Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Agnieszka J. Szczepek and Version 2 by Lindsay Dong.
Histamine is a widely distributed biogenic amine with multiple biological functions mediated by specific receptors that determine the local effects of histamine. All four types of histamine receptors were identified in the mammalian inner ear. The functional studies of histamine in the inner ear were mainly in vitro. Clinical evidence suggests that histamine and its receptors may play a role in Ménière’s disease, but the exact mechanism is not fully understood.
- histamine
- histamine receptors
- inner ear
- hearing
1. Introduction
Histamine is a bioactive amine acting as an effector molecule of the immune system and a neurotransmitter in the nervous system. Histamine is synthesized from histidine by specific decarboxylase [1][2][1,2]. The histidine decarboxylase-expressing cells include mast cells, basophils, histaminergic neurons, and enterochromaffin-like cells in the stomach [3]. Histamine can either be secreted immediately or stored in granules for later use, as is the case with mast cells and basophils [4][5][6][4,5,6], which release histamine upon stimulation [7][8][7,8]. The effects of histamine range from the involvement in innate immunity [9] and its pathologies, such as allergic rhinitis [10], to housekeeping brain and bone homeostasis [4] and depend on the target cell type and the kind of histamine receptor expressed [11].
To date, four histamine receptors have been identified: the H1 receptor (H1R), H2 receptor (H2R), H3 receptor (H3R), and H4 receptor (H4R) [12][13][12,13]. All four belong to the G-protein-coupled receptor family. H1R is expressed in many tissues and cells, including cerebral neurons, the respiratory epithelium, the adrenal medulla, and hepatic, cardiovascular, and endothelial cells [1][14][15][1,14,15]. It is involved in allergic and inflammatory responses. When stimulated, it activates phospholipase C and increases intracellular Ca2+ levels [16][17][16,17]. As a result, the smooth muscles of the respiratory tract contract, and vascular permeability increases, subsequently causing a range of symptoms associated with allergic reactions [18]. H2R is also widely distributed and highly expressed in gastric parietal cells, vascular smooth muscles, the central nervous system, and the heart [15][19][20][21][15,19,20,21]. H3R is mainly found in the central nervous system but it is also widely distributed in peripheral tissues [22]. The effects of H3R activation are diverse and include the regulation of histamine turnover, sleep–awake regulation, learning, memory, and inflammation, as well as the inhibition of the release of several other neurotransmitters, such as serotonin, GABA, and glutamate [4][22][23][4,22,23]. The H4R was first described at the beginning of the 21st century [24][25][26][24,25,26]. The gene encoding H4R was discovered via genomic homology searches and reverse pharmacology, identifying its role in immune and pruritic responses [27]. This receptor has a relatively high homology with H3R; however, its role has yet to be elucidated [27][28][27,28].
The mammalian inner ear is a complex sensory organ consisting of the cochlea, vestibule, and three semicircular canals [29]. Sound passes from the external ear canal through the middle ear to the inner ear. In the inner ear’s cochlea, sound’s mechanical energy is converted into a biochemical signal by the sensory epithelium (hair cells) in a process called mechanotransduction. Mechanotransduction induces the release of glutamate from the inner hair cells, which activates the spiral ganglion neurons by initiating an action potential that is sequentially transmitted along the auditory pathway to the auditory cortex [30][31][30,31].
The vestibular system is responsible for sensing and processing information about the position and movement of the head and body in space and maintaining balance and coordination during the movement [32]. The peripheral vestibular organs are located bilaterally in the inner ear. They consist of two otolithic organs (utricle and saccule) and three semicircular canals (anterior, posterior, and horizontal), the former sensing linear acceleration, such as head movement or gravity, and the latter sensing rotational acceleration [33]. The sensory nerve epithelium in the utricle and saccule is the macula, and in the semicircular canals, it is the crista ampullaris [32]. Both structures contain vestibular hair cells, which release glutamate upon depolarization, stimulating the vestibular ganglion’s afferent nerves. The vestibular ganglion and the cochlear spiral ganglion neurons form the eighth cranial nerve.
The endolymphatic sac is the non-sensory part of the membranous labyrinth of the inner ear. It plays several important roles, including regulating the volume and pressure of the potassium-rich endolymph fluid, participating in the immune response within the inner ear, and removing waste products from the endolymph.
In recent years, the immune function, inflammatory processes, and vascular control of the inner ear have been investigated and reviewed [34][35][36][37][34,35,36,37]. However, the specific topic of histamine and its signaling in the cochlea or vestibular organs remains scarcely addressed in the literature. A few research teams have identified the expression of histamine receptors in the inner ear of mammals [38][39][40][41][38,39,40,41]. Additionally, in 2020, our group found mast cells in the inner ear of both rats and mice [42]. Mast cells are the major source of histamine in the body, along with basophils, gastric parietal cells, and the central nervous system [43]. Upon activation, mast cells degranulate and release a number of immuno- and neuromodulatory compounds, including histamine [44][45][44,45]. Some conditions necessary for mast cell activation have already been described regarding the inner ear, including IgE antibody transcytosis across the blood–labyrinth barrier [46] and the presence of substance P [47]. However, more research is needed to understand the relationship between the presence of mast cells in the inner ear, their mode of activation, potential histamine release, and its consequences in health and disease, such as mastocytosis or IgE-mediated diseases. Clinical evidence suggests an association between elevated numbers of mast cells and inner ear disorders [48][49][48,49]. Moreover, experiments demonstrated that Meniere’s disease-like symptoms (attacks of nystagmus and hearing loss) can be induced by the experimental induction of a type I allergy in the endolymphatic sac of guinea pigs [50].
2. Histamine and Its Receptors in Mammalian Inner Ear
2.1. Expression of Histamine Receptors in the Mammalian Cochlea
Results from several studies have shown that histamine receptors were detected at multiple sites in the mammalian inner ear (Figure 13) [38][39][51][38,39,56]. The mRNA encoding histamine receptors (H1, H2, and H3R) was found to be present in the lateral portion of the cochlea, including the spiral ligament and the stria vascularis, the medial portion, including the organ of Corti, and the modiolus [38]. Takumida et al. used immunohistochemistry (IHC) to examine the mouse cochlea and found that the stria vascularis was positive for H1R, the spiral ligament for H3R and H4R, and the spiral ganglion neurons for all four types of receptors. In the latter, immunofluorescence (IF) receptor signals were observed in the cytoplasm of the spiral ganglion neurons [51][56]. In the organ of Corti, H3R was observed in both the outer and inner hair cells, whereas H1R, H2R, H3R, and H4R were observed in some supporting cells [51][56].
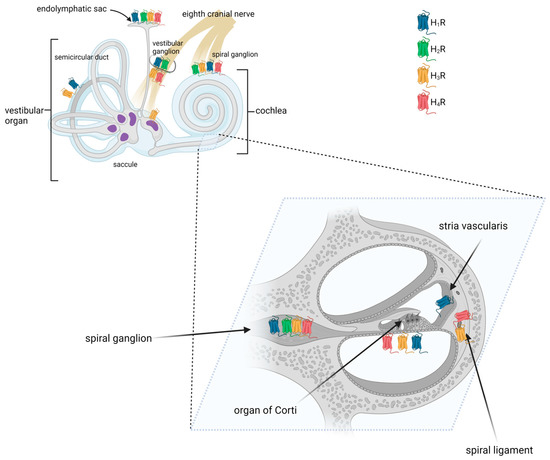
Figure 13.
Distribution of histamine receptors in the mammalian inner ear. Created with
.
2.2. Expression of Histamine Receptors in the Mammalian Vestibular System
2.1. Expression of Histamine Receptors in the Mammalian Vestibular System
2.21.1. Semicircular Canals
Using reverse RT-PCR, Western blotting, and in situ immunolabeling, Botta et al. demonstrated that the semicircular canal of the mouse expresses H1R [40]. Conversely, no clear evidence for H3R expression was found.
2.21.2. Utricle and Saccule
All four types of histamine receptors have been demonstrated in the maculae of the saccule and utricle [51][52][53,56]. More specifically, the expression of histamine receptors was found in type I hair cells, the calyx and dimorphic vestibular afferents, and subepithelial cells [52][53].
2.21.3. Vestibular Ganglion
Like in spiral ganglion neurons, H1R, H2R, H3R, and H4R were detected on vestibular ganglion cells using immunohistochemistry [51][52][53][53,54,56]. Tritto et al. examined vestibular neurons with immunofluorescence and found that approximately 30% of the nerves stained positively for H3R [52][53].
2.21.4. Endolymphatic Sac
In the murine endolymphatic sac, H1R, H2R, H3R, and H4R were detected on the epithelial cells [51][56]. H1R, H2R, and H3R were also found in the epithelial and subepithelial layers of the ducts and proximal endolymphatic sac of rabbits [41]. It has been shown that H3R is highly expressed in non-sensory epithelium [54][57], suggesting that it may play a role in maintaining cochlear fluid homeostasis. Histamine receptor expression was also found in the human endolymphatic sac. cDNA microarray data and immunohistochemical staining revealed there the presence of H1R and H3R proteins and transcripts [55]. Additionally, H1R was found in the endolymphatic sac lining, whereas H3R was present in the subepithelial capillary network [55].
