
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alessandro De Camilli | -- | 1628 | 2023-08-22 15:01:34 | | | |
| 2 | Dean Liu | Meta information modification | 1628 | 2023-08-23 02:39:52 | | | | |
| 3 | Dean Liu | Meta information modification | 1628 | 2023-08-24 03:11:41 | | |
Video Upload Options
Targeted cellular and immunotherapies have welcomed a new chapter in multi-modal cancer therapy. These agents harness our innate immune system and destroy malignant cells in a precise way as compared with “legacy” chemotherapeutic agents that largely rely on abolishing cell division. New therapies can augment the T-cell recognition of tumor antigens and effectively prevent tumor cells from their historically successful ability to evade immune recognition. These novel agents cause acute and chronic toxicities to a variety of organ systems (enteritis, pneumonitis, hypophysitis, and hepatitis), and this may masquerade as other chronic illnesses or paraneoplastic effects. As the perioperative footprint of cancer patients increases, it is essential that perioperative providers—anesthesiologists, surgeons, nurse anesthetists, and inpatient hospital medicine providers—be up to date on the physiologic mechanisms that underlie these new therapies as well as their acute and subacute toxicity profiles. Immunotherapy toxicity can significantly impact perioperative morbidity as well as influence perioperative management, such as prophylaxis for adrenal insufficiency, preoperative pulmonary assessment, and screening for thyroid dysfunction, among others.
1. Introduction
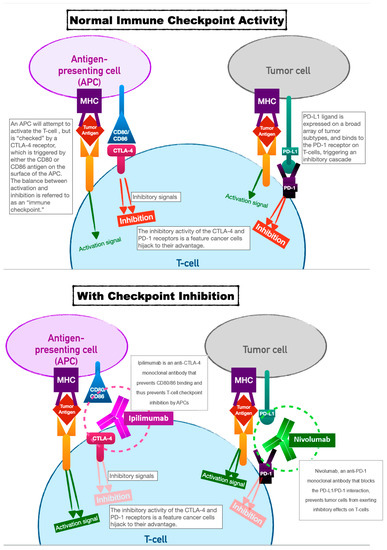
2. Immune-Checkpoint Inhibitors
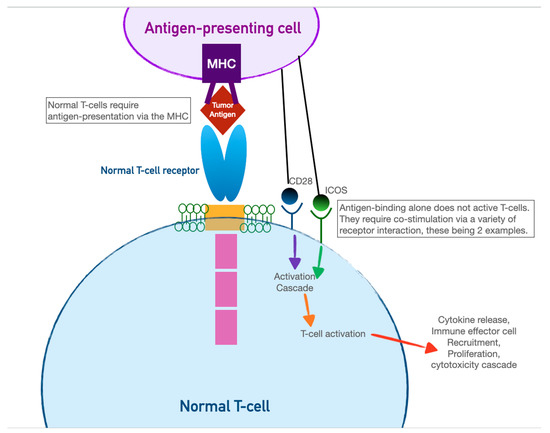
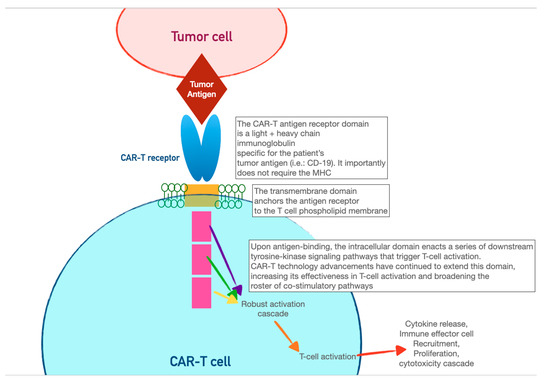
3. Chimeric Antigen-Receptor T Cells (CAR-T)
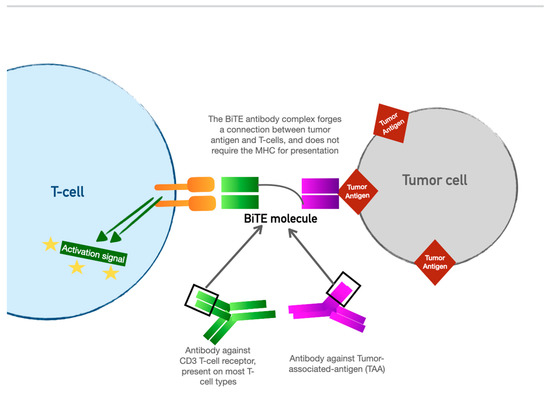
4. Bispecific T-Cell Engager (BiTE) Therapy
References
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985.
- Cascone, T.; William, W.N., Jr.; Weissferdt, A.; Leung, C.H.; Lin, H.Y.; Pataer, A.; Godoy, M.C.B.; Carter, B.W.; Federico, L.; Reuben, A.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomized NEOSTAR trial. Nat. Med. 2021, 27, 504–514.
- Mays, A.C.; Yarlagadda, B.; Achim, V.; Jackson, R.; Pipkorn, P.; Huang, A.T.; Rajasekaran, K.; Sridharan, S.; Rosko, A.J.; Orosco, R.K.; et al. Examining the relationship of immunotherapy and wound complications following flap reconstruction in patients with head and neck cancer. Head Neck 2021, 43, 1509–1520.
- Weber, J.S.; Kähler, K.C.; Hauschild, A. Management of Immune-Related Adverse Events and Kinetics of Response With Ipilimumab. J. Clin. Oncol. 2012, 30, 2691–2697.
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356, Erratum in N. Engl. J. Med. 2018, 379, 2185.
- Ikeuchi, K.; Okuma, Y.; Tabata, T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: A case report. Lung Cancer 2016, 99, 148–150.
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bhatia, S.; Budde, L.E.; Chokshi, S.; Davies, M.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 387–405.
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science 2001, 291, 319–322.
- Bonaca Marc, P.; Olenchock, B.A.; Salem, J.-E.; Wiviott, S.D.; Ederhy, S.; Cohen, A.; Stewart, G.C.; Choueiri, T.K.; Di Carli, M.; Allenbach, Y.; et al. Myocarditis in the setting of cancer therapeutics. Circulation 2019, 140, 80–91.
- Cui, K.; Wang, Z.; Zhang, Q.; Zhang, X. Immune checkpoint inhibitors and adrenal insufficiency: A large-sample case series study. Ann. Transl. Med. 2022, 10, 251.
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723, Erratum in N. Engl. J. Med. 2010, 363, 1290.
- Goswami S, Aparicio A, Subudhi SK. Immune Checkpoint Therapies in Prostate Cancer. Cancer J. 2016, 22, 117–120.
- Data Presented at AACR Support Potential of Peregrine’s PS-Targeting Immunotherapy Bavituximab to Enhance Anti-Tumor and Immune-Stimulating Effects of Anti-CTLA-4 and Anti-PD-1 Treatments in Models of Melanoma and Colon Cancer. Reuters. Archived from the Original on 2014-05-21. Available online: https://www.biospace.com/article/releases/data-presented-at-american-association-for-cancer-research-support-potential-of-peregrine-pharmaceuticals-inc-s-ps-targeting-immunotherapy-bavituxim/ (accessed on 1 August 2023).
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.-H.; Adenis, A.; et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 386, 449–462.
- Opdivo-Nivolumab Injection. DailyMed. 17 December 2019. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f570b9c4-6846-4de2-abfa-4d0a4ae4e394 (accessed on 11 March 2020).
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031.
- Haslam, A.; Gill, J.; Prasad, V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for Immune Checkpoint Inhibitor Drugs. JAMA Netw. Open 2020, 3, e200423.
- Elias, A.W.; Kasi, P.M.; Stauffer, J.A.; Thiel, D.D.; Colibaseanu, D.T.; Mody, K.; Joseph, R.W.; Bagaria, S.P. The Feasibility and Safety of Surgery in Patients Receiving Immune Checkpoint Inhibitors: A Retrospective Study. Front. Oncol. 2017, 7, 121.
- Zhang, H.; Zhao, P.; Huang, H. Engineering better chimeric antigen receptor T cells. Exp. Hematol. Oncol. 2020, 9, 34.
- Kochenderfer, J.N.; Wilson, W.H.; Janik, J.E.; Dudley, M.E.; Stetler-Stevenson, M.; Feldman, S.A.; Maric, I.; Raffeld, M.; Nathan, D.-A.N.; Lanier, B.J.; et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010, 116, 4099–4102.
- Zhang, X.; Lu, X.-A.; Yang, J.; Zhang, G.; Li, J.; Song, L.; Su, Y.; Shi, Y.; Zhang, M.; He, J.; et al. Efficacy and safety of anti-CD19 CAR T-cell therapy in 110 patients with B-cell acute lymphoblastic leukemia with high-risk features. Blood Adv. 2020, 4, 2325–2338.
- Kyte, J.A. Strategies for Improving the Efficacy of CAR T Cells in Solid Cancers. Cancers 2022, 14, 571.
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517.
- Helwick, C. Novel BiTE antibody mediates contact between T cells and cancer cells. Oncol. NEWS Int. 2008, 17.
- Dombret, H.; Topp, M.S.; Schuh, A.C.; Wei, A.H.; Durrant, S.; Bacon, C.L.; Tran, Q.; Zimmerman, Z.; Kantarjian, H. Blinatumomab versus chemotherapy in first salvage or in later salvage for B-cell precursor acute lymphoblastic leukemia. Leuk. Lymphoma 2019, 60, 2214–2222.
- Diefenbach, C.; Assouline, S.; Bosch, F.; Cheah, C.Y.; Kim, W.S.; Matasar, M.J.; Panizo, C.; Yoon, D.H.; Bender, B.; Hernandez, G.; et al. An individualized risk mitigation approach for safety: Experience from the mo-sunetuzumab (CD20/CD3 bispecific antibody) development program in relation to neurotoxicity risk . Blood 2019, 134 (Suppl. S1), 4728.
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638.




