Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Piezo1, a non-selective cation channel directly activated by mechanical forces, is widely expressed in the digestive system and participates in biological functions physiologically and pathologically.
- mechanotransduction
- Piezo1
- mechanosensitive ion channel
1. Mechanosensitive Ion Channels in the Digestive System
Mechanotransduction refers to the process of living mechanosensitive tissues or cells to detect and respond to changes in membrane tension and cytoskeleton induced by mechanical stimuli, initiating intracellular signal transduction and generating electrochemical signals [1,2]. The digestive system experiences various mechanical stimuli, including gastrointestinal peristalsis, villus movement, conduit osmotic pressure, etc, which are fundamental for initiating mechanotransduction. Mechanotransduction relies on ion channels sensitive to mechanical stimuli, which are known as mechanosensitive ion channels. The mechanosensitive ion channels in digestive system include transient receptor potential vanilloid family (TRPV) [3], Piezo1/2 [4], two pore-domain potassium channels (K2p) [5], large-conductance Ca2+-activated potassium channel (BKCa) [6] and others.
The Piezo protein, characterized as the largest plasma membrane ion channel complex with over 30 putative transmembrane domains, is a unique entity capable of inducing large mechanically-activated cationic currents unlike other known ion channels or proteins [7]. At present, Piezo channel has drawn considerable research interest [8]. Piezo protein contains two homologues in Homo sapiens, Piezo1 and Piezo2 (Table 1). Compared with Piezo1, Piezo2 has additional charged residues at the beam-carboxy-terminal domain interface and additional constriction sites at L2743, F2754 and E2757 in the central pore [9,10]. Piezo1 is widely expressed in multiple cell types, whereas Piezo2 is believed to be predominantly expressed in neurons and intestinal enterochromaffin cells.
First identified in 2010 by Coste B [11], Piezo1 (Fam38a) plays important roles in maintaining various cellular effects such as bone and epithelial homeostasis, neural stem cell differentiation, macrophage polarization, and regulating biological functions including vascular development, red blood cell volume homeostasis, inflammation response generation and etc. [12,13,14,15]. More recently, Piezo1 channel has also been identified to transduce itch in sensory neuron which is associated with Piezo2 channel as generally believed [16]. Therefore, Piezo1 participates in life activity widely and deeply. And more and more evidences have demonstrated the predominant and special contributions of the Piezo1 channel in the digestive system at present [4]. here we review current studies focused on the cellular effects of Piezo1 in digestive system, with special highlights on its importance in regulating biological function.
Table 1. Differences between Piezo1 and Piezo2.
| Piezo1 | Study | Piezo2 | Study | |
|---|---|---|---|---|
| Gene region | 16q24.3 | [12] | 18p11.22-p11.21 | [17] |
| Amino acid residues | 2520 | [12] | 2752 | [17] |
| Distribution | almost all cell types | - | mainly nerve cells and some specific cell types | [18] |
| Detection threshold (fJ) * | 213.7 ± 16.6 | [19] | 86.8 ± 7.1 | [19] |
| Work resolution (fJ) * | 1.2 ± 0.4 | [19] | 1.0 ± 0.2 | [19] |
| Transduction Speed (ms) * | 8.2 ± 2.2 | [19] | 1.5 ± 0.5 | [19] |
| Inactivation kinetics (ms) * | 16.5 ± 1.4 | [11] | 7.3 ± 0.7 | [11] |
| Activator | Yoda1, Jedi1/2 | [20,21] | - | - |
| Inhibitor | Dooku1, GsMTx4, ruthenium red (RR), gadolinium (Gd3+) | [11,22] | GsMTx4, ruthenium red, gadolinium | [23] |
| Hereditary human disorders | dehydrated hereditary stomatocytosis, congenital lymphatic dysplasia with non-immune fetal hydrops | [24,25,26] | recessive distal arthrogryposis syndrome, dominant distal arthrogryposis syndrome (type III and V), Marden-Walker Syndrome | [27,28] |
* detected in HEK293T cell line.
2. Structure and Kinetics of Piezo1
The Piezo1 gene is located on human chromosome 16q24.3 and contains 51 exons [12]. The Piezo1 monomer is about 290–320 kDa while it is a conservative trimer of about 900 kDa naturally [29]. This structure mediats nonlinear transduction of mechanical energy and detects mechanical energies as 213.7 fJ, with a resolution of 1.2 fJ [19]. As a non-selective mechanosensitive cation channel, Piezo1 has the strongest affinity to Ca2+ [11,30]. The opening of Piezo channel triggers mainly influx of Ca2+ and Na+ [31,32]. Therefore, opening of Piezo channel has two effects: it shifts membrane potential and activates other voltage-gated ion channels, which triggers an action potential; simultaneously, it alters [Ca2+]i (intracellular calcium) concentration and triggers downstream signal transduction pathways [33]. Structurally, Piezo1 can be divided into three parts: peripheral N-terminal propeller blades for sensing mechanical stimulation, beam and anchor domain for conducting mechanical signals, and the C-terminal central pore for facilitating ion transport [12] (Figure 1).
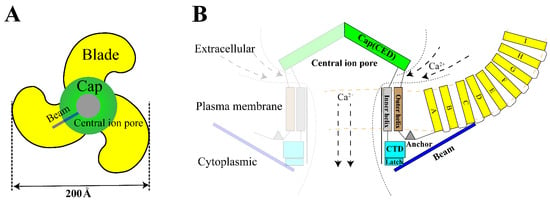
Figure 1. Structure of Piezo1. (A). The trimeric Piezo1 is a three-blade propeller with central pore from extracellular view. (B). Membrane view of the trimeric Piezo1. The propeller blade consists of several four-transmembrane helix bundles with the same topology called “Piezo Repeat” range A-I. CED. C-terminal extracellular domain, CTD. C-terminal domain.
Currently, two principal mechanisms are proposed for Piezo1 activation: the “force-from-lipids” mechanism believes mechanical force alters the membrane lipid-Piezo1 interaction and induces the activation of Piezo1; the “force-from-filaments “ mechanism suggests the force modifies the interaction between Piezo1 and extracellular matrix or cytoskeletal proteins, thereby changing their conformation and opening the channels [34,35,36]. The current induced by channel opening gradually weakens and deactivates slowly at positive membrane potential but does so rapidly at negative membrane potential [11], which may be associated to the extracellular domain and inner helix in the central pore [37,38,39].
3. The Main Cellular Effects of Piezo1 in Digestive System
First, we have reviewed the role of Piezo1 of digestive system at the cellular level, to summarize the main cellular effects of Piezo1. The overall cellular effects are list in Table 2, and the associated signal network are shown in Figure 2.
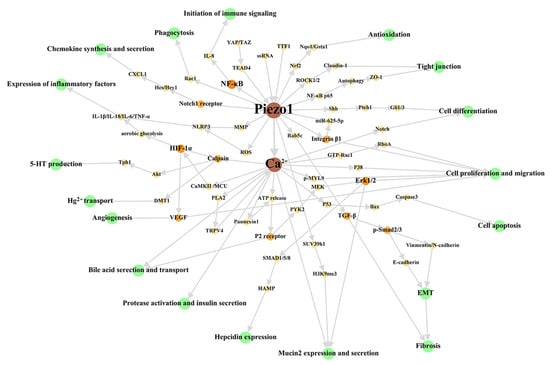
Figure 2. Cellular signal network of Piezo1 in digestive system. The green circles represent cellular effects. The orange part is the signal molecular between Piezo1/Ca2+ part and cellular effects part with gradually darkened color and increased circular diameter according to output. We dissociate the downstream Ca2+ signal pathway in studies stated clearly that Piezo1 functions through Ca2+ influx to make this figure scientific enough, although the open of Piezo1 mainly results in Ca2+ influx as general believed. The references associated with every signal pathway can be reviewed in Table 2. ROS: reactive oxygen species; MMP: Mitochondrial membrane potential; MCU: mitochondrial calcium uniporter; EMT: epithelial-mesenchymal transition.
Table 2. Cellular effects of Piezo1 in digestive system.
| Region | Distribution | Cellular Effect | Species | Intervention * | Study |
|---|---|---|---|---|---|
| Oral cavity | odontoblast cell | generate dentinal sensitivity, suppress dentinogenesis, conduct sensory | human | probe, micropipettes, fluid shear stress, shRNA | [40,41] |
| trigeminal ganglion neuron | transduct acute pain perception | human, rat | n.a. | [42] | |
| dental pulp stem cell | stimulate stem cell proliferation and migration | human, rat | LIPUS, siRNA | [43,44] | |
| squamous carcinoma cell | promote cell growth and proliferation | human | siRNA, shRNA | [45] | |
| acinar cell and duct-forming regions | modulate early differentiation | mouse | siRNA | [46] | |
| Pharyngeal | stratified squamous epithelial cell | n.a. | human | n.a. | [47] |
| pharyngeal muscle, pharyngeal gland, sensory neuron | regulate pharyngeal pumping and defecation | nematode Caenorhabditis elegans | RNAi | [48] | |
| Esophagus | squamous carcinoma cell | regulate cell apoptosis, migration, and invasion | human | shRNA | [49] |
| Stomach | G cell | stimulate gastrin secretion | mouse | n.a. | [50] |
| submucosal and myenteric plexus cell | n.a. | human, guinea pig, mouse | intraganglionic injections | [51] | |
| gastric cancer cell | promote cell proliferation, migration, invasion; suppress cell apoptosis; maintain cellular morphology | human, mouse | siRNA, in vivo xenograft | [52,53] | |
| Small intestine | enterochromaffin cell | mediate 5-HT synthesis | mouse, rat | cyclic stretching, siRNA, sgRNA | [54] |
| epithelial cell | activate NLRP3 inflammasome and initiate immune gene expression | human | beads, siRNA, gRNA | [55,56] | |
| intestinal stem cell | trigger stem-cell proliferation and differentiation | Drosophila | microfluidic chip, gRNA | [57] | |
| fibroblast reticular cell | promote lymphocyte recruitment, initiate mucosal antibody responses | mice | n.a. | [58] | |
| submucosal plexus and myenteric plexus | n.a. | human, mouse, guinea pig | intraganglionic injection | [51] | |
| Large intestine | goblet cell | promote mucin2 expression and mucus secretion | human, mouse | hydrostatic pressure, mechanical traction, shear force, siRNA | [59,60] |
| epithelial cell | activate cell autophagy, regulate expression of tight junction protein, promote Hg2+ transport | human | fluid shear stress, cyclic strain, shRNA, sgRNA | [61,62,63] | |
| adenocarcinoma cell | promote cell migration and metastasis, mediate apoptosis | human | siRNA | [64] | |
| macrophage | promote aerobic glycolysis and secretion of IL-6, TNF-α, IL-1β | mouse | static pressure, cyclic hydrostatic pressure, lps | [65] | |
| microvascular endothelial cell | promote cell migration, organization and alignment | human, mouse | shear stress | [66] | |
| Liver | hepatocyte | reduce mitochondrial ROS, mediate cell apoptosis/necrosis, regulate expression of hepcidin | human, mouse | siRNA, pLVX-EF1α-IRES-ZsGreen1-PIEZO1 mutant constructs | [67,68] |
| hepatocellular carcinoma | promote cell proliferation, migration, invasion, EMT and angiogenesis | human, mouse, rat | matrix stiffness, shRNA, in vivo xenograft | [69,70] | |
| hepatoblastoma | promote cell proliferation and migration | human | siRNA | [71] | |
| macrophage | enhance phagocytosis, regulate expression of hepcidin | mouse | membrane stretch | [72] | |
| hepatic sinus endothelial cell | promote CXCL1 generation and secretion | mouse | cyclic stretch | [73] | |
| Biliary tract | bile canaliculi | promote the contraction of peritubular actin cortex | rat | n.a. | [74] |
| cholangiocyte | trigger ATP secretion | mouse | osmotic pressure, siRNA | [75] | |
| Pancreas | pancreatic acinar cell | trigger intracellular trypsin activation and cell necrosis | mouse | pancreatic duct injection | [76] |
| islet β cell | induce insulin secretion | mouse, rat | circular shear stress, hypotonicity, siRNA | [77] | |
| pancreatic stellate cell | promote cell migration, mediate fibrogenic responses and loss of perinuclear fat droplets | human, mouse | glass pipette, fluid shear stress, spheroid traction, acidification | [78,79] |
* Intervention is not involved in reagent such as Yoda1, Jedi1/2, GsMTx4, Dooku1, RR, Gd3+ because of they are not specific enough and do not imitate mechanical microenvironment cell exposed. n.a. not applicable.
This entry is adapted from the peer-reviewed paper 10.3390/ijms241612953
This entry is offline, you can click here to edit this entry!
