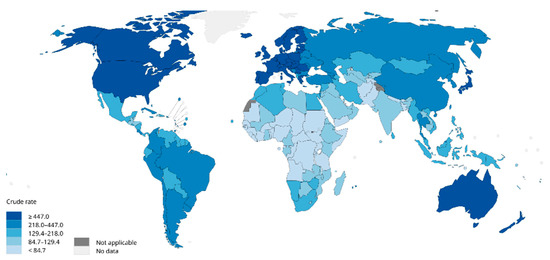
Figure 1. Worldwide estimated crude incidence rates of female breast cancer (2020) [2].
Despite considerable strides in both laboratory research and the clinical practice of breast cancer, the global incidence and mortality rates continue to rise. At the root of this persisting issue is the heterogeneous nature of breast cancer, which is not a monolithic disease, but a spectrum of distinct subtypes, each representing a unique malignancy within the breast’s cellular makeup. Current research categorizes these molecular subtypes into six major classes: (i) hormone-receptor-positive breast cancer (ER+), (ii) hormone receptor/HER2-positive breast cancer (ER+/HER2+), (iii) HER2-positive breast cancer (HER2+), (iv) basal-like breast cancer, (v) claudin-low subtype, and (vi) normal-like subtype [5]. The distinct subtypes of breast cancer each present unique clinical features and associated risk factors. This diversity extends to treatment responses and long-term patient survival, which differ significantly across the subtypes. This inherent complexity adds layers of challenge to the effective diagnosis and treatment of breast cancer. For instance, the basal-like subtype of breast cancer, characterized by high rates of cellular proliferation, is associated with distinct risk factors, which include the early onset of menstruation, a younger age at the first full-term pregnancy, and the accumulation of abdominal fat. In contrast, patients with the claudin-low subtype, marked by an enrichment of epithelial–mesenchymal transition markers, typically exhibit pronounced invasiveness. Such individuals often bear the burden of exposure to chemicals and radiation in their early years, leading to a high load of DNA damage induced by cancer genes and early chromosomal instability (CIN) [6].
The inherent heterogeneity of breast cancer presents considerable hurdles for conventional diagnostic and therapeutic approaches. Generally, traditional methods depend on analyzing bulk tumor tissue samples, a process which, by considering the average expression levels, may obscure the underlying heterogeneity, complicating accurate tumor classification. But emerging technologies such as single-cell analysis techniques offer promising alternatives, already being widely used in oncology research. These techniques, by investigating gene expression, phenotypes, protein levels, and other cellular properties at an individual cell level, are well suited to address the challenge of tumor heterogeneity (Figure 2) [7][8][9]. Particularly for highly heterogeneous cancers like breast cancer, a single-cell analysis can help to predict cellular evolution during tumor progression. The analysis of genetic and epigenetic variations as well as gene expression at the single-cell level is among the techniques that enhance the precision of predicting tumor development trends, evaluating treatment outcomes, and forecasting patient prognosis. Furthermore, single-cell analysis techniques play a crucial role in devising novel therapeutic strategies. These methods allow for the examination of genetic variations and phenotypic characteristics of tumor cells in detail, which can lead to the identification of new therapeutic targets. Consequently, this paves the way for the development of highly targeted, precise treatment strategies, enhancing the ability to predict treatment efficacy and potential drug resistance.
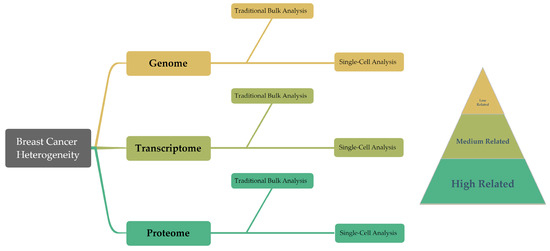
Figure 2. This diagram presents an overview of the structure of the article, summarizing the traditional bulk techniques and single-cell findings in genomics, transcriptomics, and proteomics. Moreover, these three groups demonstrate a progressive correlation with cancer cell behavior in the research on breast cancer heterogeneity.
Single-cell gene sequencing, leveraging the power of next-generation sequencing (NGS), has emerged as a pivotal tool for investigating breast cancer heterogeneity [10]. Unlike traditional Sanger sequencing, NGS systems use massive parallel sequencing to yield billions of DNA reads, from 36 to 150 base pairs, which can be aligned to the human genome. This alignment allows for the detection of various genetic variations, including single-nucleotide mutations, small insertions/deletions, and copy number variations, offering a comprehensive view for streamlining the development of targeted treatment strategies. A case in point is HER2-positive breast cancer, where single-cell sequencing detects the diversity in HER2 gene amplification across different cells, facilitating the formulation of bespoke treatment plans [11]. The versatile utility of NGS extends to RNA sequencing (RNA-seq), which is a high-throughput technique enabling quantitative and sequence analyses of diverse RNA types, along with their expression levels in cells and tissues. RNA-seq facilitates an in-depth exploration of gene expression regulation, signaling, and metabolic pathways pertinent to breast cancer, thereby enriching the understanding of its molecular mechanisms. Intriguingly, beyond the recognized impact of non-coding RNAs on breast cancer progression, even the half-life of mRNA serves as an informative marker [12].
In molecular profiling, the strength of the correlation between molecular patterns and cellular behavior is pivotal, as a higher correlation implies a more accurate reflection of the tumor’s actual condition [13]. Complementing single-cell genomics and transcriptome, the emergence of single-cell proteomics offers another potent instrument for investigating breast cancer heterogeneity. This technique enables the detection and analysis of protein expression at the single-cell level. It provides a more precise view of protein expression compared to its RNA-sequencing counterpart, revealing insights into protein localization and intra-cellular interactions. For instance, immunohistochemistry provides a more direct appraisal of a patient’s tumor condition. This technique plays a pivotal role in breast cancer diagnosis, as it involves staining clinical samples of breast cancer tissues to reveal the expression of crucial proteins, including ER, PR, and HER2. The resultant immuno-stained samples offer a visual map for clinicians, aiding them in identifying the subtype of breast cancer and enabling effective pathological staging and treatment selection. Through techniques like immunohistochemistry, resear can not only discern between these subtypes, but also detect signs of lymph node metastasis and monitor potential tumor recurrence. Such insights are pivotal for guiding treatment decisions and tracking the progression of the disease [14][15][16].
2. Genomic Profiling
2.1. Traditional Genomic Profiling
In 1994, the BRCA1 gene was identified through positional cloning, followed by the discovery of the BRCA2 gene in 1995. These genes play a crucial role in DNA damage repair and maintaining genomic stability, thereby reducing the risk of tumor development [17][18]. BRCA1 and BRCA2 are tumor suppressor genes involved in repairing dsDNA breaks. Mutations in these genes significantly increase the lifetime risk of developing breast cancer. Inherited mutations in BRCA1 and BRCA2 account for a small percentage of breast cancer cases. Tumors associated with BRCA1 mutations often exhibit a basal-like phenotype and a higher histological grade, while those linked to BRCA2 mutations resemble sporadic tumors more closely. Several specific scenarios can notably elevate the incidence of breast cancer: (1) sequence variants encoding premature termination codons such as nonsense or frameshift mutations occurring prior to the 1855th amino acid in BRCA1 and the 3309th amino acid in BRCA2; (2) mutations located at splice site consensus sequences—either the first or second base positioned upstream or downstream of an exon; (3) copy number loss mutations leading to frameshift mutations prior to the 1855th amino acid of BRCA1 and the 3309th amino acid of BRCA2, or mutations eliminating one or more exons not predicted or confirmed to produce functional in-frame RNA isoforms capable of restoring BRCA1/2 gene function; and (4) copy number repeat variations of any size resulting in the duplication of one or more exons, and proven to cause frameshift mutations before the 1855th amino acid of BRCA1 and the 3309th amino acid of BRCA2 [17][19].
Individuals harboring potential pathogenic variants in the BRCA1/2 genes can benefit significantly from timely education and early screening. According to the European Society for Medical Oncology (ESMO) guidelines [20], females identified with having mutations in BRCA1, BRCA2, or other high-penetrance genes should initiate breast cancer prevention education from the age of 18, maintain vigilant awareness of breast conditions, and comply with regular medical check-ups. Physicians advocate for annual clinical breast examinations complemented by breast X-ray imaging and magnetic resonance imaging (MRI) assessments starting from the age of 25 [21]. Despite the insights gained from early genetic knowledge, they did not immediately translate into clinical treatment strategies. The initial clinical data highlighted that BRCA-associated tumors exhibited high sensitivity to poly (ADP-ribose) polymerase (PARP) inhibitors. These inhibitors act on the PARP-mediated DNA damage repair mechanism, thereby disrupting the tumor’s ability to repair its DNA. As of now, PARP inhibitors are primarily accessible through clinical trials [22].
With the deepening understanding of breast cancer and the widespread use of NGS, more relevant genes have been discovered (Table 1).
Table 1. Breast-cancer-relevant gene, discovery year, involved process, and mutation risk.
| Gene | Discovery | Involved Process | Mutation Risk | Reference |
|---|---|---|---|---|
| PTEN | 1997 | apoptosis, cell cycle, and signal transduction | activation of proliferation and survival signals | [23] |
| STK11 | 1997 | cell cycle, metabolism, and energy balance | activation of cell proliferation and metabolic pathways | [24] |
| CHEK2 | 1999 | DNA repair and cell apoptosis | impairments in DNA repair and cell apoptosis processes | [25] |
| PIK3CA | 2004 | regulation of signaling pathway | activation of survival signals | [26] |
| AKT1 | 2007 | regulation of signaling pathway | activation of cell proliferation and survival signals | [27] |
| BARD1 | 2010 | DNA repair and cell apoptosis | increased susceptibility to breast cancer | [28] |
| NF1 | 2015 | regulation of signaling pathway | increased rate of developing breast cancer | [29] |
There was a study involving a large cohort of breast cancer patients who underwent analysis for gene mutations and copy number variations, further substantiating the prevalence of gene alterations in this population [30]. TP53 gene mutations are commonly found in the basal-like subtype, while the HER2-positive subtype also exhibits a high incidence of TP53 gene mutations. Additionally, the HER2-positive subtype shows a significant frequency of PIK3CA gene mutations. The basal-like and HER2-positive subtypes are characterized by genomic instability and susceptibility to changes in gene copy numbers. Conducting concurrent assessments of DNA copy number and gene mutations in breast cancer cells enables the prediction of the cellular subtype. For example, it is known that the amplification of Kras2 is associated with tumor progression, while insufficient Kras2 copy numbers delays tumor progression [31]. In an investigation involving 16 human basal-like breast tumors, none displayed Kras2 mutations; however, an increased DNA copy number at the Kras2 locus was observed in 9 of the tumors. These observations imply that Kras2 amplification may modulate cell phenotypes or earmark target cell types that are susceptible in basal-like tumors [32].
Furthermore, leveraging insights from patients’ DNA sequencing results proves invaluable in tailoring subsequent treatment strategies [33]. Taking the P53 gene as an example, it plays a fundamental role as a key regulator of cellular processes, participating in controlling cell proliferation and maintaining genomic integrity and stability. Activated in response to an array of stress signals, the TP53 tumor suppressor protein curbs cell transformation by precipitating cell cycle arrest, DNA repair, and apoptosis. In breast cancer patients, however, TP53 gene mutations may precipitate a partial or complete functional loss of the TP53 tumor suppressor protein, thereby undermining its capacity to inhibit tumor development. Significantly, Asian breast cancer patients exhibit a mutation frequency of 42.9% in the P53 gene, which surpasses the mutation rate of 30% observed in Western breast cancer. This suggests a potentially higher degree of endocrine therapy resistance and lower survival rates among this demographic. Hence, breast cancer patients with inactivating mutations in the P53 gene necessitate swift intervention with appropriate follow-ups, reexaminations, and immediate treatment. For those carrying TP53 mutations, related treatment strategies can be explored in a clinical setting, which might include Gendicine therapy either as a standalone treatment or in conjunction with radiation therapy, chemotherapy, or hyperthermia, among other treatment approaches. Overall, the use of such targeted treatment for TP53 mutations has achieved a complete response rate of 30–40% and a partial response rate of 50–60% in various clinical applications and studies, with an overall response rate reaching 90–96% [34].
2.2. Single-Cell Genomic Profiling
While conventional genetic profiling can offer valuable insights, it faces significant challenges, most notably its inability to differentiate between normal and tumorous tissues. Breast tumors typically comprise a heterogeneous mix of cancerous cells, healthy tissues, stromal components, and infiltrating leukocytes. Histopathological assessments have revealed that certain samples may contain a composition of around 60% normal cells and 35% cancer cells, with a significant presence of infiltrating leukocytes [35]. The information from these additional normal tissues can be considered in the results, which may potentially overshadow crucial information and even lead to erroneous results. Additionally, traditional DNA analysis can only provide vague insights into cancer development because large-scale analyses yield average DNA profiles for tumors, making it impossible to differentiate and track each tumor cell lineage within a tumor tissue [36][37]. Such issues can be addressed through single-cell DNA sequencing [7].
A pivotal step in scDNA-seq involves extracting minuscule quantities of DNA from single cells, followed by whole-genome amplification (WGA). To minimize amplification-related errors and biases, PCR-based methods are commonly utilized for copy number variation (CNV) detection due to their ability to provide more uniform coverage. In contrast, for single-nucleotide variation (SNV) detection, MDA-based techniques are favored owing to their use of high-fidelity DNA polymerases that function at room temperature and display a heightened sensitivity to single-base alterations [38][39].
CNVs are common in a wide range of cancer cell lines, with conventional genomic analyses indicating their substantial influence in the emergence and progression of breast cancer. An assessment and identification of genomic regions bearing copy number alterations—both gains and losses—in tumor cells can facilitate the construction of lineage trees, elucidating shared ancestry among tumor subpopulations [40]. Single-cell DNA sequencing enables researchers to scrutinize CNVs and discern tumor cell subpopulations within solid tumors, even at the early stages of breast cancer development [35]. By employing a comparative analysis of CNV differences, researchers have categorized roughly 100 tumor cells into three distinct subpopulations: a diploid (D) cell subpopulation characterized by a flat morphology, a pseudodiploid (P) cell subpopulation showcasing varying degrees of deviation from diploidy, and a subpopulation embodying complex genomic rearrangements, mirroring the characteristics of a ‘late-stage’ tumor subpopulation. During the late stages of breast cancer progression, liver metastasis invariably becomes a significant concern. By employing pseudodiploid cells as reference standards, researchers have identified striking similarities between the copy number profiles of primary tumors and their metastatic counterparts. This strongly suggests that the origin of metastatic cells can be traced predominantly to late-stage amplifications, as opposed to intermediate stages or entirely divergent subpopulations [35].
Conceptually, information pertaining to SNVs within breast cancer cells can be extrapolated from pre-existing WGA data. While this methodology has proven to be adequate for detecting copy number variations, it falls short in resolving whole-genome mutations at a granular base pair resolution. A commonly used approach is to increase coverage by performing deep sequencing of these libraries. Researchers have developed a high-coverage whole-genome and exome single-cell sequencing method using high-fidelity DNA polymerase to amplify 22 chromosome-specific primer pairs. The amplified DNA is then incubated with Tn5 transposase, which fragments and ligates the DNA for sequencing adapters [41]. This technology achieves a low false-positive rate for point mutations, equivalent to 1–2 errors per million base pairs [42][43]. Using this technique, researchers selected invasive ductal carcinoma from estrogen-receptor-positive (ER1/PR1/HER2) breast cancer patients for bulk and single-cell sequencing. After filtering germline variations, several non-synonymous mutations were identified in the non-diploid tumor cell population, including TBX3, NOTCH2, JAK1, ARAF, NOTCH3, MAP3K4, NTRK1, AFF4, CDH6, SETBP1, AKAP9, MAP2K7, ECM2, and ECM1 [30].
Through a comprehensive analysis of the breast cancer dataset, investigators have pinpointed two key pathways that are notably disrupted during tumor evolution: the TGF-β signaling pathway and the extracellular matrix receptor signaling pathway [7]. This revelation carries substantial clinical implications, as it enables healthcare professionals to select appropriate chemotherapy drugs that specifically target these disrupted pathways. For example, TGF-β receptor I kinase inhibitors like LY2157299 can inhibit TGF-β signaling pathway transmission, slowing down tumor growth and metastasis [44]. Additionally, drugs targeting extracellular matrix receptor signaling are already being used in clinical practice, such as anti-HER2 therapy for HER2-positive breast cancer patients [7]. These findings offer new insights and approaches for breast cancer treatment.
While single-cell gene profiling technology has greatly advanced the understanding of breast cancer tumor cells, offering high precision and efficiency and resolving some standing challenges, ongoing discoveries of genetically and phenotypically unique cellular subpopulations have prompted a shift in the perception of cancer. The cancer narrative has evolved beyond viewing it as a homogeneous aggregation of tumor cells, and researchers now embrace the reality of it as a spectrum of heterogeneous and evolving cancer subtypes [45]. This underlines that a sole reliance on DNA single-cell detection may fall short in addressing the research demands presented by breast cancer heterogeneity and in guiding clinical interventions directly. Therefore, it is necessary to utilize other single-cell omics detection technologies to collectively address research questions in breast cancer.
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.-W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403.
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789.
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23.
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921.
- Sotiriou, C.; Desmedt, C.; Durbecq, V.; Dal Lago, L.; Lacroix, M.; Cardoso, F.; Piccart-Gebhart, M.; Ross, J.S.; Hortobagyi, G.N. Genomic and molecular classification of breast cancer. In Molecular Oncology of Breast Cancer; Jones and Barlett Publishers: Sudbury, MA, USA, 2005; pp. 81–95.
- Rivenbark, A.G.; O’Connor, S.M.; Coleman, W.B. Molecular and cellular heterogeneity in breast cancer: Challenges for personalized medicine. Am. J. Pathol. 2013, 183, 1113–1124.
- Wang, Y.; Waters, J.; Leung, M.L.; Unruh, A.; Roh, W.; Shi, X.; Chen, K.; Scheet, P.; Vattathil, S.; Liang, H. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature 2014, 512, 155–160.
- Chung, W.; Eum, H.H.; Lee, H.-O.; Lee, K.-M.; Lee, H.-B.; Kim, K.-T.; Ryu, H.S.; Kim, S.; Lee, J.E.; Park, Y.H. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat. Commun. 2017, 8, 15081.
- Kim, J.J.; Liang, W.; Kang, C.-C.; Pegram, M.D.; Herr, A.E. Single-cell immunoblotting resolves estrogen receptor-α isoforms in breast cancer. PLoS ONE 2021, 16, e0254783.
- Gerlinger, M.; Swanton, C. How Darwinian models inform therapeutic failure initiated by clonal heterogeneity in cancer medicine. Br. J. Cancer 2010, 103, 1139–1143.
- Shiovitz, S.; Korde, L.A. Genetics of breast cancer: A topic in evolution. Ann. Oncol. 2015, 26, 1291–1299.
- Griseri, P.; Pagès, G. Regulation of the mRNA half-life in breast cancer. World J. Clin. Oncol. 2014, 5, 323.
- Ellis, M.J.; Perou, C.M. The genomic landscape of breast cancer as a therapeutic roadmap. Cancer Discov. 2013, 3, 27–34.
- Da Silva, L.; Lakhani, S.R. Pathology of hereditary breast cancer. Mod. Pathol. 2010, 23, S46–S51.
- Wellings, S.; Jensen, H. On the origin and progression of ductal carcinoma in the human breast. J. Natl. Cancer Inst. 1973, 50, 1111–1118.
- Sundah, N.R.; Ho, N.R.; Lim, G.S.; Natalia, A.; Ding, X.; Liu, Y.; Seet, J.E.; Chan, C.W.; Loh, T.P.; Shao, H. Barcoded DNA nanostructures for the multiplexed profiling of subcellular protein distribution. Nat. Biomed. Eng. 2019, 3, 684–694.
- Szabo, C.I.; King, M.-C. Inherited breast and ovarian cancer. Hum. Mol. Genet. 1995, 4, 1811–1817.
- Wooster, R.; Bignell, G.; Lancaster, J.; Swift, S.; Seal, S.; Mangion, J.; Collins, N.; Gregory, S.; Gumbs, C.; Micklem, G. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995, 378, 789–792.
- Ford, D.; Easton, D.; Stratton, M.; Narod, S.; Goldgar, D.; Devilee, P.; Bishop, D.; Weber, B.; Lenoir, G.; Chang-Claude, J. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am. J. Hum. Genet. 1998, 62, 676–689.
- Balmana, J.; Diez, O.; Rubio, I.; Cardoso, F. BRCA in breast cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2011, 22, vi31–vi34.
- Brooksby, B.; Jiang, S.; Dehghani, H.; Pogue, B.W.; Paulsen, K.D.; Weaver, J.; Kogel, C.; Poplack, S.P. Combining near-infrared tomography and magnetic resonance imaging to study in vivo breast tissue: Implementation of a Laplacian-type regularization to incorporate magnetic resonance structure. J. Biomed. Opt. 2005, 10, 051504.
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; Mortimer, P.; Swaisland, H.; Lau, A.; O’Connor, M.J. Inhibition of poly (ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009, 361, 123–134.
- Yamada, K.M.; Araki, M. Tumor suppressor PTEN: Modulator of cell signaling, growth, migration and apoptosis. J. Cell Sci. 2001, 114, 2375–2382.
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262.
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078.
- Samuels, Y.; Velculescu, V.E. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle 2004, 3, 1221–1224.
- Bhaskar, P.T.; Hay, N. The two TORCs and AKT. Dev. Cell 2007, 12, 487–502.
- De Brakeleer, S.; De Grève, J.; Loris, R.; Janin, N.; Lissens, W.; Sermijn, E.; Teugels, E. Cancer predisposing missense and protein truncating BARD1 mutations in non-BRCA1 or BRCA2 breast cancer families. Hum. Mutat. 2010, 31, E1175–E1185.
- Krauthammer, M.; Kong, Y.; Bacchiocchi, A.; Evans, P.; Pornputtapong, N.; Wu, C.; McCusker, J.P.; Ma, S.; Cheng, E.; Straub, R. Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat. Genet. 2015, 47, 996–1002.
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70.
- Liu, M.L.; Shibata, M.A.; Von Lintig, F.C.; Wang, W.; Cassenaer, S.; Boss, G.R.; Green, J.E. Haploid loss of Ki-ras delays mammary tumor progression in C3 (1)/SV40 Tag transgenic mice. Oncogene 2001, 20, 2044–2049.
- Herschkowitz, J.I.; Simin, K.; Weigman, V.J.; Mikaelian, I.; Usary, J.; Hu, Z.; Rasmussen, K.E.; Jones, L.P.; Assefnia, S.; Chandrasekharan, S. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007, 8, R76.
- Silwal-Pandit, L.; Vollan, H.K.; Chin, S.F.; Rueda, O.M.; McKinney, S.; Osako, T.; Quigley, D.A.; Kristensen, V.N.; Aparicio, S.; Børresen-Dale, A.L.; et al. TP53 mutation spectrum in breast cancer is subtype specific and has distinct prognostic relevance. Clin. Cancer Res. 2014, 20, 3569–3580.
- Brooks, M.D.; Burness, M.L.; Wicha, M.S. Therapeutic implications of cellular heterogeneity and plasticity in breast cancer. Cell Stem Cell 2015, 17, 260–271.
- Navin, N.; Kendall, J.; Troge, J.; Andrews, P.; Rodgers, L.; McIndoo, J.; Cook, K.; Stepansky, A.; Levy, D.; Esposito, D. Tumour evolution inferred by single-cell sequencing. Nature 2011, 472, 90–94.
- Lawson, D.A.; Kessenbrock, K.; Davis, R.T.; Pervolarakis, N.; Werb, Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat. Cell Biol. 2018, 20, 1349–1360.
- McGranahan, N.; Swanton, C. Clonal heterogeneity and tumor evolution: Past, present, and the future. Cell 2017, 168, 613–628.
- Carter, N.P.; Bebb, C.E.; Nordenskjo, M.; Ponder, B.A.; Tunnacliffe, A. Degenerate oligonucleotide-primed PCR: General amplification of target DNA by a single degenerate primer. Genomics 1992, 13, 718–725.
- Picher, A.J.; Budeus, B.; Wafzig, O.; Krüger, C.; García-Gómez, S.; Martínez-Jiménez, M.I.; Díaz-Talavera, A.; Weber, D.; Blanco, L.; Schneider, A. TruePrime is a novel method for whole-genome amplification from single cells based on Tth PrimPol. Nat. Commun. 2016, 7, 13296.
- Beroukhim, R.; Getz, G.; Nghiemphu, L.; Barretina, J.; Hsueh, T.; Linhart, D.; Vivanco, I.; Lee, J.C.; Huang, J.H.; Alexander, S. Assessing the significance of chromosomal aberrations in cancer: Methodology and application to glioma. Proc. Natl. Acad. Sci. USA 2007, 104, 20007–20012.
- Adey, A.; Morrison, H.G.; Xun, X.; Kitzman, J.O.; Turner, E.H.; Stackhouse, B.; MacKenzie, A.P.; Caruccio, N.C.; Zhang, X.; Shendure, J. Rapid, low-input, low-bias construction of shotgun fragment libraries by high-density in vitro transposition. Genome Biol. 2010, 11, R119.
- Hou, Y.; Song, L.; Zhu, P.; Zhang, B.; Tao, Y.; Xu, X.; Li, F.; Wu, K.; Liang, J.; Shao, D. Single-cell exome sequencing and monoclonal evolution of a JAK2-negative myeloproliferative neoplasm. Cell 2012, 148, 873–885.
- Zong, C.; Lu, S.; Chapman, A.R.; Xie, X.S. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science 2012, 338, 1622–1626.
- Williams, C.H., III. Chemical Genetics of Vertebrate Development; Vanderbilt University: Nashville, TN, USA, 2017.
- Powell, A.A.; Talasaz, A.H.; Zhang, H.; Coram, M.A.; Reddy, A.; Deng, G.; Telli, M.L.; Advani, R.H.; Carlson, R.W.; Mollick, J.A. Single cell profiling of circulating tumor cells: Transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS ONE 2012, 7, e33788.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
468
Revisions:
2 times
(View History)
Update Date:
28 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




