Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Agnieszka J. Szczepek | -- | 2455 | 2023-08-24 12:06:42 | | | |
| 2 | Lindsay Dong | Meta information modification | 2455 | 2023-08-25 03:09:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kong, L.; Domarecka, E.; Szczepek, A.J. Histamine and Its Receptors in Mammalian Inner Ear. Encyclopedia. Available online: https://encyclopedia.pub/entry/48435 (accessed on 07 February 2026).
Kong L, Domarecka E, Szczepek AJ. Histamine and Its Receptors in Mammalian Inner Ear. Encyclopedia. Available at: https://encyclopedia.pub/entry/48435. Accessed February 07, 2026.
Kong, Lingyi, Ewa Domarecka, Agnieszka J. Szczepek. "Histamine and Its Receptors in Mammalian Inner Ear" Encyclopedia, https://encyclopedia.pub/entry/48435 (accessed February 07, 2026).
Kong, L., Domarecka, E., & Szczepek, A.J. (2023, August 24). Histamine and Its Receptors in Mammalian Inner Ear. In Encyclopedia. https://encyclopedia.pub/entry/48435
Kong, Lingyi, et al. "Histamine and Its Receptors in Mammalian Inner Ear." Encyclopedia. Web. 24 August, 2023.
Copy Citation
Histamine is a widely distributed biogenic amine with multiple biological functions mediated by specific receptors that determine the local effects of histamine. All four types of histamine receptors were identified in the mammalian inner ear. The functional studies of histamine in the inner ear were mainly in vitro. Clinical evidence suggests that histamine and its receptors may play a role in Ménière’s disease, but the exact mechanism is not fully understood.
histamine
histamine receptors
inner ear
hearing
1. Introduction
Histamine is a bioactive amine acting as an effector molecule of the immune system and a neurotransmitter in the nervous system. Histamine is synthesized from histidine by specific decarboxylase [1][2]. The histidine decarboxylase-expressing cells include mast cells, basophils, histaminergic neurons, and enterochromaffin-like cells in the stomach [3]. Histamine can either be secreted immediately or stored in granules for later use, as is the case with mast cells and basophils [4][5][6], which release histamine upon stimulation [7][8]. The effects of histamine range from the involvement in innate immunity [9] and its pathologies, such as allergic rhinitis [10], to housekeeping brain and bone homeostasis [4] and depend on the target cell type and the kind of histamine receptor expressed [11].
To date, four histamine receptors have been identified: the H1 receptor (H1R), H2 receptor (H2R), H3 receptor (H3R), and H4 receptor (H4R) [12][13]. All four belong to the G-protein-coupled receptor family. H1R is expressed in many tissues and cells, including cerebral neurons, the respiratory epithelium, the adrenal medulla, and hepatic, cardiovascular, and endothelial cells [1][14][15]. It is involved in allergic and inflammatory responses. When stimulated, it activates phospholipase C and increases intracellular Ca2+ levels [16][17]. As a result, the smooth muscles of the respiratory tract contract, and vascular permeability increases, subsequently causing a range of symptoms associated with allergic reactions [18]. H2R is also widely distributed and highly expressed in gastric parietal cells, vascular smooth muscles, the central nervous system, and the heart [15][19][20][21]. H3R is mainly found in the central nervous system but it is also widely distributed in peripheral tissues [22]. The effects of H3R activation are diverse and include the regulation of histamine turnover, sleep–awake regulation, learning, memory, and inflammation, as well as the inhibition of the release of several other neurotransmitters, such as serotonin, GABA, and glutamate [4][22][23]. The H4R was first described at the beginning of the 21st century [24][25][26]. The gene encoding H4R was discovered via genomic homology searches and reverse pharmacology, identifying its role in immune and pruritic responses [27]. This receptor has a relatively high homology with H3R; however, its role has yet to be elucidated [27][28].
The mammalian inner ear is a complex sensory organ consisting of the cochlea, vestibule, and three semicircular canals [29]. Sound passes from the external ear canal through the middle ear to the inner ear. In the inner ear’s cochlea, sound’s mechanical energy is converted into a biochemical signal by the sensory epithelium (hair cells) in a process called mechanotransduction. Mechanotransduction induces the release of glutamate from the inner hair cells, which activates the spiral ganglion neurons by initiating an action potential that is sequentially transmitted along the auditory pathway to the auditory cortex [30][31].
The vestibular system is responsible for sensing and processing information about the position and movement of the head and body in space and maintaining balance and coordination during the movement [32]. The peripheral vestibular organs are located bilaterally in the inner ear. They consist of two otolithic organs (utricle and saccule) and three semicircular canals (anterior, posterior, and horizontal), the former sensing linear acceleration, such as head movement or gravity, and the latter sensing rotational acceleration [33]. The sensory nerve epithelium in the utricle and saccule is the macula, and in the semicircular canals, it is the crista ampullaris [32]. Both structures contain vestibular hair cells, which release glutamate upon depolarization, stimulating the vestibular ganglion’s afferent nerves. The vestibular ganglion and the cochlear spiral ganglion neurons form the eighth cranial nerve.
The endolymphatic sac is the non-sensory part of the membranous labyrinth of the inner ear. It plays several important roles, including regulating the volume and pressure of the potassium-rich endolymph fluid, participating in the immune response within the inner ear, and removing waste products from the endolymph.
In recent years, the immune function, inflammatory processes, and vascular control of the inner ear have been investigated and reviewed [34][35][36][37]. However, the specific topic of histamine and its signaling in the cochlea or vestibular organs remains scarcely addressed in the literature. A few research teams have identified the expression of histamine receptors in the inner ear of mammals [38][39][40][41]. Additionally, in 2020, our group found mast cells in the inner ear of both rats and mice [42]. Mast cells are the major source of histamine in the body, along with basophils, gastric parietal cells, and the central nervous system [43]. Upon activation, mast cells degranulate and release a number of immuno- and neuromodulatory compounds, including histamine [44][45]. Some conditions necessary for mast cell activation have already been described regarding the inner ear, including IgE antibody transcytosis across the blood–labyrinth barrier [46] and the presence of substance P [47]. However, more research is needed to understand the relationship between the presence of mast cells in the inner ear, their mode of activation, potential histamine release, and its consequences in health and disease, such as mastocytosis or IgE-mediated diseases. Clinical evidence suggests an association between elevated numbers of mast cells and inner ear disorders [48][49]. Moreover, experiments demonstrated that Meniere’s disease-like symptoms (attacks of nystagmus and hearing loss) can be induced by the experimental induction of a type I allergy in the endolymphatic sac of guinea pigs [50].
2. Histamine and Its Receptors in Mammalian Inner Ear
2.1. Expression of Histamine Receptors in the Mammalian Cochlea
Results from several studies have shown that histamine receptors were detected at multiple sites in the mammalian inner ear (Figure 1) [38][39][51]. The mRNA encoding histamine receptors (H1, H2, and H3R) was found to be present in the lateral portion of the cochlea, including the spiral ligament and the stria vascularis, the medial portion, including the organ of Corti, and the modiolus [38]. Takumida et al. used immunohistochemistry (IHC) to examine the mouse cochlea and found that the stria vascularis was positive for H1R, the spiral ligament for H3R and H4R, and the spiral ganglion neurons for all four types of receptors. In the latter, immunofluorescence (IF) receptor signals were observed in the cytoplasm of the spiral ganglion neurons [51]. In the organ of Corti, H3R was observed in both the outer and inner hair cells, whereas H1R, H2R, H3R, and H4R were observed in some supporting cells [51].
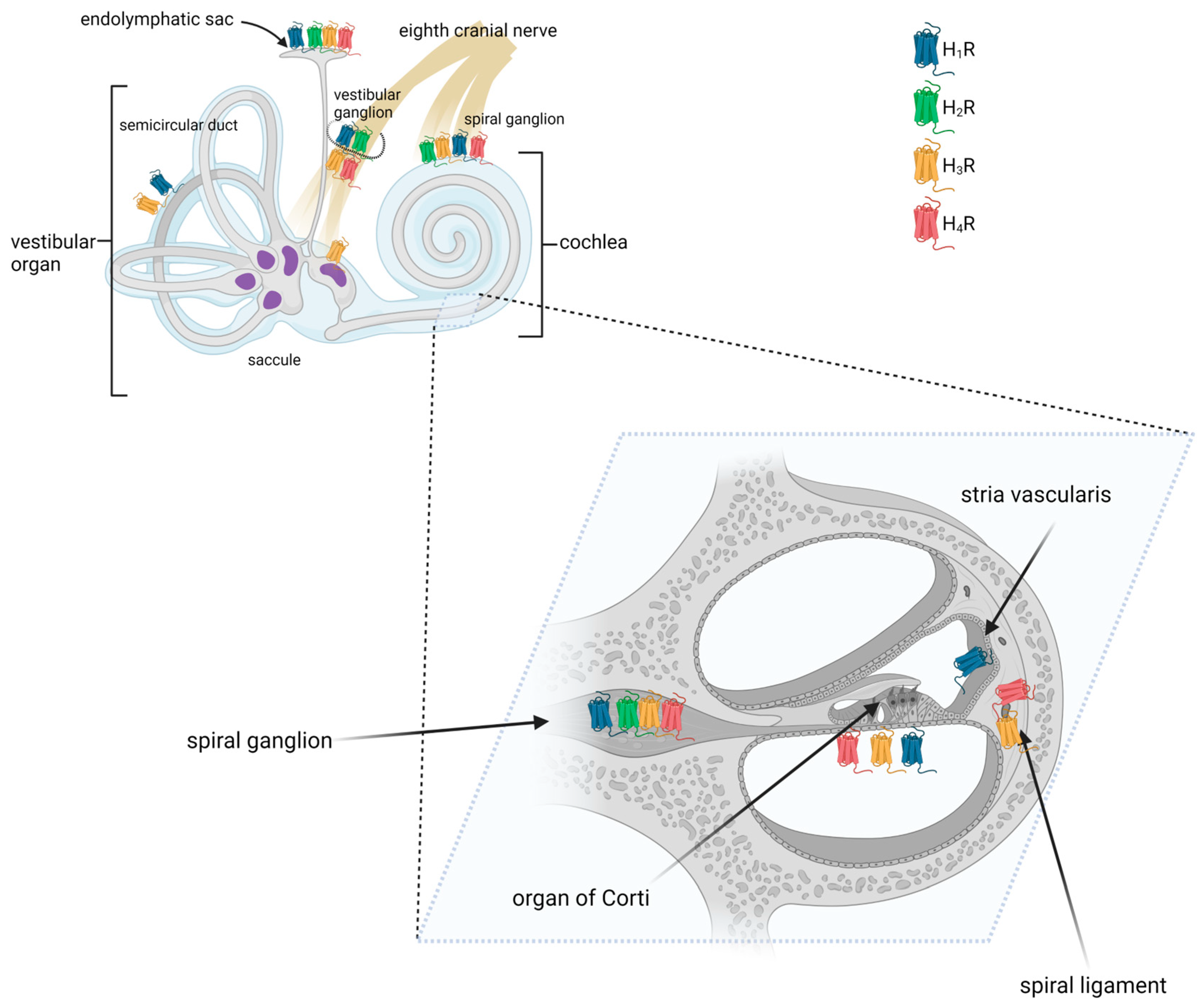
Figure 1. Distribution of histamine receptors in the mammalian inner ear. Created with BioRender.com.
2.2. Expression of Histamine Receptors in the Mammalian Vestibular System
2.2.1. Semicircular Canals
Using reverse RT-PCR, Western blotting, and in situ immunolabeling, Botta et al. demonstrated that the semicircular canal of the mouse expresses H1R [40]. Conversely, no clear evidence for H3R expression was found.
2.2.2. Utricle and Saccule
All four types of histamine receptors have been demonstrated in the maculae of the saccule and utricle [51][52]. More specifically, the expression of histamine receptors was found in type I hair cells, the calyx and dimorphic vestibular afferents, and subepithelial cells [52].
2.2.3. Vestibular Ganglion
Like in spiral ganglion neurons, H1R, H2R, H3R, and H4R were detected on vestibular ganglion cells using immunohistochemistry [51][52][53]. Tritto et al. examined vestibular neurons with immunofluorescence and found that approximately 30% of the nerves stained positively for H3R [52].
2.2.4. Endolymphatic Sac
In the murine endolymphatic sac, H1R, H2R, H3R, and H4R were detected on the epithelial cells [51]. H1R, H2R, and H3R were also found in the epithelial and subepithelial layers of the ducts and proximal endolymphatic sac of rabbits [41]. It has been shown that H3R is highly expressed in non-sensory epithelium [54], suggesting that it may play a role in maintaining cochlear fluid homeostasis. Histamine receptor expression was also found in the human endolymphatic sac. cDNA microarray data and immunohistochemical staining revealed there the presence of H1R and H3R proteins and transcripts [55]. Additionally, H1R was found in the endolymphatic sac lining, whereas H3R was present in the subepithelial capillary network [55].
2.3. Histamine Alters Vascular Permeability
One of the major physiological functions of histamine found in the inner ear is the ability to alter vascular permeability, primarily depending on H1R located on endothelial cells of the inner ear vascular lining [56][57]. A study found that histamine disrupts endothelial barrier formation in microvenules, as evidenced by changes in the localization of vascular endothelial cadherin (VE-cadherin) at endothelial cell junctions, and these manifestations can be eliminated by using H1R antagonists [57]. An increase in vascular permeability can produce beneficial physiological effects. For example, when tissue is injured or infected, an increase in vascular permeability allows white blood cells and antibodies to move out of the bloodstream and into the affected area, where they can help fight off the infection and promote healing [58]. On the other hand, an increase in vascular permeability can also help deliver nutrients and oxygen to damaged tissues, which is essential for repair and regeneration [59]. However, an excessive release of histamine can also lead to pathological effects. Koo et al. showed that vasoactive peptides, such as histamine, can enhance the ototoxicity of aminoglycosides by altering the permeability of the blood–labyrinth barrier in the mouse cochlea [56]. Like the blood–brain barrier, the blood–labyrinth barrier is composed of specialized cells and tight junctions that prevent the entry of large molecules, immune cells, and other potentially harmful substances into the inner ear from the bloodstream. These observations suggest an increased risk of ototoxicity during bacterial infections during aminoglycoside therapy, with adverse consequences for hearing function during recovery.
2.4. Electrophysiological Studies—The Role of Histamine in the Transmission of Electrical Signals of Sound
Only two studies have studied the electrophysiological function of histamine in the mammalian inner ear [53][60]. Previous results obtained using the vestibular organ of frogs and lateral line of Xenopus laevis have suggested that histamine may act as a hair cell transmitter [61][62][63]. These studies provided evidence that histamine increases the afferent firing rate in nerves and that this afferent firing could be blocked by H1R and H2R antagonists. Regarding guinea pigs, Minoda et al. reported that the infusion of histamine at low concentrations (10 and 50 μM) increased the compound action potential (CAP) amplitude without affecting the cochlear microphonic (CM), and the increase in CAP amplitude could be suppressed by H1R and H2R antagonists (50 μM) [60]. CAP represents the synchronous discharge of many cochlear afferent nerve fibers and is an indirect indicator of afferent nerve fiber activity. This result confirms previous findings in non-mammals. Therefore, histamine may act as an extracellular stimulatory signal that influences sound signaling via H1R and H2R in the cochlea.
2.5. Histamine May Affect Hair Cell Synaptic Transmission by Binding to Histamine 3 Receptor
H3R was originally described as an autoreceptor, inhibiting the release of histamine from histaminergic neurons in the brain [4]. H3R was shown to modulate inflammatory processes in the brain and the properties of neuronal synapses and has also been associated with the emergence of neurodevelopmental disorders [4]. Recent evidence suggests that the H3R is a pre- and postsynaptic receptor, regulating the release of several important neurotransmitters (such as acetylcholine, dopamine, GABA, norepinephrine, and serotonin) both in the peripheral and central nervous systems [4][22]. In the mammalian inner ear, the H3R has already been detected in many locations along the vestibulocochlear pathway, including the spiral ganglion and vestibular ganglion, the stria vascularis, and the endolymphatic sac [38][39][41][51]. However, it is worth mentioning that in the organ of Corti of the adult mouse, hair cells and supporting cells were also found to express H3R [51]. Glutamate is the main neurotransmitter at the hair cell afferent synapse [64]. Studies have found that histamine causes glutamate release from cultured astrocytes [4].
2.6. Clinical Application
The endolymphatic sac is a non-sensory segment of the inner ear and a part of the membranous labyrinth [65]. Its main function is to maintain the fragile endostasis of the endolymphatic and ectolymphatic vessels and to remove endolymphatic waste products [65][66]. One of the most common pathologies of the endolymphatic compartment (cochlear duct, also known as scala media) is endolymphatic hydrops. In this condition, characteristic for Menière’s disease, the excessive pressure in the cochlear duct ruptures physical barriers of the endolymphatic space causing a temporary loss of hearing and vestibular function [67][68]. The proposed causes of endolymphatic hydrops appear to be heterogeneous. Interestingly, experiments in the guinea pig have shown that histamine, released from the mast cells of the endolymphatic sac, induces a calcium response in the vestibular hair cells that is mediated by H1R, H2R, and H3R [41][69]. The histamine-mediated vasodilation of the endolymphatic sac could also lead to the deposition of immune complexes and endolymphatic effusion. These histamine-induced functional changes may be involved in the pathophysiology of Ménière’s disease.
Betahistine, a structural analog of histamine, is a weak H1R agonist and a strong H3R antagonist [70][71]. It is used to treat Ménière’s disease, particularly in central Europe [68]. Clinical trials found that repetitive daily doses of betahistine reduce the number and severity of attacks during the course of the disease [70][72]. Bertlich et al. studied the effects of betahistine on cochlear pericapillary cells and precapillary arteries and showed that the main mode of action was apparently the active dilation of the precapillary arteries [70]. Some researchers have suggested that the dilation may be due to the activation of H1R and H2R on the cochlear vasculature. The H1R and H2R expressed in the vascular smooth muscles contribute to vascular contraction and dilation [73]. A subsequent study examined changes in cochlear blood flow and blood pressure by separately blocking specific histamine receptors and found that the activation of H3R caused the decrease in cochlear blood flow and blood pressure, rather than H1R or H2R [70]. The infusion of the histaminergic H3R antagonist thioperamide prior to betahistine infusion completely reversed the effects of betahistine on cochlear blood flow [74].
Two main mechanisms of action of betahistine have been proposed. One is the counter-antagonistic effect of betahistine on H3R, which is thought to contribute to central neural compensation in the presence of peripheral vestibular imbalance [70]. The second involves an enhanced cochlear microcirculation in the stria vascularis [70][72][75]. However, betahistine may also cause clinical adverse effects, including flushing, headache, skin reactions, and hypotension, which are typical of H1R-related reactions and challenge the selectivity of the drug. The current findings provide a vision for future studies to optimize drug efficacy, for example, by targeting a specific symptom of Ménière’s disease by reducing drug side effects while maintaining efficacy.
References
- Parsons, M.E.; Ganellin, C.R. Histamine and its receptors. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S127–S135.
- Lieberman, P. The basics of histamine biology. Ann. Allergy Asthma Immunol. 2011, 106, S2–S5.
- Huang, H.; Li, Y.; Liang, J.; Finkelman, F.D. Molecular Regulation of Histamine Synthesis. Front. Immunol. 2018, 9, 1392.
- Haas, H.L.; Sergeeva, O.A.; Selbach, O. Histamine in the nervous system. Physiol. Rev. 2008, 88, 1183–1241.
- O’Mahony, L.; Akdis, M.; Akdis, C.A. Regulation of the immune response and inflammation by histamine and histamine receptors. J. Allergy Clin. Immunol. 2011, 128, 1153–1162.
- Tanaka, S.; Furuta, K. Roles of IgE and Histamine in Mast Cell Maturation. Cells 2021, 10, 2170.
- Uvnäs, B. The molecular basis for the storage and release of histamine in rat mast cell granules. Life Sci. 1974, 14, 2355–2366.
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873.
- Ferstl, R.; Akdis, C.A.; O’Mahony, L. Histamine regulation of innate and adaptive immunity. FBL 2012, 17, 40–53.
- Melvin, T.A.; Ramanathan, M., Jr. Role of innate immunity in the pathogenesis of allergic rhinitis. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 194–198.
- Panula, P. Histamine receptors, agonists, and antagonists in health and disease. Handb. Clin. Neurol. 2021, 180, 377–387.
- Cataldi, M.; Borriello, F.; Granata, F.; Annunziato, L.; Marone, G. Histamine receptors and antihistamines: From discovery to clinical applications. Chem. Immunol. Allergy 2014, 100, 214–226.
- Sadek, B.; Stark, H. Cherry-picked ligands at histamine receptor subtypes. Neuropharmacology 2016, 106, 56–73.
- Hargrove, L.; Graf-Eaton, A.; Kennedy, L.; Demieville, J.; Owens, J.; Hodges, K.; Ladd, B.; Francis, H. Isolation and characterization of hepatic mast cells from cholestatic rats. Lab. Investig. 2016, 96, 1198–1210.
- Hattori, Y.; Hattori, K.; Matsuda, N. Regulation of the Cardiovascular System by Histamine. Handb. Exp. Pharmacol. 2017, 241, 239–258.
- Wu, G.Y.; Zhuang, Q.X.; Zhang, X.Y.; Li, H.Z.; Wang, J.J.; Zhu, J.N. Facilitation of spinal α-motoneuron excitability by histamine and the underlying ionic mechanisms. Sheng Li Xue Bao 2019, 71, 809–823.
- Ramírez-Ponce, M.P.; Sola-García, A.; Balseiro-Gómez, S.; Maldonado, M.D.; Acosta, J.; Alés, E.; Flores, J.A. Mast Cell Changes the Phenotype of Microglia via Histamine and ATP. Cell. Physiol. Biochem. 2021, 55, 17–32.
- MacGlashan, D., Jr. Histamine: A mediator of inflammation. J. Allergy Clin. Immunol. 2003, 112, S53–S59.
- Orsini, J.A.; Spencer, P.A. Effects of a histamine type 2 receptor antagonist, BMY-26539-01, on equine gastric acid secretion. Can. J. Vet. Res. 2001, 65, 55–59.
- Wendell, S.G.; Fan, H.; Zhang, C. G Protein-Coupled Receptors in Asthma Therapy: Pharmacology and Drug Action. Pharmacol. Rev. 2020, 72, 1–49.
- Neumann, J.; Kirchhefer, U.; Dhein, S.; Hofmann, B.; Gergs, U. The Roles of Cardiovascular H(2)-Histamine Receptors under Normal and Pathophysiological Conditions. Front. Pharmacol. 2021, 12, 732842.
- Schlicker, E.; Kathmann, M. Role of the Histamine H(3) Receptor in the Central Nervous System. Handb. Exp. Pharmacol. 2017, 241, 277–299.
- Passani, M.B.; Giannoni, P.; Bucherelli, C.; Baldi, E.; Blandina, P. Histamine in the brain: Beyond sleep and memory. Biochem. Pharmacol. 2007, 73, 1113–1122.
- Nakamura, T.; Itadani, H.; Hidaka, Y.; Ohta, M.; Tanaka, K. Molecular cloning and characterization of a new human histamine receptor, HH4R. Biochem. Biophys. Res. Commun. 2000, 279, 615–620.
- Liu, C.; Ma, X.; Jiang, X.; Wilson, S.J.; Hofstra, C.L.; Blevitt, J.; Pyati, J.; Li, X.; Chai, W.; Carruthers, N.; et al. Cloning and pharmacological characterization of a fourth histamine receptor (H(4)) expressed in bone marrow. Mol. Pharmacol. 2001, 59, 420–426.
- Nguyen, T.; Shapiro, D.A.; George, S.R.; Setola, V.; Lee, D.K.; Cheng, R.; Rauser, L.; Lee, S.P.; Lynch, K.R.; Roth, B.L.; et al. Discovery of a novel member of the histamine receptor family. Mol. Pharmacol. 2001, 59, 427–433.
- Thurmond, R.L. The histamine H4 receptor: From orphan to the clinic. Front. Pharmacol. 2015, 6, 65.
- Connelly, W.M.; Shenton, F.C.; Lethbridge, N.; Leurs, R.; Waldvogel, H.J.; Faull, R.L.; Lees, G.; Chazot, P.L. The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. Br. J. Pharmacol. 2009, 157, 55–63.
- Ekdale, E.G. Form and function of the mammalian inner ear. J. Anat. 2016, 228, 324–337.
- Nayagam, B.A.; Muniak, M.A.; Ryugo, D.K. The spiral ganglion: Connecting the peripheral and central auditory systems. Hear. Res. 2011, 278, 2–20.
- Goutman, J.D.; Elgoyhen, A.B.; Gómez-Casati, M.E. Cochlear hair cells: The sound-sensing machines. FEBS Lett. 2015, 589, 3354–3361.
- Day, B.L.; Fitzpatrick, R.C. The vestibular system. Curr. Biol. 2005, 15, R583–R586.
- Khan, S.; Chang, R. Anatomy of the vestibular system: A review. NeuroRehabilitation 2013, 32, 437–443.
- Keithley, E.M. Inner ear immunity. Hear. Res. 2022, 419, 108518.
- Ishibashi, Y.; Sung, C.Y.W.; Grati, M.; Chien, W. Immune responses in the mammalian inner ear and their implications for AAV-mediated inner ear gene therapy. Hear. Res. 2023, 432, 108735.
- Miwa, T.; Okano, T. Role of Inner Ear Macrophages and Autoimmune/Autoinflammatory Mechanisms in the Pathophysiology of Inner Ear Disease. Front. Neurol. 2022, 13, 861992.
- Shi, X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear. Res. 2016, 338, 52–63.
- Azuma, H.; Sawada, S.; Takeuchi, S.; Higashiyama, K.; Kakigi, A.; Takeda, T. Expression of mRNA encoding the H1, H2, and H3 histamine receptors in the rat cochlea. Neuroreport 2003, 14, 423–425.
- Azuma, H.; Sawada, S.; Takeuchi, S.; Higashiyama, K.; Kakigi, A.; Takeda, T. Immunohistochemical localization of histamine receptors in rat cochlea. Laryngoscope 2004, 114, 2249–2251.
- Botta, L.; Tritto, S.; Perin, P.; Laforenza, U.; Gastaldi, G.; Zampini, V.; Zucca, G.; Valli, S.; Masetto, S.; Valli, P. Histamine H1 receptors are expressed in mouse and frog semicircular canal sensory epithelia. Neuroreport 2008, 19, 425–429.
- Dagli, M.; Goksu, N.; Eryilmaz, A.; Mocan Kuzey, G.; Bayazit, Y.; Gun, B.D.; Gocer, C. Expression of histamine receptors (H(1), H(2), and H(3)) in the rabbit endolymphatic sac: An immunohistochemical study. Am. J. Otolaryngol. 2008, 29, 20–23.
- Szczepek, A.J.; Dudnik, T.; Karayay, B.; Sergeeva, V.; Olze, H.; Smorodchenko, A. Mast Cells in the Auditory Periphery of Rodents. Brain Sci. 2020, 10, 697.
- Scholar, E. Histamine. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2009; pp. 1–6.
- Church, M.K.; Kolkhir, P.; Metz, M.; Maurer, M. The role and relevance of mast cells in urticaria. Immunol. Rev. 2018, 282, 232–247.
- Kolkhir, P.; Elieh-Ali-Komi, D.; Metz, M.; Siebenhaar, F.; Maurer, M. Understanding human mast cells: Lesson from therapies for allergic and non-allergic diseases. Nat. Rev. Immunol. 2022, 22, 294–308.
- Zhang, N.; Lyu, Y.; Guo, J.; Liu, J.; Song, Y.; Fan, Z.; Li, X.; Li, N.; Zhang, D.; Wang, H. Bidirectional Transport of IgE by CD23 in the Inner Ear of Patients with Meniere’s Disease. J. Immunol. 2022, 208, 827–838.
- Felix, H.; Oestreicher, E.; Felix, D.; Ehrenberger, K. Role of substance P in the peripheral vestibular and auditory system. Adv. Otorhinolaryngol. 2002, 59, 26–34.
- Ina, A.; Altintaş, D.U.; Yilmaz, M.; Uğuz, A.; Tuncer, U.; Kiroğlu, M.; Hergüner, O.; Bicakci, K. Congenital mastocytosis associated with neurosensory deafness. Pediatr. Dermatol. 2007, 24, 460–462.
- Trevisan, G.; Pauluzzi, P.; Gatti, A.; Semeraro, A. Familial mastocytosis associated with neurosensory deafness. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 119–122.
- Miyamura, K.; Kanzaki, Y.; Nagata, M.; Ishikawa, T. Provocation of nystagmus and deviation by type I allergy in the inner ear of the guinea pig. Ann. Allergy 1987, 58, 36–40.
- Takumida, M.; Takumida, H.; Anniko, M. Localization of histamine (H1, H2, H3 and H4) receptors in mouse inner ear. Acta Otolaryngol. 2016, 136, 537–544.
- Tritto, S.; Botta, L.; Zampini, V.; Zucca, G.; Valli, P.; Masetto, S. Calyx and dimorphic neurons of mouse Scarpa’s ganglion express histamine H3 receptors. BMC Neurosci. 2009, 10, 70.
- Desmadryl, G.; Gaboyard-Niay, S.; Brugeaud, A.; Travo, C.; Broussy, A.; Saleur, A.; Dyhrfjeld-Johnsen, J.; Wersinger, E.; Chabbert, C. Histamine H4 receptor antagonists as potent modulators of mammalian vestibular primary neuron excitability. Br. J. Pharmacol. 2012, 167, 905–916.
- Eberhard, K.E.; Kirkeby, S.; Hansen, L.J.; Cayé-Thomasen, P. Neurotransmitter and neurotransmitter receptor expression in the saccule of the human vestibular system. Prog. Neurobiol. 2022, 212, 102238.
- Møller, M.N.; Kirkeby, S.; Vikeså, J.; Nielsen, F.C.; Caye-Thomasen, P. Expression of histamine receptors in the human endolymphatic sac: The molecular rationale for betahistine use in Menieres disease. Eur. Arch. Otorhinolaryngol. 2016, 273, 1705–1710.
- Koo, J.W.; Wang, Q.; Steyger, P.S. Infection-mediated vasoactive peptides modulate cochlear uptake of fluorescent gentamicin. Audiol. Neurootol. 2011, 16, 347–358.
- Ashina, K.; Tsubosaka, Y.; Nakamura, T.; Omori, K.; Kobayashi, K.; Hori, M.; Ozaki, H.; Murata, T. Histamine Induces Vascular Hyperpermeability by Increasing Blood Flow and Endothelial Barrier Disruption In Vivo. PLoS ONE 2015, 10, e0132367.
- Park-Windhol, C.; D’Amore, P.A. Disorders of Vascular Permeability. Annu. Rev. Pathol. 2016, 11, 251–281.
- Dvorak, H.F. Vascular permeability to plasma, plasma proteins, and cells: An update. Curr. Opin. Hematol. 2010, 17, 225–229.
- Minoda, R.; Toriya, T.; Masuyama, K.; Yumoto, E. The effects of histamine and its antagonists on the cochlear microphonic and the compound action potential of the guinea pig. Auris Nasus Larynx 2001, 28, 219–222.
- Housley, G.D.; Norris, C.H.; Guth, P.S. Histamine and related substances influence neurotransmission in the semicircular canal. Hear. Res. 1988, 35, 87–97.
- Bledsoe, S.C., Jr.; Sinard, R.J.; Allen, S.J. Analysis of histamine as a hair-cell transmitter in the lateral line of Xenopus laevis. Hear. Res. 1989, 38, 81–93.
- Botta, L.; Mira, E.; Valli, S.; Perin, P.; Zucca, G.; Valli, P. Effects of betahistine on vestibular receptors of the frog. Acta Otolaryngol. 1998, 118, 519–523.
- Glowatzki, E.; Grant, L.; Fuchs, P. Hair cell afferent synapses. Curr. Opin. Neurobiol. 2008, 18, 389–395.
- Kim, S.H.; Nam, G.S.; Choi, J.Y. Pathophysiologic Findings in the Human Endolymphatic Sac in Endolymphatic Hydrops: Functional and Molecular Evidence. Ann. Otol. Rhinol. Laryngol. 2019, 128, 76s–83s.
- Lo, W.W.; Daniels, D.L.; Chakeres, D.W.; Linthicum, F.H., Jr.; Ulmer, J.L.; Mark, L.P.; Swartz, J.D. The endolymphatic duct and sac. AJNR Am. J. Neuroradiol. 1997, 18, 881–887.
- Bertlich, M.; Ihler, F.; Weiss, B.G.; Freytag, S.; Strupp, M.; Jakob, M.; Canis, M. Role of capillary pericytes and precapillary arterioles in the vascular mechanism of betahistine in a guinea pig inner ear model. Life Sci. 2017, 187, 17–21.
- Nakashima, T.; Pyykkö, I.; Arroll, M.A.; Casselbrant, M.L.; Foster, C.A.; Manzoor, N.F.; Megerian, C.A.; Naganawa, S.; Young, Y.H. Meniere’s disease. Nat. Rev. Dis. Primers 2016, 2, 16028.
- Tomoda, K.; Nagata, M.; Harada, N.; Iwai, H.; Yamashita, T. Effect of histamine on intracellular Ca2+ concentration in guinea pig isolated vestibular hair cells. Acta Otolaryngol. Suppl. 1997, 528, 37–40.
- Bertlich, M.; Ihler, F.; Freytag, S.; Weiss, B.G.; Strupp, M.; Canis, M. Histaminergic H3-Heteroreceptors as a Potential Mediator of Betahistine-Induced Increase in Cochlear Blood Flow. Audiol. Neurootol. 2015, 20, 283–293.
- Holmes, S.; Lalwani, A.K.; Mankekar, G. Is Betahistine Effective in the Treatment of Ménière’s Disease? Laryngoscope 2021, 131, 2639–2640.
- Ihler, F.; Bertlich, M.; Sharaf, K.; Strieth, S.; Strupp, M.; Canis, M. Betahistine exerts a dose-dependent effect on cochlear stria vascularis blood flow in guinea pigs in vivo. PLoS ONE 2012, 7, e39086.
- Miller, D.J.; O’Dowd, A. Vascular smooth muscle actions of carnosine as its zinc complex are mediated by histamine H(1) and H(2) receptors. Biochemistry 2000, 65, 798–806.
- Laurikainen, E.; Miller, J.F.; Pyykkö, I. Betahistine effects on cochlear blood flow: From the laboratory to the clinic. Acta Otolaryngol. Suppl. 2000, 544, 5–7.
- Bertlich, M.; Ihler, F.; Sharaf, K.; Weiss, B.G.; Strupp, M.; Canis, M. Betahistine metabolites, aminoethylpyridine, and hydroxyethylpyridine increase cochlear blood flow in guinea pigs in vivo. Int. J. Audiol. 2014, 53, 753–759.
More
Information
Subjects:
Otorhinolaryngology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
25 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




