
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yuchen Bai | -- | 4620 | 2023-08-24 04:05:14 | | | |
| 2 | Peter Tang | Meta information modification | 4620 | 2023-08-24 04:54:38 | | |
Video Upload Options
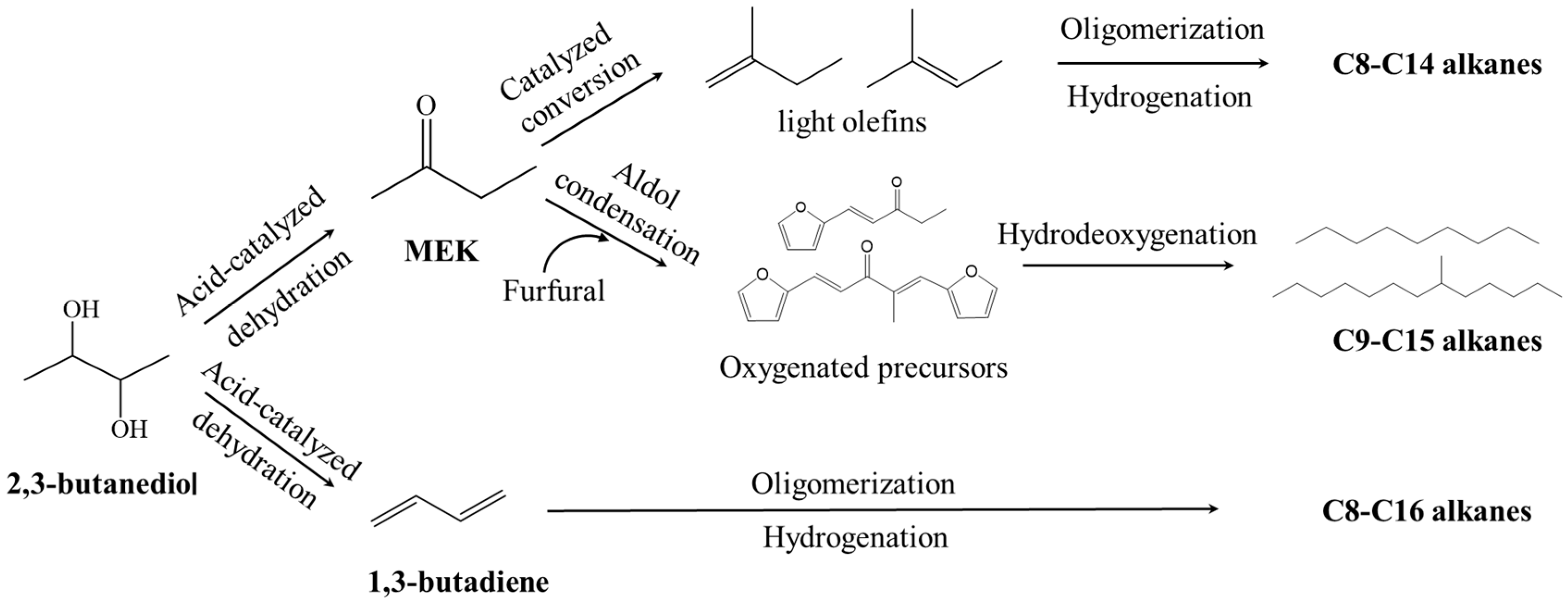
2,3-butanediol (2,3-BDO) is an important biomass-derived platform chemical with various applications. The biological conversion of renewable carbon sources with bacteria or yeasts is a sustainable way to produce 2,3-BDO. Various carbon sources including glucose, glycerol, molasses and lignocellulose hydrolysate have been used for 2,3-BDO production, and the 2,3-BDO concentration in the fermentation broth can be higher than 150 g/L by optimizing the operating parameters with fed-batch operations. Various derivatives can be produced from 2,3-BDO, including isobutyraldehyde, 1,3-butadiene, methyl ethyl ketone (MEK), diacetyl, etc.; among these, there is a large market demand for MEK and 1,3-butadiene each year. Some of the derivatives can be used as fuel additives or to produce biofuels. Generally, there are three ways to produce hydrocarbon fuels from 2,3-BDO, which are via the steps of dehydration, carbon chain extension, and hydrogenation (or hydrodeoxygenation), with MEK or 1,3-butadiene as the intermediates. C8–C16 alkanes can be produced by these routes, which can be potentially used as bio-jet fuels.
1. Introduction
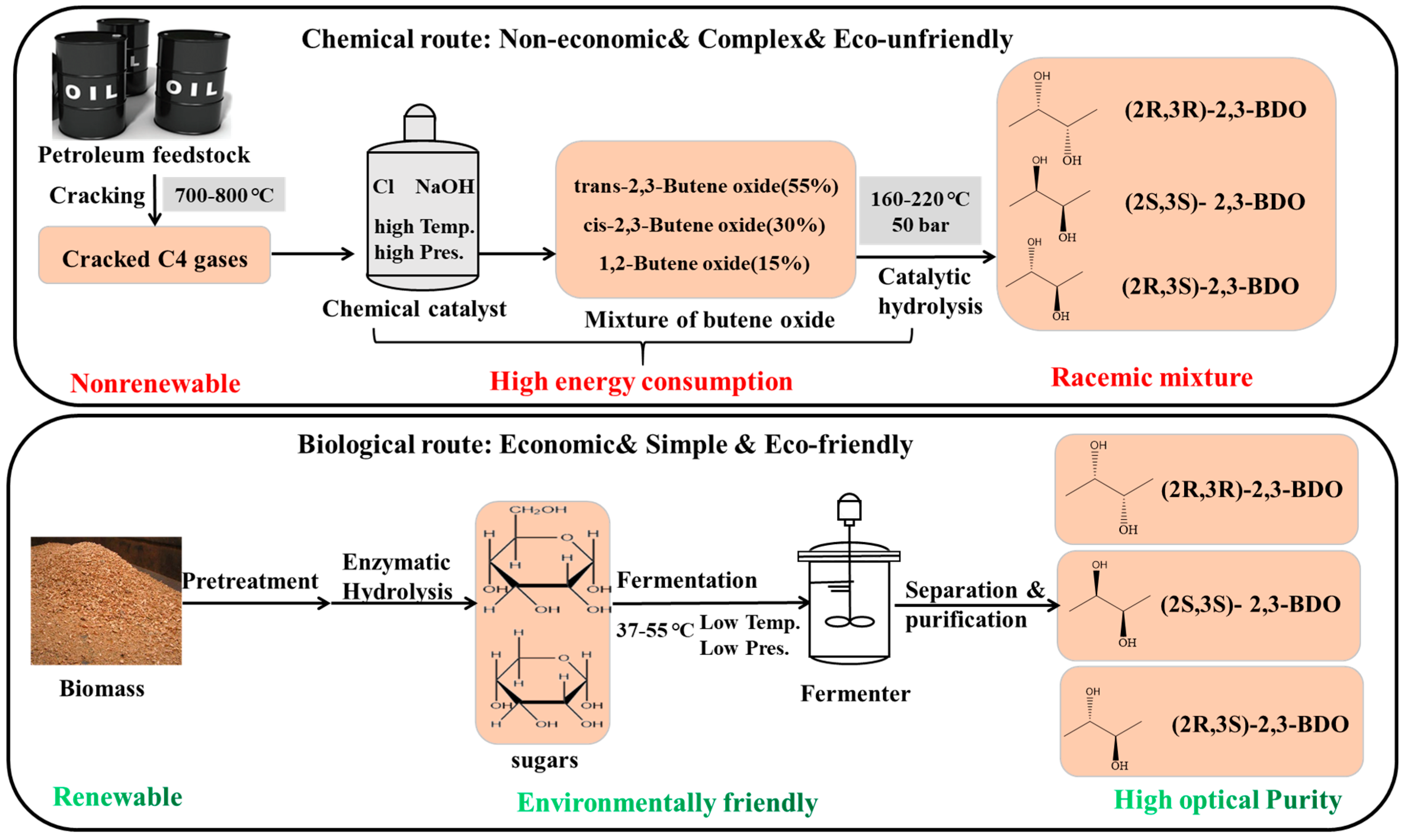
2. Metabolic Pathways for Microbial Production of 2,3-BDO
2.1. Biological Function of 2,3-BDO
2.2. Metabolic Pathway of 2,3-BDO Biosynthesis
2.3. Mechanisms of Biosynthesis of the 2,3-BDO Stereoisomer
2.4. Microorganisms to Produce 2,3-BDO
3. Feedstocks for 2,3-BDO Production
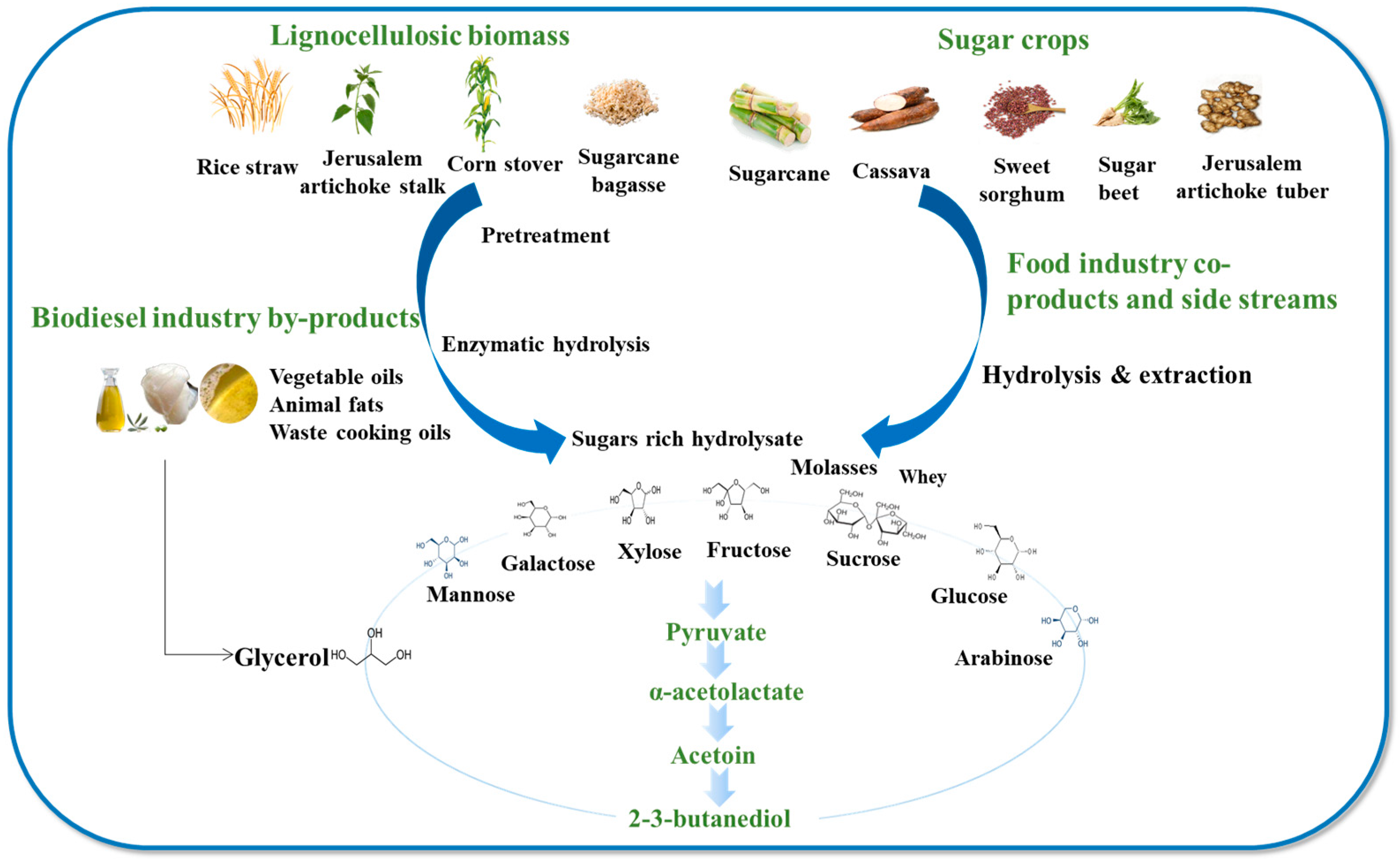
4. 2,3-Butanediol Recovery and Purification Strategies
5. Derivatives of 2,3-Butanediol and Their Applications
5.1. Applications of 2,3-Butanediol
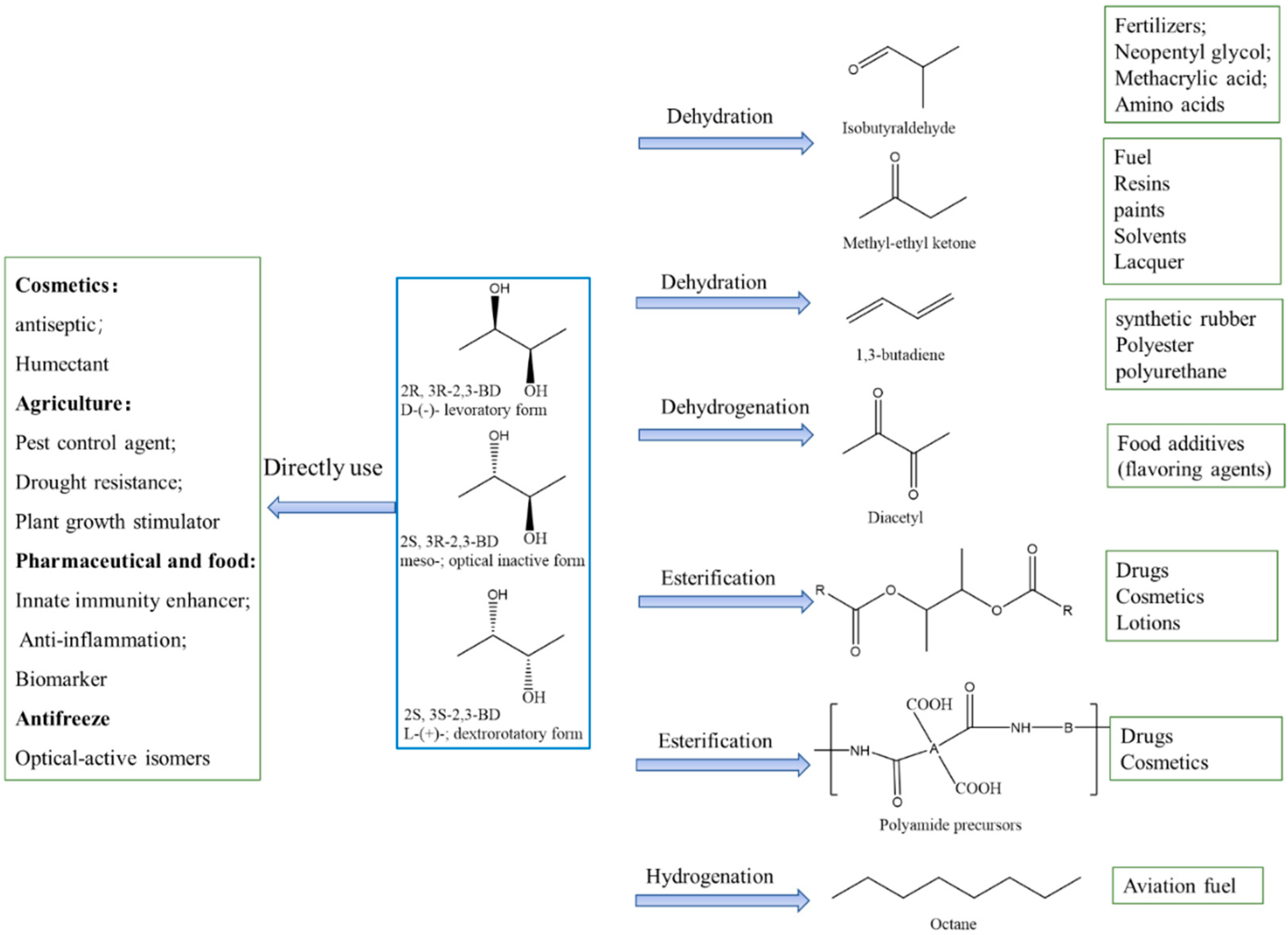
5.2. Applications in Biofuel Production and Fuel Additives
References
- Sohn, Y.J.; Son, J.; Jo, S.Y.; Park, S.Y.; Yoo, J.I.; Baritugo, K.A.; Na, J.G.; Choi, J.I.; Kim, H.T.; Joo, J.C. Chemoautotroph Cupriavidus necator as a potential game-changer for global warming and plastic waste problem: A review. Bioresour. Technol. 2021, 340, 125693.
- Sohn, Y.J.; Son, J.; Lim, H.J.; Lim, S.H.; Park, S.J. Valorization of lignocellulosic biomass for polyhydroxyalkanoate production: Status and perspectives. Bioresour. Technol. 2022, 360, 127575.
- Son, J.; Joo, J.C.; Baritugo, K.A.; Jeong, S.; Lee, J.Y.; Lim, H.J.; Lim, S.H.; Yoo, J.I.; Park, S.J. Consolidated microbial production of four-, five-, and six-carbon organic acids from crop residues: Current status and perspectives. Bioresour. Technol. 2022, 351, 127001.
- Lee, H.; Jung Sohn, Y.; Jeon, S.; Yang, H.; Son, J.; Jin Kim, Y.; Jae Park, S. Sugarcane wastes as microbial feedstocks: A review of the biorefinery framework from resource recovery to production of value-added products. Bioresour. Technol. 2023, 376, 128879.
- Syu, M.J. Biological production of 2,3-butanediol. Appl. Microbiol. Biotechnol. 2001, 55, 10–18.
- Kim, S.J.; Kim, J.W.; Lee, Y.G.; Park, Y.C.; Seo, J.H. Metabolic engineering of Saccharomyces cerevisiae for 2,3-butanediol production. Appl. Microbiol. Biotechnol. 2017, 101, 2241–2250.
- Harden, A.; Walpole, G.S. Chemical action of Bacillus lactis aerogenes (Escherich) on glucose and mannitol: Production of 2 3-butyleneglycol and acetylmethylcarbinol. Proc. R. Soc. London. Ser. B Contain. Pap. A Biol. Character 1997, 77, 399–405.
- Sabra, W.; Groeger, C.; Zeng, A.P. Microbial Cell Factories for Diol Production. In Bioreactor Engineering Research and Industrial Applications i: Cell Factories; Ye, Q., Bao, J., Zhong, J.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 155, pp. 165–197.
- Rehman, S.; Islam, M.K.; Khanzada, N.K.; Zhuang, H.; Wang, H.; Chaiprapat, S.; Leu, S.-Y. Sustainability index accounting food and carbon benefits on circular 2,3-butanediol biorefinery with oil palm empty fruit bunches. Appl. Energy 2021, 303, 117667.
- Shi, L.; Gao, S.; Yu, Y.; Yang, H. Microbial production of 2,3-butanediol by a newly-isolated strain of Serratia marcescens. Biotechnol. Lett. 2014, 36, 969–973.
- Bialkowska, A.M. Strategies for efficient and economical 2,3-butanediol production: New trends in this field. World J. Microbiol. Biotechnol. 2016, 32, 200.
- Van Houdt, R.; Aertsen, A.; Michiels, C.W. Quorum-sensing-dependent switch to butanediol fermentation prevents lethal medium acidification in Aeromonas hydrophila AH-1N. Res. Microbiol. 2007, 158, 379–385.
- Ji, X.J.; Huang, H.; Ouyang, P.K. Microbial 2,3-butanediol production: A state-of-the-art review. Biotechnol. Adv. 2011, 29, 351–364.
- Ji, X.J.; Huang, H.; Li, S.; Du, J.; Lian, M. Enhanced 2,3-butanediol production by altering the mixed acid fermentation pathway in Klebsiella oxytoca. Biotechnol. Lett. 2008, 30, 731–734.
- Blomqvist, K.; Nikkola, M.; Lehtovaara, P.; Suihko, M.L.; Airaksinen, U.; Straby, K.B.; Knowles, J.K.; Penttila, M.E. Characterization of the genes of the 2,3-butanediol operons from Klebsiella terrigena and Enterobacter aerogenes. J. Bacteriol. 1993, 175, 1392–1404.
- Nakashimada, Y.; Marwoto, B.; Kashiwamura, T.; Kakizono, T.; Nishio, N. Enhanced 2,3-butanediol production by addition of acetic acid in Paenibacillus polymyxa. J. Biosci. Bioeng. 2000, 90, 661–664.
- Bryn, K.; Ulstrup, J.C.; Størmer, F.C. Effect of Acetate upon the Formation of Acetoin in Klebsiella and Enterobacter and Its Possible Practical Application in a Rapid Voges-Proskauer Test. Appl. Microbiol. 1973, 25, 511–512.
- Celinska, E.; Grajek, W. Biotechnological production of 2,3-butanediol-current state and prospects. Biotechnol. Adv. 2009, 27, 715–725.
- Xiao, Z.; Xu, P. Acetoin metabolism in bacteria. Crit. Rev. Microbiol. 2007, 33, 127–140.
- Nigam, P.S.; Singh, A. Production of liquid biofuels from renewable resources. Prog. Energy Combust. 2011, 37, 52–68.
- Yang, T.W.; Rao, Z.M.; Zhang, X.; Xu, M.J.; Xu, Z.H.; Yang, S.T. Enhanced 2,3-butanediol production from biodiesel-derived glycerol by engineering of cofactor regeneration and manipulating carbon flux in Bacillus amyloliquefaciens. Microb. Cell Fact. 2015, 14, 122.
- Stormer, F.C. Evidence for induction of the 2,3-butanediol-forming enzymes in Aerobacter aerogenes. FEBS. Lett. 1968, 2, 36–38.
- Taylor, M.B.; Juni, E. Stereoisomeric specificities of 2,3-butanediol dehydrogenases. Biochim. Biophys. Acta 1960, 39, 448–457.
- Voloch, M.; Ladisch, M.R.; Rodwell, V.W.; Tsao, G.T. Reduction of acetoin to 2,3-butanediol in Klebsiella pneumoniae: A new model. Biotechnol. Bioeng. 1983, 25, 173–183.
- Ui, S.; Masuda, H.; Muraki, H. Separation and quantitation of acetoin isomers (D(-) and L(+)) by a combined use of enzyme and gas-chromatography. Agric. Biol. Chem. 1984, 48, 2835–2836.
- Ui, S.; Masuda, T.; Masuda, H.; Muraki, H. Mechanism for the formation of 2,3-butanediol stereoisomers in Bacillus polymyxa. J. Ferment. Bioeng. 1986, 64, 481–486.
- Ui, S.; Matsuyama, N.; Masuda, H.; Muraki, H. Mechanism for the formation of 2,3-butanediol stereoisomers in klebsiella-pneumoniae. J. Ferment. Bioeng. 1984, 62, 551–559.
- Rathnasingh, C.; Park, J.M.; Kim, D.K.; Song, H.; Chang, Y.K. Metabolic engineering of Klebsiella pneumoniae and in silico investigation for enhanced 2,3-butanediol production. Biotechnol. Lett. 2016, 38, 975–982.
- Ji, X.J.; Huang, H.; Zhu, J.G.; Ren, L.J.; Nie, Z.K.; Du, J.; Li, S. Engineering Klebsiella oxytoca for efficient 2, 3-butanediol production through insertional inactivation of acetaldehyde dehydrogenase gene. Appl. Microbiol. Biotechnol. 2010, 85, 1751–1758.
- Cho, S.; Kim, T.; Woo, H.M.; Lee, J.; Kim, Y.; Um, Y. Enhanced 2,3-Butanediol Production by Optimizing Fermentation Conditions and Engineering Klebsiella oxytoca M1 through Overexpression of Acetoin Reductase. PLoS ONE 2015, 10, e0138109.
- Jantama, K.; Polyiam, P.; Khunnonkwao, P.; Chan, S.; Sangproo, M.; Khor, K.; Jantama, S.S.; Kanchanatawee, S. Efficient reduction of the formation of by-products and improvement of production yield of 2,3-butanediol by a combined deletion of alcohol dehydrogenase, acetate kinase-phosphotransacetylase, and lactate dehydrogenase genes in metabolically engineered Klebsiella oxytoca in mineral salts medium. Metab. Eng. 2015, 30, 16–26.
- Kim, J.W.; Kim, J.; Seo, S.O.; Kim, K.H.; Jin, Y.S.; Seo, J.H. Enhanced production of 2,3-butanediol by engineered Saccharomyces cerevisiae through fine-tuning of pyruvate decarboxylase and NADH oxidase activities. Biotechnol. Biofuels 2016, 9, 265.
- Yang, T.W.; Rao, Z.M.; Zhang, X.; Xu, M.J.; Xu, Z.H.; Yang, S.T. Improved Production of 2,3-Butanediol in Bacillus amyloliquefaciens by Over-Expression of Glyceraldehyde-3-Phosphate Dehydrogenase and 2,3-butanediol Dehydrogenase. PLoS ONE 2013, 8, e76149.
- Bai, F.; Dai, L.; Fan, J.; Truong, N.; Rao, B.; Zhang, L.; Shen, Y. Engineered Serratia marcescens for efficient (3R)-acetoin and (2R,3R)-2,3-butanediol production. J. Ind. Microbiol. Biotechnol. 2015, 42, 779–786.
- Ge, Y.; Li, K.; Li, L.; Gao, C.; Zhang, L.; Ma, C.; Xu, P. Contracted but effective: Production of enantiopure 2,3-butanediol by thermophilic and GRAS Bacillus licheniformis. Green Chem. 2016, 18, 4693–4703.
- Li, L.; Li, K.; Wang, Y.; Chen, C.; Xu, Y.; Zhang, L.; Han, B.; Gao, C.; Tao, F.; Ma, C.; et al. Metabolic engineering of Enterobacter cloacae for high-yield production of enantiopure (2R,3R)-2,3-butanediol from lignocellulose-derived sugars. Metab. Eng. 2015, 28, 19–27.
- Zhang, L.; Cao, C.; Jiang, R.; Xu, H.; Xue, F.; Huang, W.; Ni, H.; Gao, J. Production of R,R-2,3-butanediol of ultra-high optical purity from Paenibacillus polymyxa ZJ-9 using homologous recombination. Bioresour. Technol. 2018, 261, 272–278.
- Hassler, T.; Schieder, D.; Pfaller, R.; Faulstich, M.; Sieber, V. Enhanced fed-batch fermentation of 2,3-butanediol by Paenibacillus polymyxa DSM 365. Bioresour. Technol. 2012, 124, 237–244.
- Jang, M.-O.; Choi, G. Techno-economic analysis of butanol production from lignocellulosic biomass by concentrated acid pretreatment and hydrolysis plus continuous fermentation. Biochem. Eng. J. 2018, 134, 30–43.
- Cha, J.W.; Jang, S.H.; Kim, Y.J.; Chang, Y.K.; Jeong, K.J. Engineering of Klebsiella oxytoca for production of 2,3-butanediol using mixed sugars derived from lignocellulosic hydrolysates. GCB Bioenergy 2020, 12, 275–286.
- Salakkam, A.; Webb, C. Production of poly(3-hydroxybutyrate) from a complete feedstock derived from biodiesel by-products (crude glycerol and rapeseed meal). Biochem. Eng. J. 2018, 137, 358–364.
- Kubic, W.L.; Tan, E.C.D. Reactive Extraction Process for Separating 2,3-Butanediol from Fermentation Broth. Ind. Eng. Chem. Res. 2023, 62, 5241–5251.
- Xiao, Z.; Lu, J.R. Strategies for enhancing fermentative production of acetoin: A review. Biotechnol. Adv. 2014, 32, 492–503.
- Sabra, W.; Quitmann, H.; Zeng, A.P.; Dai, J.Y.; Xiu, Z.L. Microbial Production of 2,3-Butanediol. Compr. Biotechnol. 2011, 3, 87–97.
- Hong, J.; Van Duc Long, N.; Harvianto, G.R.; Haider, J.; Lee, M. Design and optimization of multi-effect-evaporation-assisted distillation configuration for recovery of 2,3-butanediol from fermentation broth. Chem. Eng. Process. 2019, 136, 107–115.
- Garg, S.K.; Jain, A. Fermentative production of 2,3-butanediol: A review. Bioresour. Technol. 1995, 51, 103–109.
- Xiu, Z.L.; Zeng, A.P. Present state and perspective of downstream processing of biologically produced 1,3-propanediol and 2,3-butanediol. Appl. Microbiol. Biotechnol. 2008, 78, 917–926.
- Qureshi, N.; Meagher, M.M.; Hutkins, R.W. Recovery of 2,3-Butanediol by Vacuum Membrane Distillation∗. Sep. Sci. Technol. 1994, 29, 1733–1748.
- Shao, P.; Kumar, A. Process energy efficiency in pervaporative and vacuum membrane distillation separation of 2,3-butanediol. Can. J. Chem. Eng. 2011, 89, 1255–1265.
- Afschar, A.S.; Vaz Rossell, C.E.; Jonas, R.; Quesada Chanto, A.; Schaller, K. Microbial production and downstream processing of 2,3-butanediol. J. Biotechnol. 1993, 27, 317–329.
- Harvianto, G.R.; Kang, K.J.; Lee, M. Process Design and Optimization of an Acetic Acid Recovery System in Terephthalic Acid Production via Hybrid Extraction–Distillation Using a Novel Mixed Solvent. Ind. Eng. Chem. Res. 2017, 56, 2168–2176.
- Haider, J.; Harvianto, G.R.; Qyyum, M.A.; Lee, M. Cost- and Energy-Efficient Butanol-Based Extraction-Assisted Distillation Designs for Purification of 2,3-Butanediol for Use as a Drop-in Fuel. ACS Sustain. Chem. Eng. 2018, 6, 14901–14910.
- Shao, P.; Kumar, A. Recovery of 2,3-butanediol from water by a solvent extraction and pervaporation separation scheme. J. Membr. Sci. 2009, 329, 160–168.
- Jeon, S.; Kim, D.K.; Song, H.; Lee, H.J.; Park, S.; Seung, D.; Chang, Y.K. 2,3-Butanediol recovery from fermentation broth by alcohol precipitation and vacuum distillation. J. Biosci. Bioeng. 2014, 117, 464–470.
- Harvianto, G.R.; Haider, J.; Hong, J.; Van Duc Long, N.; Shim, J.J.; Cho, M.H.; Kim, W.K.; Lee, M. Purification of 2,3-butanediol from fermentation broth: Process development and techno-economic analysis. Biotechnol. Biofuels 2018, 11, 18.
- Li, Z.; Teng, H.; Xiu, Z. Aqueous two-phase extraction of 2,3-butanediol from fermentation broths using an ethanol/ammonium sulfate system. Process Biochem. 2010, 45, 731–737.
- Jiang, B.; Li, Z.-G.; Dai, J.-Y.; Zhang, D.-J.; Xiu, Z.-L. Aqueous two-phase extraction of 2,3-butanediol from fermentation broths using an ethanol/phosphate system. Process Biochem. 2009, 44, 112–117.
- Wan, F.; Kang, T.; Liu, A.; Zhou, C.; Liu, S.; Xu, Y.; Si, S. Salt induced phase separation extraction of 2,3-Butanediol from aqueous solutions: Recovery and recycling of potassium triphosphate. Process Biochem. 2023, 125, 222–231.
- Silva, F.L.; Pinheiro, J.C.; Leite, M.J.L.; Proner, M.C.; da Silva, A.F.V.; Freire, D.M.G.; Treichel, H.; Ambrosi, A.; Di Luccio, M. Influence of different PEG/salt aqueous two-phase system on the extraction of 2,3-butanediol. Prep. Biochem. Biotechnol. 2022, 52, 1051–1059.
- Birajdar, S.D.; Rajagopalan, S.; Sawant, J.S.; Padmanabhan, S. Continuous countercurrent liquid–liquid extraction method for the separation of 2,3-butanediol from fermentation broth using n-butanol and phosphate salt. Process Biochem. 2015, 50, 1449–1458.
- Hao, J.; Xu, F.; Liu, H.; Liu, D. Downstream processing of 1,3-propanediol fermentation broth. J. Chem. Technol. Biotechnol. 2006, 81, 102–108.
- Li, Y.; Wu, Y.; Zhu, J.; Liu, J. Separation of 2,3-butanediol from fermentation broth by reactive-extraction using acetaldehyde-cyclohexane system. Biotechnol. Bioproc. E 2012, 17, 337–345.
- Li, Y.; Zhu, J.; Wu, Y.; Liu, J. Reactive extraction of 2,3-butanediol from fermentation broth. Korean J. Chem. Eng. 2013, 30, 154–159.
- Maina, S.; Dheskali, E.; Papapostolou, H.; Castro, A.M.d.; Guimaraes Freire, D.M.; Nychas, G.J.E.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A. Bioprocess Development for 2,3-Butanediol Production from Crude Glycerol and Conceptual Process Design for Aqueous Conversion into Methyl Ethyl Ketone. ACS Sustain. Chem. Eng. 2021, 9, 8692–8705.
- Soltys, K.A.; Batta, A.K.; Koneru, B. Successful nonfreezing, subzero preservation of rat liver with 2,3-butanediol and type I antifreeze protein. J. Surg. Res. 2001, 96, 30–34.
- Duraisamy, K.; Ha, A.; Kim, J.; Park, A.R.; Kim, B.; Song, C.W.; Song, H.; Kim, J.C. Enhancement of Disease Control Efficacy of Chemical Fungicides Combined with Plant Resistance Inducer 2,3-Butanediol against Turfgrass Fungal Diseases. Plant Pathol. J. 2022, 38, 182–193.
- Kim, B.; Park, A.R.; Song, C.W.; Song, H.; Kim, J.C. Biological Control Efficacy and Action Mechanism of Klebsiella pneumoniae JCK-2201 Producing Meso-2,3-Butanediol against Tomato Bacterial Wilt. Front. Microbiol. 2022, 13, 914589.
- Romano, P.; Granchi, L.; Caruso, M.; Borra, G.; Palla, G.; Fiore, C.; Ganucci, D.; Caligiani, A.; Brandolini, V. The species-specific ratios of 2,3-butanediol and acetoin isomers as a tool to evaluate wine yeast performance. Int. J. Food Microbiol. 2003, 86, 163–168.
- Maina, S.; Prabhu, A.A.; Vivek, N.; Vlysidis, A.; Koutinas, A.; Kumar, V. Prospects on bio-based 2,3-butanediol and acetoin production: Recent progress and advances. Biotechnol. Adv. 2022, 54, 107783.
- Song, C.W.; Park, J.M.; Chung, S.C.; Lee, S.Y.; Song, H. Microbial production of 2,3-butanediol for industrial applications. J. Ind. Microbiol. Biotechnol. 2019, 46, 1583–1601.
- Allen, J.G.; Flanigan, S.S.; LeBlanc, M.; Vallarino, J.; MacNaughton, P.; Stewart, J.H.; Christiani, D.C. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ. Health Perspect 2016, 124, 733–739.
- Park, J.M.; Song, H.; Lee, H.J.; Seung, D. In silico aided metabolic engineering of Klebsiella oxytoca and fermentation optimization for enhanced 2,3-butanediol production. J. Ind. Microbiol. Biotechnol. 2013, 40, 1057–1066.
- Hoppe, F.; Heuser, B.; Thewes, M.; Kremer, F.; Pischinger, S.; Dahmen, M.; Hechinger, M.; Marquardt, W. Tailor-made fuels for future engine concepts. Int. J. Engine Res. 2015, 17, 16–27.
- Dagle, V.L.; Dagle, R.A.; Kovarik, L.; Baddour, F.; Habas, S.E.; Elander, R. Single-step Conversion of Methyl Ethyl Ketone to Olefins over ZnxZryOz Catalysts in Water. Chemcatchem 2019, 11, 3393–3400.
- Affandy, M.; Zhu, C.; Swita, M.; Hofstad, B.; Cronin, D.; Elander, R.; Lebarbier Dagle, V. Production and catalytic upgrading of 2,3-butanediol fermentation broth into sustainable aviation fuel blendstock and fuel properties measurement. Fuel 2023, 333, 126328.
- Cui, X.K.; Zhao, X.B.; Liu, D.H. A novel route for the flexible preparation of hydrocarbon jet fuels from biomass-based platform chemicals: A case of using furfural and 2,3-butanediol as feedstocks. Green Chem. 2018, 20, 2018–2026.
- Nakazono, K.; Takahashi, R.; Yamada, Y.; Sato, S. Dehydration of 2,3-butanediol to produce 1,3-butadiene over Sc2O3 catalyst prepared through hydrothermal aging. Mol. Catal. 2021, 516, 111996.
- Liu, X.; Fabos, V.; Taylor, S.; Knight, D.W.; Whiston, K.; Hutchings, G.J. One-Step Production of 1,3-Butadiene from 2,3-Butanediol Dehydration. Chem.-Eur. J. 2016, 22, 12290–12294.
- Pittman, C.U.; Wuu, S.K.; Jacobson, S.E. 1,3-Butadiene oligomerization catalyzed by polymer-attached palladium complexes—comparison with homogeneous catalysis. J. Catal. 1976, 44, 87–100.





