
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | RICHARD-FORGET Florence | -- | 2382 | 2023-08-25 09:18:04 | | | |
| 2 | Jessie Wu | Meta information modification | 2382 | 2023-08-25 10:43:29 | | | | |
| 3 | Jessie Wu | -6 word(s) | 2376 | 2023-09-08 10:21:26 | | |
Video Upload Options
Tocochromanols, which encompass tocopherols and tocotrienols and constitute the vitamin E family, are widely distributed in cereal kernels; their biosynthetic pathway has been extensively studied with the aim to enrich plant oils and combat vitamin E deficiency in humans. Here researchers provide strong assumptions arguing in favor of an involvement of tocochromanols in plant–fungal pathogen interactions. Tocochromanols are plant compounds with a strong antioxidant potential. The biosynthesis of this class of compounds draws on metabolites from the terpenoid and shikimate pathways. Tocochromanols are acknowledged to efficiently quench singlet oxygen and scavenge various radicals, especially lipid peroxyl radicals derived from polyunsaturated fatty acids, thereby terminating lipid peroxidation chain reactions.
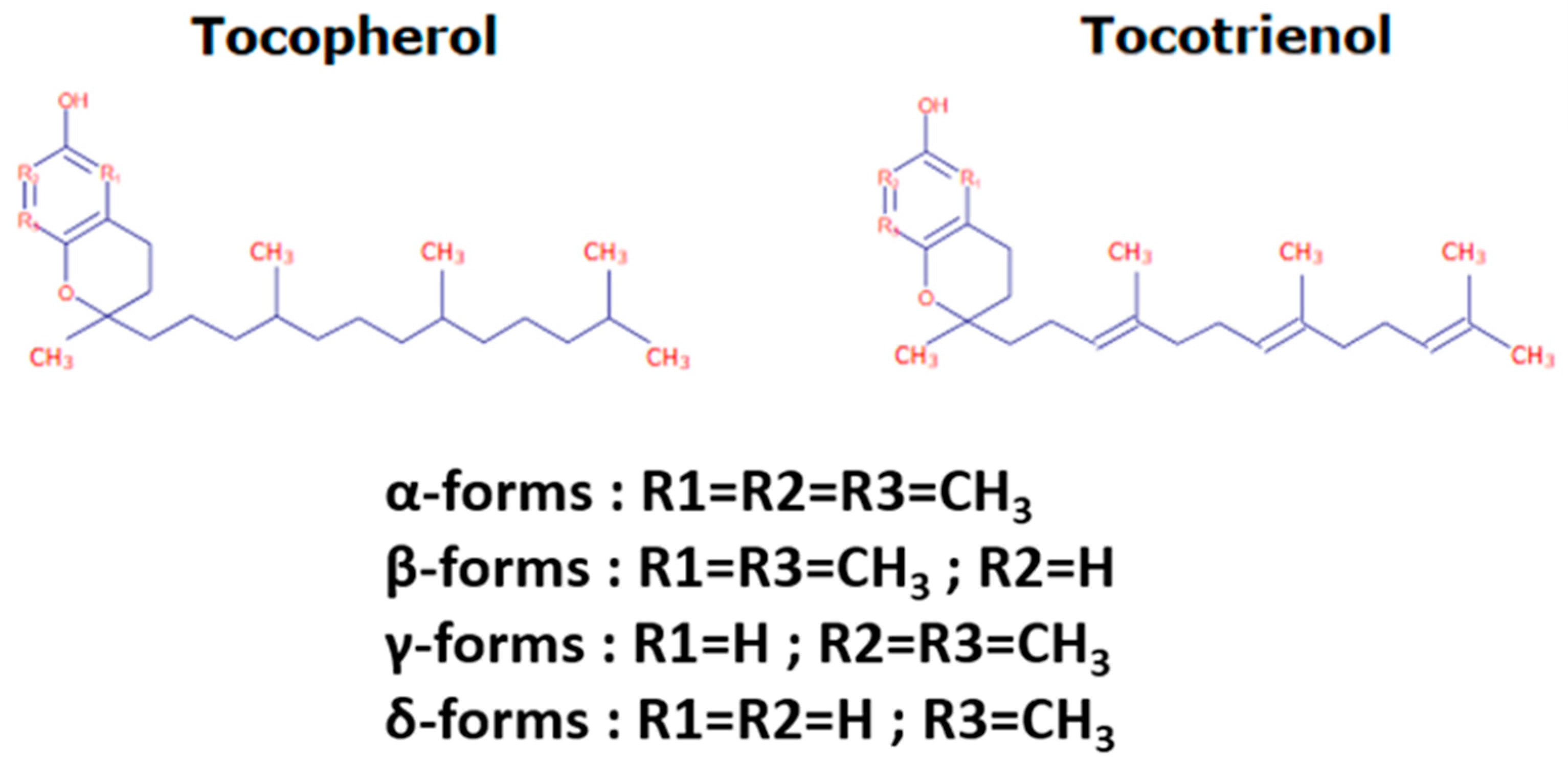
1. Tocochromanol Structure
2. Tocochromanol Biosynthesis
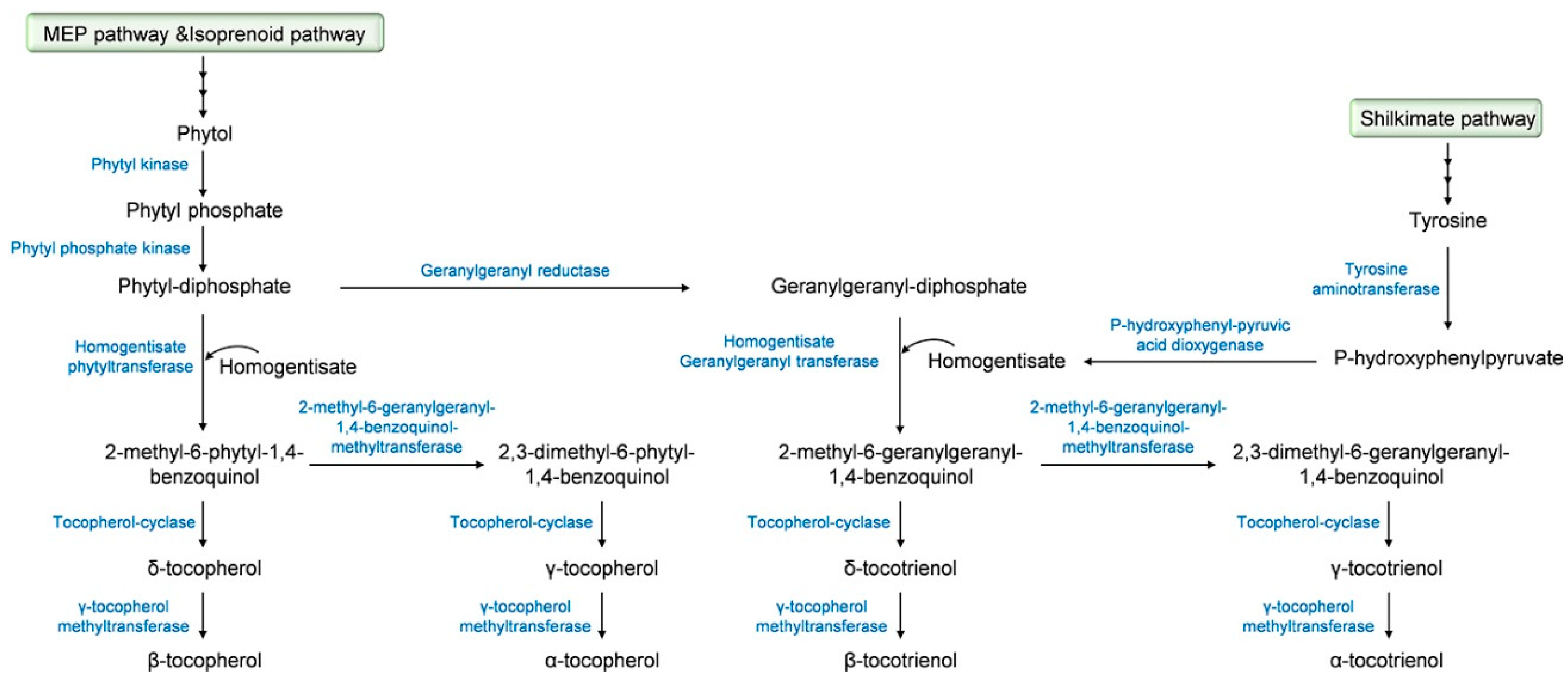
| Plant Species | Gene | Enzyme 1 | Ref. |
|---|---|---|---|
| Arabidopsis thaliana | |||
| PSD1 | HPPD | [14] | |
| GGR | GGR | [15] | |
| VTE1 | Tocopherol cyclase | [12] | |
| VTE2 | HPT | [12] | |
| VTE3 | MPBQ/MGGBQ MT | [16] | |
| VTE4 | γ-TMT | [11] | |
| VTE5 | Phytol kinase | [9] | |
| VTE6 | Phytyl-P kinase | [10] | |
| HGGT | HGGT | [17] | |
| Barley | |||
| VTE1 | Tocopherol cyclase | [18] | |
| VTE4 | γ-TMT | [18] | |
| HPT-7H | HPT | [19] | |
| HGGT | HGGT | [19] | |
| Maize | |||
| ZmVTE1 | Tocopherol cyclase | [5] | |
| ZmVTE2 | HPT | [5] | |
| ZmVTE3 | MPBQ/MGGBQ MT | [5] | |
| ZmVTE4 | γ-TMT | [5] | |
| ZmVTE5 | Phytol kinase | [5] | |
| ZmHPPD | HPPD | [5] | |
| Rice | |||
| OsGGR1 | GGR | [20] | |
| OsGGR2 | GGR | [20] | |
| OsγTMT | γ-TMT | [21] | |
| SGD1 | HPT | [22] | |
| RTD1 | HPT | [23] | |
| Oat | |||
| HPPD (3 homo) | HPPD | [24] | |
| VTE2_3/VTE2_4 | HPT | [24] | |
| VTE4_1 | γ-TMT | [24] | |
3. Tocochromanol Composition of Major Cereal Crops

4. Distribution of Tocochromanols within Cereal Kernels
| α-T 1 (%) |
β-T (%) |
γ-T (%) |
δ-T (%) |
α-T3 1 (%) |
β-T3 (%) |
γ-T3 (%) |
δ-T3 (%) |
Total Tocopherol (%) |
Total Tocotrienol (%) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Maize | ||||||||||
| Germ | 30 | 1 | 65 | 3 | 1 | 0 | 1 | 0 | 98 | 2 |
| Endosperm | 5 | 0 | 19 | 0 | 29 | 0 | 45 | 2 | 24 | 76 |
| Pericarp | 18 | 0 | 52 | 6 | 9 | 1 | 14 | 1 | 75 | 25 |
| Wheat | ||||||||||
| Germ | 69 | 26 | 0 | 0 | 2 | 3 | ND 2 | 0 | 95 | 5 |
| Endosperm | 5 | 3 | 13 | 0 | 11 | 68 | ND | 0 | 21 | 79 |
| Pericarp | 7 | 3 | 8 | 0 | 21 | 61 | ND | 0 | 18 | 82 |
| Barley | ||||||||||
| Germ | 68 | 3 | 16 | 1 | 6 | 2 | 3 | 0 | 89 | 11 |
| Endosperm | 14 | 1 | 2 | 1 | 41 | 25 | 15 | 3 | 17 | 83 |
| Pericarp | 15 | 1 | 5 | 1 | 47 | 12 | 17 | 3 | 21 | 79 |
| Rice | ||||||||||
| Germ | 81 | 3 | 5 | 0 | 6 | ND | 4 | 0 | 89 | 11 |
| Endosperm | 33 | 2 | 6 | 2 | 21 | ND | 33 | 4 | 42 | 58 |
| Pericarp | 37 | 1 | 4 | 0 | 27 | ND | 29 | 2 | 43 | 57 |
5. Kinetics of Tocochromanol Accumulation during Maturation of Cereal Kernels
References
- Muñoz, P.; Munné-Bosch, S. Vitamin E in Plants: Biosynthesis, Transport, and Function. Trends Plant Sci. 2019, 24, 1040–1051.
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative Antioxidant Activities of Carotenoids Measured by Ferric Reducing Antioxidant Power (FRAP), ABTS Bleaching Assay (ATEAC), DPPH Assay and Peroxyl Radical Scavenging Assay. Food Chem. 2011, 129, 139–148.
- Mène-Saffrané, L.; DellaPenna, D. Biosynthesis, Regulation and Functions of Tocochromanols in Plants. Plant Physiol. Biochem. 2010, 48, 301–309.
- Hussain, N.; Irshad, F.; Jabeen, Z.; Shamsi, I.H.; Li, Z.; Jiang, L. Biosynthesis, Structural, and Functional Attributes of Tocopherols in Planta; Past, Present, and Future Perspectives. J. Agric. Food Chem. 2013, 61, 6137–6149.
- Lipka, A.E.; Gore, M.A.; Magallanes-Lundback, M.; Mesberg, A.; Lin, H.; Tiede, T.; Chen, C.; Buell, C.R.; Buckler, E.S.; Rocheford, T.; et al. Genome-Wide Association Study and Pathway-Level Analysis of Tocochromanol Levels in Maize Grain. G3 GenesGenomesGenetics 2013, 3, 1287–1299.
- Fritsche, S.; Wang, X.; Jung, C. Recent Advances in Our Understanding of Tocopherol Biosynthesis in Plants: An Overview of Key Genes, Functions, and Breeding of Vitamin E Improved Crops. Antioxidants 2017, 6, 99.
- Ma, J.; Qiu, D.; Pang, Y.; Gao, H.; Wang, X.; Qin, Y. Diverse Roles of Tocopherols in Response to Abiotic and Biotic Stresses and Strategies for Genetic Biofortification in Plants. Mol. Breed. 2020, 40, 18.
- Mène-Saffrané, L. Vitamin E Biosynthesis and Its Regulation in Plants. Antioxidants 2017, 7, 2.
- Valentin, H.E.; Lincoln, K.; Moshiri, F.; Jensen, P.K.; Qi, Q.; Venkatesh, T.V.; Karunanandaa, B.; Baszis, S.R.; Norris, S.R.; Savidge, B.; et al. The Arabidopsis Vitamin E Pathway Gene5-1 Mutant Reveals a Critical Role for Phytol Kinase in Seed Tocopherol Biosynthesis. Plant Cell 2006, 18, 212–224.
- Vom Dorp, K.; Hölzl, G.; Plohmann, C.; Eisenhut, M.; Abraham, M.; Weber, A.P.; Hanson, A.D.; Dörmann, P. Remobilization of Phytol from Chlorophyll Degradation Is Essential for Tocopherol Synthesis and Growth of Arabidopsis. Plant Cell 2015, 27, 2846–2859.
- Bergmüller, E.; Porfirova, S.; Dörmann, P. Characterization of an Arabidopsis Mutant Deficient in γ-Tocopherol Methyltransferase. Plant Mol. Biol. 2003, 52, 1181–1190.
- Porfirova, S.; Bergmüller, E.; Tropf, S.; Lemke, R.; Dörmann, P. Isolation of an Arabidopsis Mutant Lacking Vitamin E and Identification of a Cyclase Essential for All Tocopherol Biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 12495–12500.
- Diepenbrock, C.H.; Kandianis, C.B.; Lipka, A.E.; Magallanes-Lundback, M.; Vaillancourt, B.; Góngora-Castillo, E.; Wallace, J.G.; Cepela, J.; Mesberg, A.; Bradbury, P.J.; et al. Novel Loci Underlie Natural Variation in Vitamin E Levels in Maize Grain. Plant Cell 2017, 29, 2374–2392.
- Norris, S.R.; Shen, X.; Della Penna, D. Complementation of the Arabidopsis Pds1 Mutation with the Gene Encoding P-Hydroxyphenylpyruvate Dioxygenase. Plant Physiol. 1998, 117, 1317–1323.
- Keller, Y.; Bouvier, F.; d’Harlingue, A.; Camara, B. Metabolic Compartmentation of Plastid Prenyllipid Biosynthesis. Eur. J. Biochem. 1998, 251, 413–417.
- Cheng, Z.; Sattler, S.; Maeda, H.; Sakuragi, Y.; Bryant, D.A.; DellaPenna, D. Highly Divergent Methyltransferases Catalyze a Conserved Reaction in Tocopherol and Plastoquinone Synthesis in Cyanobacteria and Photosynthetic Eukaryotes. Plant Cell 2003, 15, 2343–2356.
- Zhang, C.; Cahoon, R.E.; Hunter, S.C.; Chen, M.; Han, J.; Cahoon, E.B. Genetic and Biochemical Basis for Alternative Routes of Tocotrienol Biosynthesis for Enhanced Vitamin E Antioxidant Production. Plant J. 2013, 73, 628–639.
- Graebner, R.C.; Wise, M.; Cuesta-Marcos, A.; Geniza, M.; Blake, T.; Blake, V.C.; Butler, J.; Chao, S.; Hole, D.J.; Horsley, R.; et al. Quantitative Trait Loci Associated with the Tocochromanol (Vitamin E) Pathway in Barley. PLoS ONE 2015, 10, e0133767.
- Schuy, C.; Groth, J.; Ammon, A.; Eydam, J.; Baier, S.; Schweizer, G.; Hanemann, A.; Herz, M.; Voll, L.M.; Sonnewald, U. Deciphering the Genetic Basis for Vitamin E Accumulation in Leaves and Grains of Different Barley Accessions. Sci. Rep. 2019, 9, 9470.
- Kimura, E.; Abe, T.; Murata, K.; Kimura, T.; Otoki, Y.; Yoshida, T.; Miyazawa, T.; Nakagawa, K. Identification of OsGGR2, a Second Geranylgeranyl Reductase Involved in α-Tocopherol Synthesis in Rice. Sci. Rep. 2018, 8, 1870.
- Wang, X.-Q.; Yoon, M.-Y.; He, Q.; Kim, T.-S.; Tong, W.; Choi, B.-W.; Lee, Y.-S.; Park, Y.-J. Natural Variations in OsγTMT Contribute to Diversity of the α-Tocopherol Content in Rice. Mol. Genet. Genom. 2015, 290, 2121–2135.
- Wang, D.; Wang, Y.; Long, W.; Niu, M.; Zhao, Z.; Teng, X.; Zhu, X.; Zhu, J.; Hao, Y.; Wang, Y.; et al. SGD1, a Key Enzyme in Tocopherol Biosynthesis, Is Essential for Plant Development and Cold Tolerance in Rice. Plant Sci. Int. J. Exp. Plant Biol. 2017, 260, 90–100.
- Yunhui, Z.; Kai, L.; Xiaomei, Z.; Yan, W.; Suobing, Z.; Haiyuan, C.; Jing, L.; Yingjie, W.; Xianwen, F. Rice Tocopherol Deficiency 1 Encodes a Homogentisate Phytyltransferase Essential for Tocopherol Biosynthesis and Plant Development in Rice. Plant Cell Rep. 2018, 37, 775–787.
- Gutierrez-Gonzalez, J.J.; Garvin, D.F. Subgenome-Specific Assembly of Vitamin E Biosynthesis Genes and Expression Patterns during Seed Development Provide Insight into the Evolution of Oat Genome. Plant Biotechnol. J. 2016, 14, 2147–2157.
- Chaudhary, N.; Khurana, P. Vitamin E Biosynthesis Genes in Rice: Molecular Characterization, Expression Profiling and Comparative Phylogenetic Analysis. Plant Sci. 2009, 177, 479–491.
- Tanaka, R.; Rothbart, M.; Oka, S.; Takabayashi, A.; Takahashi, K.; Shibata, M.; Myouga, F.; Motohashi, R.; Shinozaki, K.; Grimm, B.; et al. LIL3, a Light-Harvesting-like Protein, Plays an Essential Role in Chlorophyll and Tocopherol Biosynthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 16721–16725.
- Zhan, W.; Liu, J.; Pan, Q.; Wang, H.; Yan, S.; Li, K.; Deng, M.; Li, W.; Liu, N.; Kong, Q.; et al. An Allele of ZmPORB2 Encoding a Protochlorophyllide Oxidoreductase Promotes Tocopherol Accumulation in Both Leaves and Kernels of Maize. Plant J. 2019, 100, 114–127.
- Wang, H.; Xu, S.; Fan, Y.; Liu, N.; Zhan, W.; Liu, H.; Xiao, Y.; Li, K.; Pan, Q.; Li, W.; et al. Beyond Pathways: Genetic Dissection of Tocopherol Content in Maize Kernels by Combining Linkage and Association Analyses. Plant Biotechnol. J. 2018, 16, 1464–1475.
- Lachman, J.; Hejtmánková, A.; Orsák, M.; Popov, M.; Martinek, P. Tocotrienols and Tocopherols in Colored-Grain Wheat, Tritordeum and Barley. Food Chem. 2018, 240, 725–735.
- Sookwong, P.; Murata, K.; Nakagawa, K.; Shibata, A.; Kimura, T.; Yamaguchi, M.; Kojima, Y.; Miyazawa, T. Cross-Fertilization for Enhancing Tocotrienol Biosynthesis in Rice Plants and QTL Analysis of Their F2 Progenies. J. Agric. Food Chem. 2009, 57, 4620–4625.
- Hidalgo, A.; Brandolini, A. Protein, Ash, Lutein and Tocols Distribution in Einkorn (Triticum monococcum L. Subsp. Monococcum) Seed Fractions. Food Chem. 2008, 107, 444–448.
- Goufo, P.; Trindade, H. Rice Antioxidants: Phenolic Acids, Flavonoids, Anthocyanins, Proanthocyanidins, Tocopherols, Tocotrienols, γ-Oryzanol, and Phytic Acid. Food Sci. Nutr. 2014, 2, 75–104.
- Xie, L.; Yu, Y.; Mao, J.; Liu, H.; Hu, J.; Li, T.; Guo, X.; Liu, R. Evaluation of Biosynthesis, Accumulation and Antioxidant Activity of Vitamin E in Sweet Corn (Zea mays L.) during Kernel Development. Int. J. Mol. Sci. 2017, 18, 2780.
- Kurilich, A.C.; Juvik, J.A. Quantification of Carotenoid and Tocopherol Antioxidants in Zea mays. J. Agric. Food Chem. 1999, 47, 1948–1955.
- Sun, X.; Ma, L.; Lux, P.E.; Wang, X.; Stuetz, W.; Frank, J.; Liang, J. The Distribution of Phosphorus, Carotenoids and Tocochromanols in Grains of Four Chinese Maize (Zea mays L.) Varieties. Food Chem. 2022, 367, 130725.
- Franzen, J.; Haaß, M.M. Vitamin E Content during Development of Some Seedlings. Phytochemistry 1991, 30, 2911–2913.
- Horvath, G.; Wessjohann, L.; Bigirimana, J.; Jansen, M.; Guisez, Y.; Caubergs, R.; Horemans, N. Differential Distribution of Tocopherols and Tocotrienols in Photosynthetic and Non-Photosynthetic Tissues. Phytochemistry 2006, 67, 1185–1195.
- Zieliński, H.; Ciska, E.; Kozlowska, H. The Cereal Grains: Focus on Vitamin E. Czech J. Food Sci. 2001, 19, 182–188.
- Yu, L.; Li, G.; Li, M.; Xu, F.; Beta, T.; Bao, J. Genotypic Variation in Phenolic Acids, Vitamin E and Fatty Acids in Whole Grain Rice. Food Chem. 2016, 197, 776–782.
- Heinemann, R.J.B.; Xu, Z.; Godber, J.S.; Lanfer-Marquez, U.M. Tocopherols, Tocotrienols, and γ-Oryzanol Contents in Japonica and Indica Subspecies of Rice (Oryza sativa L.) Cultivated in Brazil. Cereal Chem. 2008, 85, 243–247.
- Kim, N.H.; Kwak, J.; Baik, J.Y.; Yoon, M.-R.; Lee, J.-S.; Yoon, S.W.; Kim, I.-H. Changes in Lipid Substances in Rice during Grain Development. Phytochemistry 2015, 116, 170–179.
- Lampi, A.-M.; Nurmi, T.; Ollilainen, V.; Piironen, V. Tocopherols and Tocotrienols in Wheat Genotypes in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9716–9721.
- Labuschagne, M.; Mkhatywa, N.; Johansson, E.; Wentzel, B.; van Biljon, A. The Content of Tocols in South African Wheat; Impact on Nutritional Benefits. Foods 2017, 6, 95.
- Gutierrez-Gonzalez, J.J.; Wise, M.L.; Garvin, D.F. A Developmental Profile of Tocol Accumulation in Oat Seeds. J. Cereal Sci. 2013, 57, 79–83.
- Bergman, C.J.; Xu, Z. Genotype and Environment Effects on Tocopherol, Tocotrienol, and γ-Oryzanol Contents of Southern U.S. Rice. Cereal Chem. 2003, 80, 446–449.
- Munne-Bosch, S. The Role of alpha-Tocopherol in Plant Stress Tolerance. J. Plant Physiol. 2005, 162, 743–748.
- Fisk, I.D.; White, D.A.; Carvalho, A.; Gray, D.A. Tocopherol—An Intrinsic Component of Sunflower Seed Oil Bodies. J. Am. Oil Chem. Soc. 2006, 83, 341–344.
- Siles, L.; Cela, J.; Munné-Bosch, S. Vitamin E Analyses in Seeds Reveal a Dominant Presence of Tocotrienols over Tocopherols in the Arecaceae Family. Phytochemistry 2013, 95, 207–214.
- Grams, G.W.; Blessin, C.W.; Inglett, G.E. Distribution of Tocopherols within the Corn Kernel. J. Am. Oil Chem. Soc. 1970, 47, 337–339.
- Ko, S.-N.; Kim, C.-J.; Kim, H.; Kim, C.-T.; Chung, S.-H.; Tae, B.-S.; Kim, I.-H. Tocol Levels in Milling Fractions of Some Cereal Grains and Soybean. J. Am. Oil Chem. Soc. 2003, 80, 585–589.
- Morrison, W.R.; Coventry, A.M.; Barnes, P.J. The Distribution of Acyl Lipids and Tocopherols in Flours Millstreams. J. Sci. Food Agric. 1982, 33, 925–933.
- Picot, A.; Atanasova-Pénichon, V.; Pons, S.; Marchegay, G.; Barreau, C.; Pinson-Gadais, L.; Roucolle, J.; Daveau, F.; Caron, D.; Richard-Forget, F. Maize Kernel Antioxidants and Their Potential Involvement in Fusarium Ear Rot Resistance. J. Agric. Food Chem. 2013, 61, 3389–3395.
- Falk, J.; Krahnstöver, A.; van der Kooij, T.A.W.; Schlensog, M.; Krupinska, K. Tocopherol and Tocotrienol Accumulation during Development of Caryopses from Barley (Hordeum vulgare L.). Phytochemistry 2004, 65, 2977–2985.





