Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
He, J.; Xie, X.; Xiao, Z.; Qian, W.; Zhang, L.; Hou, X. Piezo1 in Digestive System Function and Dysfunction. Encyclopedia. Available online: https://encyclopedia.pub/entry/48494 (accessed on 07 February 2026).
He J, Xie X, Xiao Z, Qian W, Zhang L, Hou X. Piezo1 in Digestive System Function and Dysfunction. Encyclopedia. Available at: https://encyclopedia.pub/entry/48494. Accessed February 07, 2026.
He, Jing, Xiaotian Xie, Zhuanglong Xiao, Wei Qian, Lei Zhang, Xiaohua Hou. "Piezo1 in Digestive System Function and Dysfunction" Encyclopedia, https://encyclopedia.pub/entry/48494 (accessed February 07, 2026).
He, J., Xie, X., Xiao, Z., Qian, W., Zhang, L., & Hou, X. (2023, August 25). Piezo1 in Digestive System Function and Dysfunction. In Encyclopedia. https://encyclopedia.pub/entry/48494
He, Jing, et al. "Piezo1 in Digestive System Function and Dysfunction." Encyclopedia. Web. 25 August, 2023.
Copy Citation
Piezo1, a non-selective cation channel directly activated by mechanical forces, is widely expressed in the digestive system and participates in biological functions physiologically and pathologically.
mechanotransduction
Piezo1
mechanosensitive ion channel
1. Mechanosensitive Ion Channels in the Digestive System
Mechanotransduction refers to the process of living mechanosensitive tissues or cells to detect and respond to changes in membrane tension and cytoskeleton induced by mechanical stimuli, initiating intracellular signal transduction and generating electrochemical signals [1][2]. The digestive system experiences various mechanical stimuli, including gastrointestinal peristalsis, villus movement, conduit osmotic pressure, etc, which are fundamental for initiating mechanotransduction. Mechanotransduction relies on ion channels sensitive to mechanical stimuli, which are known as mechanosensitive ion channels. The mechanosensitive ion channels in digestive system include transient receptor potential vanilloid family (TRPV) [3], Piezo1/2 [4], two pore-domain potassium channels (K2p) [5], large-conductance Ca2+-activated potassium channel (BKCa) [6] and others.
The Piezo protein, characterized as the largest plasma membrane ion channel complex with over 30 putative transmembrane domains, is a unique entity capable of inducing large mechanically-activated cationic currents unlike other known ion channels or proteins [7]. At present, Piezo channel has drawn considerable research interest [8]. Piezo protein contains two homologues in Homo sapiens, Piezo1 and Piezo2 (Table 1). Compared with Piezo1, Piezo2 has additional charged residues at the beam-carboxy-terminal domain interface and additional constriction sites at L2743, F2754 and E2757 in the central pore [9][10]. Piezo1 is widely expressed in multiple cell types, whereas Piezo2 is believed to be predominantly expressed in neurons and intestinal enterochromaffin cells.
First identified in 2010 by Coste B [11], Piezo1 (Fam38a) plays important roles in maintaining various cellular effects such as bone and epithelial homeostasis, neural stem cell differentiation, macrophage polarization, and regulating biological functions including vascular development, red blood cell volume homeostasis, inflammation response generation and etc. [12][13][14][15]. More recently, Piezo1 channel has also been identified to transduce itch in sensory neuron which is associated with Piezo2 channel as generally believed [16]. Therefore, Piezo1 participates in life activity widely and deeply. And more and more evidences have demonstrated the predominant and special contributions of the Piezo1 channel in the digestive system at present [4]. Researchers focused on the cellular effects of Piezo1 in digestive system, with special highlights on its importance in regulating biological function.
Table 1. Differences between Piezo1 and Piezo2.
| Piezo1 | Study | Piezo2 | Study | |
|---|---|---|---|---|
| Gene region | 16q24.3 | [12] | 18p11.22-p11.21 | [17] |
| Amino acid residues | 2520 | [12] | 2752 | [17] |
| Distribution | almost all cell types | - | mainly nerve cells and some specific cell types | [18] |
| Detection threshold (fJ) * | 213.7 ± 16.6 | [19] | 86.8 ± 7.1 | [19] |
| Work resolution (fJ) * | 1.2 ± 0.4 | [19] | 1.0 ± 0.2 | [19] |
| Transduction Speed (ms) * | 8.2 ± 2.2 | [19] | 1.5 ± 0.5 | [19] |
| Inactivation kinetics (ms) * | 16.5 ± 1.4 | [11] | 7.3 ± 0.7 | [11] |
| Activator | Yoda1, Jedi1/2 | [20][21] | - | - |
| Inhibitor | Dooku1, GsMTx4, ruthenium red (RR), gadolinium (Gd3+) | [11][22] | GsMTx4, ruthenium red, gadolinium | [23] |
| Hereditary human disorders | dehydrated hereditary stomatocytosis, congenital lymphatic dysplasia with non-immune fetal hydrops | [24][25][26] | recessive distal arthrogryposis syndrome, dominant distal arthrogryposis syndrome (type III and V), Marden-Walker Syndrome | [27][28] |
* detected in HEK293T cell line.
2. Structure and Kinetics of Piezo1
The Piezo1 gene is located on human chromosome 16q24.3 and contains 51 exons [12]. The Piezo1 monomer is about 290–320 kDa while it is a conservative trimer of about 900 kDa naturally [29]. This structure mediats nonlinear transduction of mechanical energy and detects mechanical energies as 213.7 fJ, with a resolution of 1.2 fJ [19]. As a non-selective mechanosensitive cation channel, Piezo1 has the strongest affinity to Ca2+ [11][30]. The opening of Piezo channel triggers mainly influx of Ca2+ and Na+ [31][32]. Therefore, opening of Piezo channel has two effects: it shifts membrane potential and activates other voltage-gated ion channels, which triggers an action potential; simultaneously, it alters [Ca2+]i (intracellular calcium) concentration and triggers downstream signal transduction pathways [33]. Structurally, Piezo1 can be divided into three parts: peripheral N-terminal propeller blades for sensing mechanical stimulation, beam and anchor domain for conducting mechanical signals, and the C-terminal central pore for facilitating ion transport [12] (Figure 1).
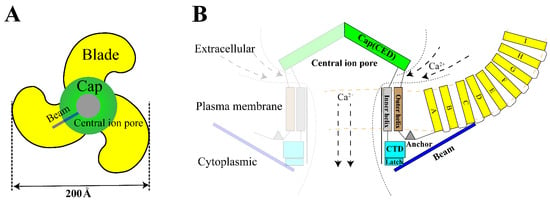
Figure 1. Structure of Piezo1. (A). The trimeric Piezo1 is a three-blade propeller with central pore from extracellular view. (B). Membrane view of the trimeric Piezo1. The propeller blade consists of several four-transmembrane helix bundles with the same topology called “Piezo Repeat” range A-I. CED. C-terminal extracellular domain, CTD. C-terminal domain.
Currently, two principal mechanisms are proposed for Piezo1 activation: the “force-from-lipids” mechanism believes mechanical force alters the membrane lipid-Piezo1 interaction and induces the activation of Piezo1; the “force-from-filaments “ mechanism suggests the force modifies the interaction between Piezo1 and extracellular matrix or cytoskeletal proteins, thereby changing their conformation and opening the channels [34][35][36]. The current induced by channel opening gradually weakens and deactivates slowly at positive membrane potential but does so rapidly at negative membrane potential [11], which may be associated to the extracellular domain and inner helix in the central pore [37][38][39].
3. The Main Cellular Effects of Piezo1 in Digestive System
First, researchers have reviewed the role of Piezo1 of digestive system at the cellular level, to summarize the main cellular effects of Piezo1. The overall cellular effects are list in Table 2, and the associated signal network are shown in Figure 2.
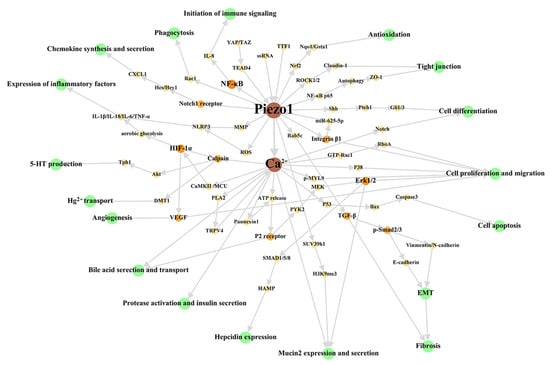
Figure 2. Cellular signal network of Piezo1 in digestive system. The green circles represent cellular effects. The orange part is the signal molecular between Piezo1/Ca2+ part and cellular effects part with gradually darkened color and increased circular diameter according to output. Researchers dissociate the downstream Ca2+ signal pathway in studies stated clearly that Piezo1 functions through Ca2+ influx to make this figure scientific enough, although the open of Piezo1 mainly results in Ca2+ influx as general believed. The references associated with every signal pathway can be reviewed in Table 2. ROS: reactive oxygen species; MMP: Mitochondrial membrane potential; MCU: mitochondrial calcium uniporter; EMT: epithelial-mesenchymal transition.
Table 2. Cellular effects of Piezo1 in digestive system.
| Region | Distribution | Cellular Effect | Species | Intervention * | Study |
|---|---|---|---|---|---|
| Oral cavity | odontoblast cell | generate dentinal sensitivity, suppress dentinogenesis, conduct sensory | human | probe, micropipettes, fluid shear stress, shRNA | [40][41] |
| trigeminal ganglion neuron | transduct acute pain perception | human, rat | n.a. | [42] | |
| dental pulp stem cell | stimulate stem cell proliferation and migration | human, rat | LIPUS, siRNA | [43][44] | |
| squamous carcinoma cell | promote cell growth and proliferation | human | siRNA, shRNA | [45] | |
| acinar cell and duct-forming regions | modulate early differentiation | mouse | siRNA | [46] | |
| Pharyngeal | stratified squamous epithelial cell | n.a. | human | n.a. | [47] |
| pharyngeal muscle, pharyngeal gland, sensory neuron | regulate pharyngeal pumping and defecation | nematode Caenorhabditis elegans | RNAi | [48] | |
| Esophagus | squamous carcinoma cell | regulate cell apoptosis, migration, and invasion | human | shRNA | [49] |
| Stomach | G cell | stimulate gastrin secretion | mouse | n.a. | [50] |
| submucosal and myenteric plexus cell | n.a. | human, guinea pig, mouse | intraganglionic injections | [51] | |
| gastric cancer cell | promote cell proliferation, migration, invasion; suppress cell apoptosis; maintain cellular morphology | human, mouse | siRNA, in vivo xenograft | [52][53] | |
| Small intestine | enterochromaffin cell | mediate 5-HT synthesis | mouse, rat | cyclic stretching, siRNA, sgRNA | [54] |
| epithelial cell | activate NLRP3 inflammasome and initiate immune gene expression | human | beads, siRNA, gRNA | [55][56] | |
| intestinal stem cell | trigger stem-cell proliferation and differentiation | Drosophila | microfluidic chip, gRNA | [57] | |
| fibroblast reticular cell | promote lymphocyte recruitment, initiate mucosal antibody responses | mice | n.a. | [58] | |
| submucosal plexus and myenteric plexus | n.a. | human, mouse, guinea pig | intraganglionic injection | [51] | |
| Large intestine | goblet cell | promote mucin2 expression and mucus secretion | human, mouse | hydrostatic pressure, mechanical traction, shear force, siRNA | [59][60] |
| epithelial cell | activate cell autophagy, regulate expression of tight junction protein, promote Hg2+ transport | human | fluid shear stress, cyclic strain, shRNA, sgRNA | [61][62][63] | |
| adenocarcinoma cell | promote cell migration and metastasis, mediate apoptosis | human | siRNA | [64] | |
| macrophage | promote aerobic glycolysis and secretion of IL-6, TNF-α, IL-1β | mouse | static pressure, cyclic hydrostatic pressure, lps | [65] | |
| microvascular endothelial cell | promote cell migration, organization and alignment | human, mouse | shear stress | [66] | |
| Liver | hepatocyte | reduce mitochondrial ROS, mediate cell apoptosis/necrosis, regulate expression of hepcidin | human, mouse | siRNA, pLVX-EF1α-IRES-ZsGreen1-PIEZO1 mutant constructs | [67][68] |
| hepatocellular carcinoma | promote cell proliferation, migration, invasion, EMT and angiogenesis | human, mouse, rat | matrix stiffness, shRNA, in vivo xenograft | [69][70] | |
| hepatoblastoma | promote cell proliferation and migration | human | siRNA | [71] | |
| macrophage | enhance phagocytosis, regulate expression of hepcidin | mouse | membrane stretch | [72] | |
| hepatic sinus endothelial cell | promote CXCL1 generation and secretion | mouse | cyclic stretch | [73] | |
| Biliary tract | bile canaliculi | promote the contraction of peritubular actin cortex | rat | n.a. | [74] |
| cholangiocyte | trigger ATP secretion | mouse | osmotic pressure, siRNA | [75] | |
| Pancreas | pancreatic acinar cell | trigger intracellular trypsin activation and cell necrosis | mouse | pancreatic duct injection | [76] |
| islet β cell | induce insulin secretion | mouse, rat | circular shear stress, hypotonicity, siRNA | [77] | |
| pancreatic stellate cell | promote cell migration, mediate fibrogenic responses and loss of perinuclear fat droplets | human, mouse | glass pipette, fluid shear stress, spheroid traction, acidification | [78][79] |
* Intervention is not involved in reagent such as Yoda1, Jedi1/2, GsMTx4, Dooku1, RR, Gd3+ because of they are not specific enough and do not imitate mechanical microenvironment cell exposed. n.a. not applicable.
References
- Matsuyama, S.; Tanaka, Y.; Hasebe, R.; Hojyo, S.; Murakami, M. Gateway Reflex and Mechanotransduction. Front. Immunol. 2021, 12, 780451.
- Tschumperlin, D.J. Mechanotransduction. Compr. Physiol. 2011, 1, 1057–1073.
- Holzer, P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol. Ther. 2011, 131, 142–170.
- Alcaino, C.; Farrugia, G.; Beyder, A. Mechanosensitive Piezo Channels in the Gastrointestinal Tract. Curr. Top. Membr. 2017, 79, 219–244.
- La, J.H.; Gebhart, G.F. Colitis decreases mechanosensitive K2P channel expression and function in mouse colon sensory neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G165–G174.
- Wang, W.; Huang, H.; Hou, D.; Liu, P.; Wei, H.; Fu, X.; Niu, W. Mechanosensitivity of STREX-lacking BKCa channels in the colonic smooth muscle of the mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1231–G1240.
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E.; et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012, 483, 176–181.
- Guo, J.; Gu, D.; Zhao, T.; Zhao, Z.; Xiong, Y.; Sun, M.; Xin, C.; Zhang, Y.; Pei, L.; Sun, J. Trends in Piezo Channel Research Over the Past Decade: A Bibliometric Analysis. Front. Pharmacol. 2021, 12, 668714.
- Wang, L.; Zhou, H.; Zhang, M.; Liu, W.; Deng, T.; Zhao, Q.; Li, Y.; Lei, J.; Li, X.; Xiao, B. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature 2019, 573, 225–229.
- Fang, X.Z.; Zhou, T.; Xu, J.Q.; Wang, Y.X.; Sun, M.M.; He, Y.J.; Pan, S.W.; Xiong, W.; Peng, Z.K.; Gao, X.H.; et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell Biosci. 2021, 11, 13.
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60.
- Tang, H.; Zeng, R.; He, E.; Zhang, I.; Ding, C.; Zhang, A. Piezo-Type Mechanosensitive Ion Channel Component 1 (Piezo1): A Promising Therapeutic Target and Its Modulators. J. Med. Chem. 2022, 65, 6441–6453.
- Delmas, P.; Parpaite, T.; Coste, B. PIEZO channels and newcomers in the mammalian mechanosensitive ion channel family. Neuron 2022, 110, 2713–2727.
- Atcha, H.; Jairaman, A.; Holt, J.R.; Meli, V.S.; Nagalla, R.R.; Veerasubramanian, P.K.; Brumm, K.T.; Lim, H.E.; Othy, S.; Cahalan, M.D.; et al. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat. Commun. 2021, 12, 3256.
- Liu, H.; Hu, J.; Zheng, Q.; Feng, X.; Zhan, F.; Wang, X.; Xu, G.; Hua, F. Piezo1 Channels as Force Sensors in Mechanical Force-Related Chronic Inflammation. Front. Immunol. 2022, 13, 816149.
- Hill, R.Z.; Loud, M.C.; Dubin, A.E.; Peet, B.; Patapoutian, A. PIEZO1 transduces mechanical itch in mice. Nature 2022, 607, 104–110.
- Qin, L.; He, T.; Chen, S.; Yang, D.; Yi, W.; Cao, H.; Xiao, G. Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues. Bone Res. 2021, 9, 44.
- Szczot, M.; Nickolls, A.R.; Lam, R.M.; Chesler, A.T. The Form and Function of PIEZO2. Annu. Rev. Biochem. 2021, 90, 507–534.
- Young, M.N.; Sindoni, M.J.; Lewis, A.H.; Zauscher, S.; Grandl, J. The energetics of rapid cellular mechanotransduction. Proc. Natl. Acad. Sci. USA 2023, 120, e2215747120.
- Wijerathne, T.D.; Ozkan, A.D.; Lacroix, J.J. Yoda1’s energetic footprint on Piezo1 channels and its modulation by voltage and temperature. Proc. Natl. Acad. Sci. USA 2022, 119, e2202269119.
- Wang, Y.; Chi, S.; Guo, H.; Li, G.; Wang, L.; Zhao, Q.; Rao, Y.; Zu, L.; He, W.; Xiao, B. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat. Commun. 2018, 9, 1300.
- Evans, E.L.; Cuthbertson, K.; Endesh, N.; Rode, B.; Blythe, N.M.; Hyman, A.J.; Hall, S.J.; Gaunt, H.J.; Ludlow, M.J.; Foster, R.; et al. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br. J. Pharmacol. 2018, 175, 1744–1759.
- Wang, F.; Knutson, K.; Alcaino, C.; Linden, D.R.; Gibbons, S.J.; Kashyap, P.; Grover, M.; Oeckler, R.; Gottlieb, P.A.; Li, H.J.; et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J. Physiol. 2017, 595, 79–91.
- Jankovsky, N.; Caulier, A.; Demagny, J.; Guitton, C.; Djordjevic, S.; Lebon, D.; Ouled-Haddou, H.; Picard, V.; Garçon, L. Recent advances in the pathophysiology of PIEZO1-related hereditary xerocytosis. Am. J. Hematol. 2021, 96, 1017–1026.
- Fotiou, E.; Martin-Almedina, S.; Simpson, M.A.; Lin, S.; Gordon, K.; Brice, G.; Atton, G.; Jeffery, I.; Rees, D.C.; Mignot, C.; et al. Novel mutations in PIEZO1 cause an autosomal recessive generalized lymphatic dysplasia with non-immune hydrops fetalis. Nat. Commun. 2015, 6, 8085.
- Chen, Y.; Jiang, Y.; Chen, B.; Qian, Y.; Liu, J.; Yang, M.; Zhao, B.; Luo, Q. Case Report: Whole Exome Sequencing Revealed Two Novel Mutations of PIEZO1 Implicated in Nonimmune Hydrops Fetalis. Front. Genet. 2021, 12, 684555.
- McMillin, M.J.; Beck, A.E.; Chong, J.X.; Shively, K.M.; Buckingham, K.J.; Gildersleeve, H.I.; Aracena, M.I.; Aylsworth, A.S.; Bitoun, P.; Carey, J.C.; et al. Mutations in PIEZO2 cause Gordon syndrome, Marden-Walker syndrome, and distal arthrogryposis type 5. Am. J. Hum. Genet. 2014, 94, 734–744.
- Seidahmed, M.Z.; Maddirevula, S.; Miqdad, A.M.; Al Faifi, A.; Al Samadi, A.; Alkuraya, F.S. Confirming the involvement of PIEZO2 in the etiology of Marden-Walker syndrome. Am. J. Med. Genet. A 2021, 185, 945–948.
- Ge, J.; Li, W.; Zhao, Q.; Li, N.; Chen, M.; Zhi, P.; Li, R.; Gao, N.; Xiao, B.; Yang, M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 2015, 527, 64–69.
- Gnanasambandam, R.; Bae, C.; Gottlieb, P.A.; Sachs, F. Ionic Selectivity and Permeation Properties of Human PIEZO1 Channels. PLoS ONE 2015, 10, e0125503.
- Hirata, Y.; Cai, R.; Volchuk, A.; Steinberg, B.E.; Saito, Y.; Matsuzawa, A.; Grinstein, S.; Freeman, S.A. Lipid peroxidation increases membrane tension, Piezo1 gating, and cation permeability to execute ferroptosis. Curr. Biol. 2023, 33, 1282–1294.
- Guo, Y.; Merten, A.L.; Schöler, U.; Yu, Z.Y.; Cvetkovska, J.; Fatkin, D.; Feneley, M.P.; Martinac, B.; Friedrich, O. In vitro cell stretching technology (IsoStretcher) as an approach to unravel Piezo1-mediated cardiac mechanotransduction. Prog. Biophys. Mol. Biol. 2021, 159, 22–33.
- Wang, Z.; Chen, J.; Babicheva, A.; Jain, P.P.; Rodriguez, M.; Ayon, R.J.; Ravellette, K.S.; Wu, L.; Balistrieri, F.; Tang, H.; et al. Endothelial upregulation of mechanosensitive channel Piezo1 in pulmonary hypertension. Am J. Physiol. Cell Physiol. 2021, 321, C1010–C1027.
- Murthy, S.E.; Dubin, A.E.; Patapoutian, A. Piezos thrive under pressure: Mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 771–783.
- Vasileva, V.; Chubinskiy-Nadezhdin, V. Regulation of PIEZO1 channels by lipids and the structural components of extracellular matrix/cell cytoskeleton. J. Cell Physiol. 2023, 238, 918–930.
- Lin, Y.C.; Guo, Y.R.; Miyagi, A.; Levring, J.; MacKinnon, R.; Scheuring, S. Force-induced conformational changes in PIEZO1. Nature 2019, 573, 230–234.
- Bae, C.; Gottlieb, P.A.; Sachs, F. Human PIEZO1: Removing inactivation. Biophys. J. 2013, 105, 880–886.
- Wu, J.; Young, M.; Lewis, A.H.; Martfeld, A.N.; Kalmeta, B.; Grandl, J. Inactivation of Mechanically Activated Piezo1 Ion Channels Is Determined by the C-Terminal Extracellular Domain and the Inner Pore Helix. Cell Rep. 2017, 21, 2357–2366.
- Zheng, W.; Gracheva, E.O.; Bagriantsev, S.N. A hydrophobic gate in the inner pore helix is the major determinant of inactivation in mechanosensitive Piezo channels. Elife 2019, 8, e44003.
- Matsunaga, M.; Kimura, M.; Ouchi, T.; Nakamura, T.; Ohyama, S.; Ando, M.; Nomura, S.; Azuma, T.; Ichinohe, T.; Shibukawa, Y. Mechanical Stimulation-Induced Calcium Signaling by Piezo1 Channel Activation in Human Odontoblast Reduces Dentin Mineralization. Front. Physiol. 2021, 12, 704518.
- Sun, X.F.; Qiao, W.W.; Meng, L.Y.; Bian, Z. PIEZO1 Ion Channels Mediate Mechanotransduction in Odontoblasts. J. Endod. 2022, 48, 749–758.
- Cho, Y.S.; Han, H.M.; Jeong, S.Y.; Kim, T.H.; Choi, S.Y.; Kim, Y.S.; Bae, Y.C. Expression of Piezo1 in the Trigeminal Neurons and in the Axons That Innervate the Dental Pulp. Front. Cell Neurosci. 2022, 16, 945948.
- Gao, Q.; Cooper, P.R.; Walmsley, A.D.; Scheven, B.A. Role of Piezo Channels in Ultrasound-stimulated Dental Stem Cells. J. Endod. 2017, 43, 1130–1136.
- Mousawi, F.; Peng, H.; Li, J.; Ponnambalam, S.; Roger, S.; Zhao, H.; Yang, X.; Jiang, L.H. Chemical activation of the Piezo1 channel drives mesenchymal stem cell migration via inducing ATP release and activation of P2 receptor purinergic signaling. Stem Cells 2020, 38, 410–421.
- Hasegawa, K.; Fujii, S.; Matsumoto, S.; Tajiri, Y.; Kikuchi, A.; Kiyoshima, T. YAP signaling induces PIEZO1 to promote oral squamous cell carcinoma cell proliferation. J. Pathol. 2021, 253, 80–93.
- Pokharel, E.; Aryal, Y.P.; Kim, T.Y.; Kim, A.; Kim, J.Y.; Yamamoto, H.; Cho, S.W.; Sohn, W.J.; Kim, J.Y.; Jung, J.K. Developmental function of Piezo1 in mouse submandibular gland morphogenesis. Histochem. Cell Biol. 2023, 159, 477–487.
- Foote, A.G.; Tibbetts, J.; Bartley, S.M.; Thibeault, S.L. Localization of TRPV3/4 and PIEZO1/2 sensory receptors in murine and human larynges. Laryngoscope Investig. Otolaryngol. 2022, 7, 1963–1972.
- Hughes, K.; Shah, A.; Bai, X.; Adams, J.; Bauer, R.; Jackson, J.; Harris, E.; Ficca, A.; Freebairn, P.; Mohammed, S.; et al. Distinct mechanoreceptor pezo-1 isoforms modulate food intake in the nematode Caenorhabditis elegans. G3 2022, 12, jkab429.
- Gao, L.; Ji, Y.; Wang, L.; He, M.; Yang, X.; Qiu, Y.; Sun, X.; Ji, Z.; Yang, G.; Zhang, J.; et al. Suppression of Esophageal Squamous Cell Carcinoma Development by Mechanosensitive Protein Piezo1 Downregulation. ACS Omega 2021, 6, 10196–10206.
- Lang, K.; Breer, H.; Frick, C. Mechanosensitive ion channel Piezo1 is expressed in antral G cells of murine stomach. Cell Tissue Res. 2018, 371, 251–260.
- Mazzuoli-Weber, G.; Kugler, E.M.; Bühler, C.I.; Kreutz, F.; Demir, I.E.; Ceyhan, O.G.; Zeller, F.; Schemann, M. Piezo proteins: Incidence and abundance in the enteric nervous system. Is there a link with mechanosensitivity? Cell Tissue Res. 2019, 375, 605–618.
- Zhang, J.; Zhou, Y.; Huang, T.; Wu, F.; Liu, L.; Kwan, J.S.H.; Cheng, A.S.L.; Yu, J.; To, K.F.; Kang, W. PIEZO1 functions as a potential oncogene by promoting cell proliferation and migration in gastric carcinogenesis. Mol. Carcinog. 2018, 57, 1144–1155.
- Wang, X.; Cheng, G.; Miao, Y.; Qiu, F.; Bai, L.; Gao, Z.; Huang, Y.; Dong, L.; Niu, X.; Wang, X.; et al. Piezo type mechanosensitive ion channel component 1 facilitates gastric cancer omentum metastasis. J. Cell. Mol. Med. 2021, 25, 2238–2253.
- Sugisawa, E.; Takayama, Y.; Takemura, N.; Kondo, T.; Hatakeyama, S.; Kumagai, Y.; Sunagawa, M.; Tominaga, M.; Maruyama, K. RNA Sensing by Gut Piezo1 Is Essential for Systemic Serotonin Synthesis. Cell 2020, 182, 609–624.e621.
- Tadala, L.; Langenbach, D.; Dannborg, M.; Cervantes-Rivera, R.; Sharma, A.; Vieth, K.; Rieckmann, L.M.; Wanders, A.; Cisneros, D.A.; Puhar, A. Infection-induced membrane ruffling initiates danger and immune signaling via the mechanosensor PIEZO1. Cell Rep. 2022, 40, 111173.
- Liu, Q.; Wang, D.; Yang, X.; Ma, F.; Han, W.; Hu, J.; Mei, Q. The Mechanosensitive Ion Channel PIEZO1 in Intestinal Epithelial Cells Mediates Inflammation through the NOD-Like Receptor 3 Pathway in Crohn’s Disease. Inflamm. Bowel Dis. 2023, 29, 103–115.
- He, L.; Si, G.; Huang, J.; Samuel, A.D.T.; Perrimon, N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature 2018, 555, 103–106.
- Chang, J.E.; Buechler, M.B.; Gressier, E.; Turley, S.J.; Carroll, M.C. Mechanosensing by Peyer’s patch stroma regulates lymphocyte migration and mucosal antibody responses. Nat. Immunol. 2019, 20, 1506–1516.
- Xu, Y.; Bai, T.; Xiong, Y.; Liu, C.; Liu, Y.; Hou, X.; Song, J. Mechanical stimulation activates Piezo1 to promote mucin2 expression in goblet cells. J. Gastroenterol. Hepatol. 2021, 36, 3127–3139.
- Xu, Y.; Xiong, Y.; Liu, Y.; Li, G.; Bai, T.; Zheng, G.; Hou, X.; Song, J. Activation of goblet cell Piezo1 alleviates mucus barrier damage in mice exposed to WAS by inhibiting H3K9me3 modification. Cell Biosci. 2023, 13, 7.
- Jiang, Y.; Song, J.; Xu, Y.; Liu, C.; Qian, W.; Bai, T.; Hou, X. Piezo1 regulates intestinal epithelial function by affecting the tight junction protein claudin-1 via the ROCK pathway. Life Sci. 2021, 275, 119254.
- Niu, R.; Lan, J.; Chen, H.; Ye, L.; Huang, K.; Zeng, L.; Gong, S.; Xu, W.; Yang, M. GZMA-PIEZO1 Suppressed development of inflammatory bowel disease through autophagy. J. Cell Commun. Signal. 2023.
- Wang, L.; Han, J.; Su, W.; Li, A.; Zhang, W.; Li, H.; Hu, H.; Song, W.; Xu, C.; Chen, J. Gut-on-a-chip for exploring the transport mechanism of Hg(II). Microsyst. Nanoeng. 2023, 9, 2.
- Sun, Y.; Li, M.; Liu, G.; Zhang, X.; Zhi, L.; Zhao, J.; Wang, G. The function of Piezo1 in colon cancer metastasis and its potential regulatory mechanism. J. Cancer Res. Clin. Oncol. 2020, 146, 1139–1152.
- Leng, S.; Zhang, X.; Wang, S.; Qin, J.; Liu, Q.; Liu, A.; Sheng, Z.; Feng, Q.; Hu, X.; Peng, J. Ion channel Piezo1 activation promotes aerobic glycolysis in macrophages. Front. Immunol. 2022, 13, 976482.
- Li, J.; Hou, B.; Tumova, S.; Muraki, K.; Bruns, A.; Ludlow, M.J.; Sedo, A.; Hyman, A.J.; McKeown, L.; Young, R.S.; et al. Piezo1 integration of vascular architecture with physiological force. Nature 2014, 515, 279–282.
- Wang, Q.; Peng, X.; Chen, Y.; Tang, X.; Qin, Y.; He, M.; Chen, W.; Chen, H. Piezo1 alleviates acetaminophen-induced acute liver injury by activating Nrf2 and reducing mitochondrial reactive oxygen species. Biochem. Biophys. Res. Commun. 2023, 652, 88–94.
- Andolfo, I.; Rosato, B.E.; Manna, F.; De Rosa, G.; Marra, R.; Gambale, A.; Girelli, D.; Russo, R.; Iolascon, A. Gain-of-function mutations in PIEZO1 directly impair hepatic iron metabolism via the inhibition of the BMP/SMADs pathway. Am. J. Hematol. 2020, 95, 188–197.
- Li, Y.M.; Xu, C.; Sun, B.; Zhong, F.J.; Cao, M.; Yang, L.Y. Piezo1 promoted hepatocellular carcinoma progression and EMT through activating TGF-β signaling by recruiting Rab5c. Cancer Cell Int. 2022, 22, 162.
- Li, M.; Zhang, X.; Wang, M.; Wang, Y.; Qian, J.; Xing, X.; Wang, Z.; You, Y.; Guo, K.; Chen, J.; et al. Activation of Piezo1 contributes to matrix stiffness-induced angiogenesis in hepatocellular carcinoma. Cancer Commun. 2022, 42, 1162–1184.
- Ye, X.; Xia, Y.; Zheng, Y.; Chen, W.; Chen, Z.; Cheng, Z.; Wang, B. The function of Piezo1 in hepatoblastoma metastasis and its potential transduction mechanism. Heliyon 2022, 8, e10301.
- Ma, S.; Dubin, A.E.; Zhang, Y.; Mousavi, S.A.R.; Wang, Y.; Coombs, A.M.; Loud, M.; Andolfo, I.; Patapoutian, A. A role of PIEZO1 in iron metabolism in mice and humans. Cell 2021, 184, 969–982.e913.
- Hilscher, M.B.; Sehrawat, T.; Arab, J.P.; Zeng, Z.; Gao, J.; Liu, M.; Kostallari, E.; Gao, Y.; Simonetto, D.A.; Yaqoob, U.; et al. Mechanical Stretch Increases Expression of CXCL1 in Liver Sinusoidal Endothelial Cells to Recruit Neutrophils, Generate Sinusoidal Microthombi, and Promote Portal Hypertension. Gastroenterology 2019, 157, 193–209.e199.
- Gupta, K.; Ng, I.C.; Balachander, G.M.; Nguyen, B.P.; Tucker-Kellogg, L.; Low, B.C.; Yu, H. Bile canaliculi contract autonomously by releasing calcium into hepatocytes via mechanosensitive calcium channel. Biomaterials 2020, 259, 120283.
- Desplat, A.; Penalba, V.; Gros, E.; Parpaite, T.; Coste, B.; Delmas, P. Piezo1-Pannexin1 complex couples force detection to ATP secretion in cholangiocytes. J. Gen. Physiol. 2021, 153, e202112871.
- Romac, J.M.; Shahid, R.A.; Swain, S.M.; Vigna, S.R.; Liddle, R.A. Piezo1 is a mechanically activated ion channel and mediates pressure induced pancreatitis. Nat. Commun. 2018, 9, 1715.
- Deivasikamani, V.; Dhayalan, S.; Abudushalamu, Y.; Mughal, R.; Visnagri, A.; Cuthbertson, K.; Scragg, J.L.; Munsey, T.S.; Viswambharan, H.; Muraki, K.; et al. Piezo1 channel activation mimics high glucose as a stimulator of insulin release. Sci. Rep. 2019, 9, 16876.
- Kuntze, A.; Goetsch, O.; Fels, B.; Najder, K.; Unger, A.; Wilhelmi, M.; Sargin, S.; Schimmelpfennig, S.; Neumann, I.; Schwab, A.; et al. Protonation of Piezo1 Impairs Cell-Matrix Interactions of Pancreatic Stellate Cells. Front. Physiol. 2020, 11, 89.
- Swain, S.M.; Romac, J.M.; Vigna, S.R.; Liddle, R.A. Piezo1-mediated stellate cell activation causes pressure-induced pancreatic fibrosis in mice. JCI Insight 2022, 7, e158288.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
583
Revisions:
2 times
(View History)
Update Date:
28 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




