
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Satoru Matsuda | -- | 2173 | 2023-08-21 18:23:09 | | | |
| 2 | Peter Tang | + 1 word(s) | 2174 | 2023-08-22 04:22:37 | | |
Video Upload Options
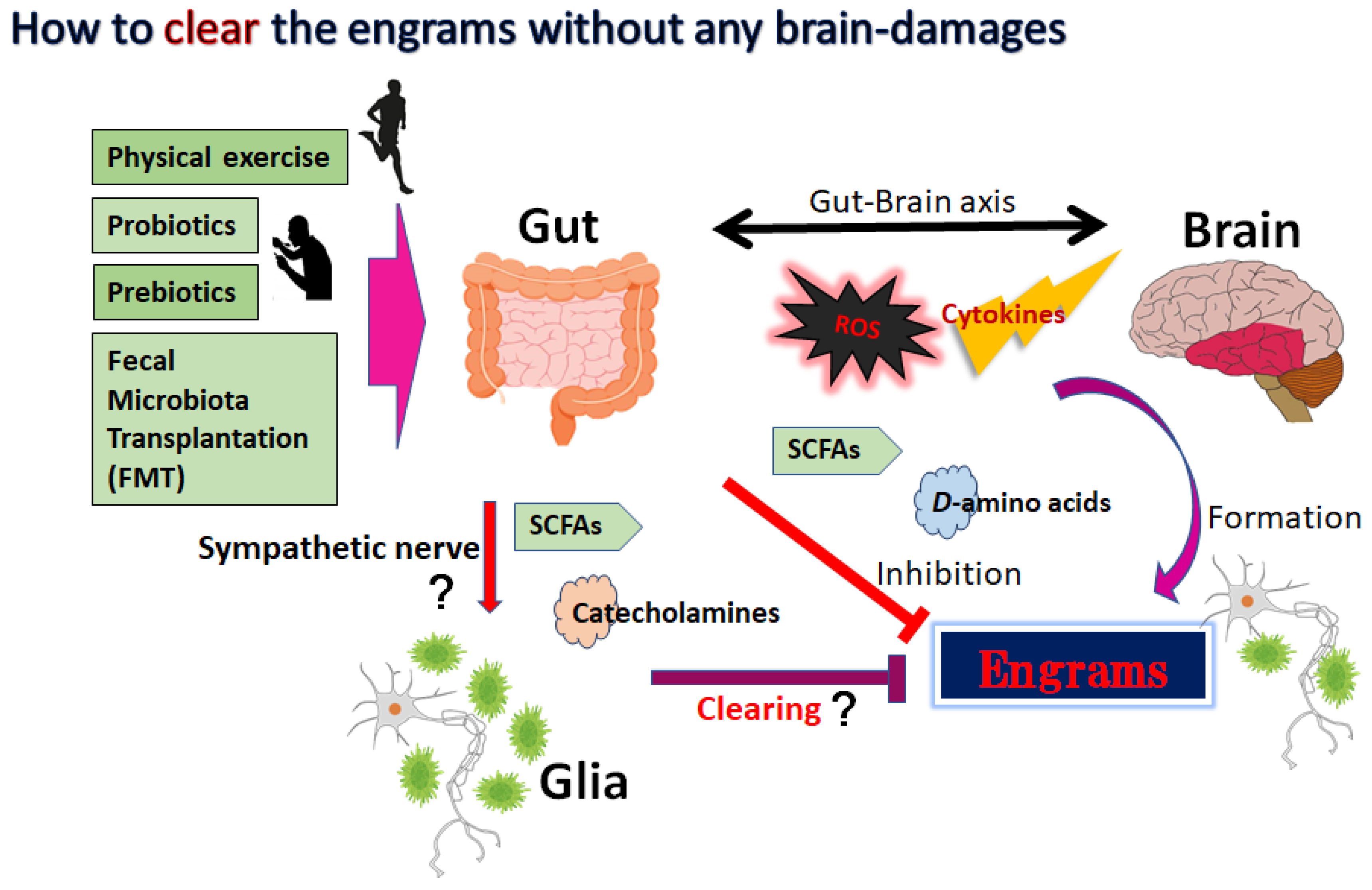
Nerve cell death accounts for various neurodegenerative disorders, in which altered immunity to the integrated central nervous system (CNS) might have destructive consequences. This undesirable immune response often affects the progressive neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, schizophrenia and/or amyotrophic lateral sclerosis (ALS). It has been shown that commensal gut microbiota could influence the brain and/or several machineries of immune function. In other words, neurodegenerative disorders may be connected to the gut–brain–immune correlational system. The engrams in the brain could retain the information of a certain inflammation in the body which might be involved in the pathogenesis of neurodegenerative disorders. Tactics involving the use of probiotics and/or fecal microbiota transplantation (FMT) are now evolving as the most promising and/or valuable for the modification of the gut–brain–immune axis.
1. Introduction
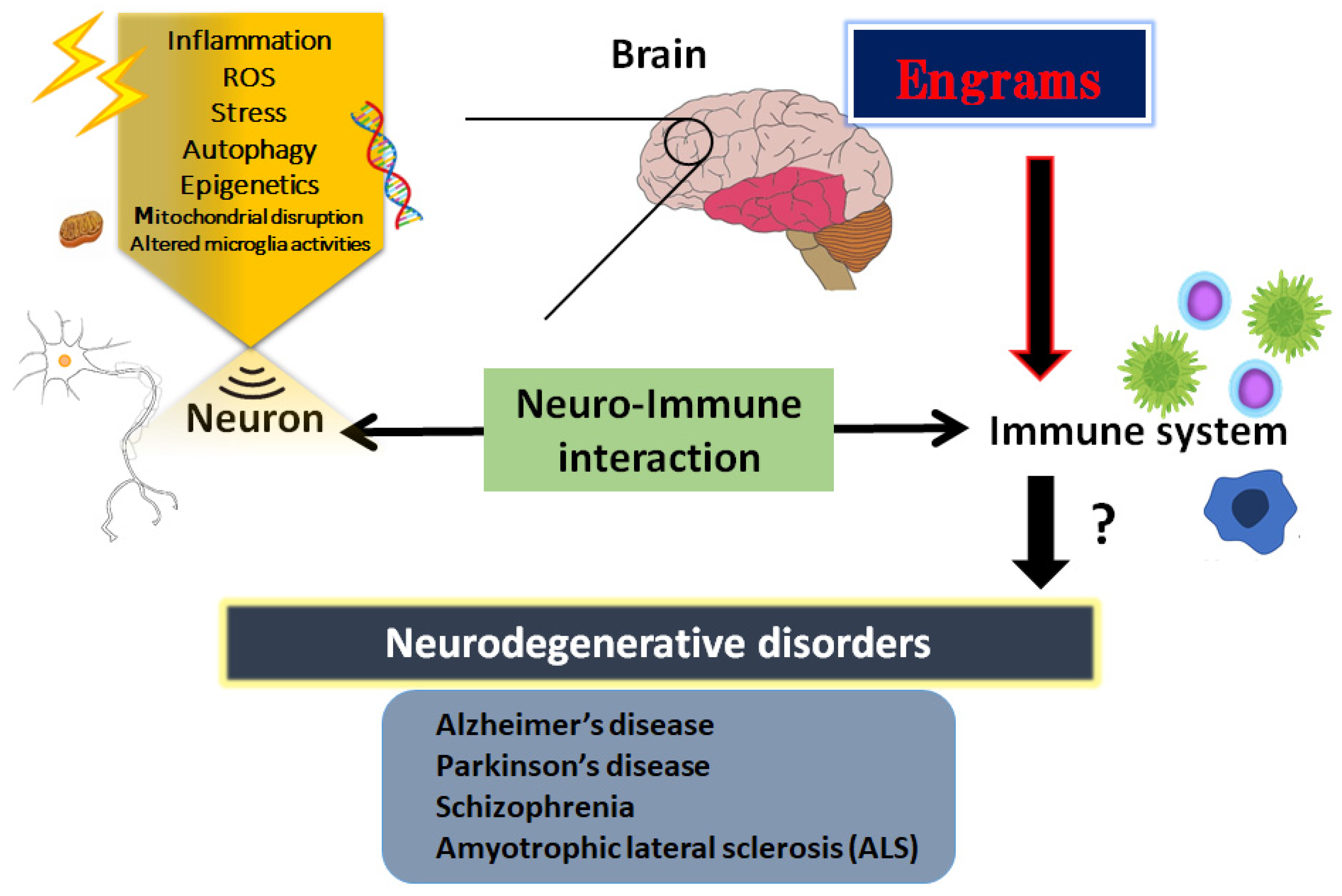
2. Inflammatory Neuro-Immune Responses
3. Engrams and Neuro-Immune Responses in the Pathogenesis of Neurodegenerative Disorders
4. How to Modulate the Engrams
5. Utilization of Gut–Brain Axis for the Treatment of Neurodegenerative Disorders
References
- Heneka, M.T.; McManus, R.M.; Latz, E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018, 19, 610–621.
- Gilodi, M.; Lisi, S.; Dudás, E.F.; Fantini, M.; Puglisi, R.; Louka, A.; Marcatili, P.; Cattaneo, A.; Pastore, A. Selection and Modelling of a New Single-Domain Intrabody Against TDP-43. Front. Mol. Biosci. 2022, 8, 773234.
- Ikeda, Y.; Nagase, N.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Neuroprotection by dipeptidyl-peptidase-4 inhibitors and glucagon-like peptide-1 analogs via the modulation of AKT-signaling pathway in Alzheimer’s disease. World. J. Biol. Chem. 2021, 12, 104–113.
- Ogino, M.; Ichimura, M.; Nakano, N.; Minami, A.; Kitagishi, Y.; Matsuda, S. Roles of PTEN with DNA Repair in Parkinson’s Disease. Int. J. Mol. Sci. 2016, 17, 954.
- Kitagishi, Y.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer’s disease. Alzheimers. Res. Ther. 2014, 6, 35.
- Abbaszadeh, F.; Fakhri, S.; Khan, H. Targeting apoptosis and autophagy following spinal cord injury: Therapeutic approaches to polyphenols and candidate phytochemicals. Pharmacol. Res. 2020, 160, 105069.
- Matsuda, S.; Ikeda, Y.; Murakami, M.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y. Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses. Diseases 2019, 7, 22.
- Kitagishi, Y.; Minami, A.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Neuron membrane trafficking and protein kinases involved in autism and ADHD. Int. J. Mol. Sci. 2015, 16, 3095–3115.
- Singh, A.; Kukretim, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950.
- Hitzeroth, A.; Niehaus, D.J.; Koen, L.; Botes, W.C.; Deleuze, J.F.; Warnich, L. Association between the MnSOD Ala-9Val polymorphism and development of schizophrenia and abnormal involuntary movements in the Xhosa population. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 664–672.
- Le Gall, L.; Anakor, E.; Connolly, O.; Vijayakumar, U.G.; Duddy, W.J.; Duguez, S. Molecular and Cellular Mechanisms Affected in ALS. J. Pers. Med. 2020, 10, 101.
- Godoy, J.A.; Rios, J.A.; Picón-Pagès, P.; Herrera-Fernández, V.; Swaby, B.; Crepin, G.; Vicente, R.; Fernández-Fernández, J.M.; Muñoz, F.J. Mitostasis, Calcium and Free Radicals in Health, Aging and Neurodegeneration. Biomolecules 2021, 11, 1012.
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152.
- Fakhri, S.; Abbaszadeh, F.; Jorjanim, M. On the therapeutic targets and pharmacological treatments for pain relief following spinal cord injury: A mechanistic review. Biomed. Pharmacother. 2021, 139, 111563.
- Zhong, S.R.; Kuang, Q.; Zhang, F.; Chen, B.; Zhong, Z.G. Functional roles of the microbiota-gut-brain axis in Alzheimer’s disease: Implications of gut microbiota-targeted therapy. Transl. Neurosci. 2021, 12, 581–600.
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503.
- Bruce-Keller, A.J.; Salbaum, J.M.; Berthoud, H.R. Harnessing Gut Microbes for Mental Health: Getting from Here to There. Biol. Psychiatry 2018, 83, 214–223.
- Noss, M.M.; Millwood, S.N.; Kuhlman, K.R. Women with lower systemic inflammation demonstrate steeper cognitive decline with age: Results from a large prospective, longitudinal sample. Brain. Behav. Immun. Health 2022, 22, 100465.
- Arsenault, D.; Coulombe, K.; Zhu, A.; Gong, C.; Kil, K.E.; Choi, J.K.; Poutiainen, P.; Brownell, A.L. Loss of Metabotropic Glutamate Receptor 5 Function on Peripheral Benzodiazepine Receptor in Mice Prenatally Exposed to LPS. PLoS ONE 2015, 10, e0142093.
- Chavan, S.S.; Pavlov, V.A.; Tracey, K.J. Mechanisms and Therapeutic Relevance of Neuro-immune Communication. Immunity 2017, 46, 927–942.
- Clark, S.M.; Vaughn, C.N.; Soroka, J.A.; Li, X.; Tonelli, L.H. Neonatal adoptive transfer of lymphocytes rescues social behaviour during adolescence in immune-deficient mice. Eur. J. Neurosci. 2018, 47, 968–978.
- Theodoropoulou, S.; Spanakos, G.; Baxevanis, C.N.; Economou, M.; Gritzapis, A.D.; Papamichail, M.P.; Stefanis, C.N. Cytokine serum levels, autologous mixed lymphocyte reaction and surface marker analysis in never medicated and chronically medicated schizophrenic patients. Schizophr. Res. 2001, 47, 13–25.
- Lupaescu, A.V.; Iavorschi, M.; Covasa, M. The Use of Bioactive Compounds in Hyperglycemia- and Amyloid Fibrils-Induced Toxicity in Type 2 Diabetes and Alzheimer’s Disease. Pharmaceutics 2022, 14, 235.
- Chang, M.C.; Kwak, S.G.; Park, J.S.; Park, D. The effectiveness of nonsteroidal anti-inflammatory drugs and acetaminophen in reduce the risk of amyotrophic lateral sclerosis? A meta-analysis. Sci. Rep. 2020, 10, 14759.
- Csabai, D.; Sebők-Tornai, A.; Wiborg, O.; Czéh, B. A Preliminary Quantitative Electron Microscopic Analysis Reveals Reduced Number of Mitochondria in the Infralimbic Cortex of Rats Exposed to Chronic Mild Stress. Front. Behav. Neurosci. 2022, 16, 885849.
- Karmakar, J.; Mukherjee, K.; Mandal, C. Siglecs Modulate Activities of Immune Cells Through Positive and Negative Regulation of ROS Generation. Front. Immunol. 2021, 12, 758588.
- Scherz-Shouval, R.; Elazar, Z. Regulation of autophagy by ROS: Physiology and pathology. Trends. Biochem. Sci. 2011, 36, 30–38.
- Zhu, L.; Liu, L. New Insights into the Interplay Among Autophagy, the NLRP3 Inflammasome and Inflammation in Adipose Tissue. Front. Endocrinol. 2022, 13, 739882.
- Marcucci, F.; Bellone, M.; Caserta, C.A.; Corti, A. Pushing tumor cells towards a malignant phenotype: Stimuli from the microenvironment, intercellular communications and alternative roads. Int. J. Cancer. 2014, 135, 1265–1276.
- Yan, L.S.; Zhang, S.F.; Luo, G.; Cheng, B.C.; Zhang, C.; Wang, Y.W.; Qiu, X.Y.; Zhou, X.H.; Wang, Q.G.; Song, X.L.; et al. Schisandrin B mitigates hepatic steatosis and promotes fatty acid oxidation by inducing autophagy through AMPK/mTOR signaling pathway. Metabolism 2022, 131, 155200.
- Nagy, S.; Maurer, G.W.; Hentze, J.L.; Rose, M.; Werge, T.M.; Rewitz, K. AMPK signaling linked to the schizophrenia-associated 1q21.1 deletion is required for neuronal and sleep maintenance. PLoS Genet. 2018, 14, e1007623.
- Koren, T.; Yifa, R.; Amer, M.; Krot, M.; Boshnak, N.; Ben-Shaanan, T.L.; Azulay-Debby, H.; Zalayat, I.; Avishai, E.; Hajjo, H.; et al. Insular cortex neurons encode and retrieve specific immune responses. Cell 2021, 184, 5902–5915.e17.
- Gogolla, N. The brain remembers where and how inflammation struck. Cell 2021, 184, 5851–5853.
- Roy, D.S.; Park, Y.G.; Kim, M.E.; Zhang, Y.; Ogawa, S.K.; DiNapoli, N.; Gu, X.; Cho, J.H.; Choi, H.; Kamentsky, L.; et al. Brain-wide mapping reveals that engrams for a single memory are distributed across multiple brain regions. Nat. Commun. 2022, 13, 1799.
- Sakaguchi, M.; Hayashi, Y. Catching the engram: Strategies to examine the memory trace. Mol. Brain. 2012, 5, 32.
- Gebicke-Haerter, P.J. Engram formation in psychiatric disorders. Front. Neurosci. 2014, 8, 118.
- Fuentes-Ramos, M.; Alaiz-Noya, M.; Barco, A. Transcriptome and epigenome analysis of engram cells: Next-generation sequencing technologies in memory research. Neurosci. Biobehav. Rev. 2021, 127, 865–875.
- Bachmann, S.; Linde, J.; Bell, M.; Spehr, M.; Zempel, H.; Zimmer-Bensch, G. DNA Methyltransferase 1 (DNMT1) Shapes Neuronal Activity of Human iPSC-Derived Glutamatergic Cortical Neurons. Int. J. Mol. Sci. 2021, 22, 2034.
- Gulmez Karaca, K.; Kupke, J.; Brito, D.V.C.; Zeuch, B.; Thome, C.; Weichenhan, D.; Lutsik, P.; Plass, C.; Oliveira, A.M.M. Neuronal ensemble-specific DNA methylation strengthens engram stability. Nat. Commun. 2020, 11, 639.
- Niemi, M.B.; Härting, M.; Kou, W.; Del Rey, A.; Besedovsky, H.O.; Schedlowski, M.; Pacheco-López, G. Taste-immunosuppression engram: Reinforcement and extinction. J. Neuroimmunol. 2007, 188, 74–79.
- Pacheco-López, G.; Niemi, M.B.; Kou, W.; Baum, S.; Hoffman, M.; Altenburger, P.; del Rey, A.; Besedovsky, H.O.; Schedlowski, M. Central blockade of IL-1 does not impair taste-LPS associative learning. Neuroimmunomodulation 2007, 14, 150–156.
- Kyrke-Smith, M.; Williams, J.M. Bridging Synaptic and Epigenetic Maintenance Mechanisms of the Engram. Front. Mol. Neurosci. 2018, 11, 369.
- Manea, S.A.; Vlad, M.L.; Fenyo, I.M.; Lazar, A.G.; Raicu, M.; Muresian, H.; Simionescu, M.; Manea, A. Pharmacological inhibition of histone deacetylase reduces NADPH oxidase expression, oxidative stress and the progression of atherosclerotic lesions in hypercholesterolemic apolipoprotein E-deficient mice; potential implications for human atherosclerosis. Redox Biol. 2020, 28, 101338.
- Qing, L.; Liu, L.; Zhou, L.; Zhang, F.; Gao, C.; Hu, L.; Nie, S. Sex-dependent association of mineralocorticoid receptor gene (NR3C2) DNA methylation and schizophrenia. Psychiatry Res. 2020, 292, 113318.
- Bostancıklıoğlu, M. An update on memory formation and retrieval: An engram-centric approach. Alzheimers. Dement. 2020, 16, 926–937.
- Wang, C.; Yue, H.; Hu, Z.; Shen, Y.; Ma, J.; Li, J.; Wang, X.D.; Wang, L.; Sun, B.; Shi, P.; et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science 2020, 367, 688–694.
- Wang, Y.Y.; Deng, Y.S.; Dai, S.K.; Mi, T.W.; Li, R.Y.; Liu, P.P.; Liu, C.; He, B.D.; He, X.C.; Du, H.Z.; et al. Loss of microglial EED impairs synapse density, learning, and memory. Mol. Psychiatry 2022, 27, 2999–3009.
- Wang, X.; Chang, L.; Wan, X.; Tan, Y.; Qu, Y.; Shan, J.; Yang, Y.; Ma, L.; Hashimoto, K. (R)-ketamine ameliorates demyelination and facilitates remyelination in cuprizone-treated mice: A role of gut-microbiota-brain axis. Neurobiol. Dis. 2022, 165, 105635.
- Ghezzi, L.; Cantoni, C.; Pinget, G.V.; Zhou, Y.; Piccio, L. Targeting the gut to treat multiple sclerosis. J. Clin. Investig. 2021, 131, e143774.
- Klann, E.M.; Dissanayake, U.; Gurrala, A.; Farrer, M.; Shukla, A.W.; Ramirez-Zamora, A.; Mai, V.; Vedam-Mai, V. The Gut-Brain Axis and Its Relation to Parkinson’s Disease: A Review. Front. Aging Neurosci. 2022, 13, 782082.
- Lecomte, A.; Barateau, L.; Pereira, P.; Paulin, L.; Auvinen, P.; Scheperjans, F.; Dauvilliers, Y. Gut microbiota composition is associated with narcolepsy type 1. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e896.
- Wiley, N.C.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Stanton, C. Production of Psychoactive Metabolites by Gut Bacteria. Mod. Trends Psychiatry 2021, 32, 74–99.
- Caputi, V.; Popov, J.; Giron, M.C.; O’Mahony, S. Gut Microbiota as a Mediator of Host Neuro-Immune Interactions: Implications in Neuroinflammatory Disorders. Mod. Trends. Psychiatry 2021, 32, 40–57.
- Benakis, C.; Martin-Gallausiaux, C.; Trezzi, J.P.; Melton, P.; Liesz, A.; Wilmes, P. The microbiome-gut-brain axis in acute and chronic brain diseases. Curr. Opin. Neurobiol. 2020, 61, 1–9.
- Castanon, N.; Luheshi, G.; Layé, S. Role of neuroinflammation in the emotional and cognitive alterations displayed by animal models of obesity. Front. Neurosci. 2015, 9, 229.
- Hertzberg, V.S.; Singh, H.; Fournier, C.N.; Moustafa, A.; Polak, M.; Kuelbs, C.A.; Torralba, M.G.; Tansey, M.G.; Nelson, K.E.; Glass, J.D. Gut microbiome differences between amyotrophic lateral sclerosis patients and spouse controls. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2022, 23, 91–99.
- Kim, H.S.; Son, J.; Lee, D.; Tsai, J.; Wang, D.; Chocron, E.S.; Jeong, S.; Kittrell, P.; Murchison, C.F.; Kennedy, R.E.; et al. Gut- and oral-dysbiosis differentially impact spinal- and bulbar-onset ALS, predicting ALS severity and potentially determining the location of disease onset. BMC. Neurol. 2022, 22, 62.
- Chidambaram, S.B.; Essa, M.M.; Rathipriya, A.G.; Bishir, M.; Ray, B.; Mahalakshmi, A.M.; Tousif, A.H.; Sakharkar, M.K.; Kashyap, R.S.; Friedland, R.P.; et al. Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: Tales of a vicious cycle. Pharmacol. Ther. 2022, 231, 107988.
- Trujillo-Del Río, C.; Tortajada-Pérez, J.; Gómez-Escribano, A.P.; Casterá, F.; Peiró, C.; Millán, J.M.; Herrero, M.J.; Vázquez-Manrique, R.P. Metformin to treat Huntington disease: A pleiotropic drug against a multi-system disorder. Mech. Ageing Dev. 2022, 204, 111670.
- Matsuda, S.; Nakagawa, Y.; Amano, K.; Ikeda, Y.; Tsuji, A.; Kitagishi, Y. By using either endogenous or transplanted stem cells, which could you prefer for neural regeneration? Neural. Regen. Res. 2018, 13, 1731–1732.
- Taniguchi, K.; Ikeda, Y.; Nagase, N.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Implications of Gut-Brain axis in the pathogenesis of Psychiatric disorders. AIMS. Bioeng. 2021, 8, 243–256.
- Matsuda, S.; Nakagawa, Y.; Kitagishi, Y.; Nakanishi, A.; Murai, T. Reactive Oxygen Species, Superoxide Dimutases, and PTEN-p53-AKT-MDM2 Signaling Loop Network in Mesenchymal Stem/Stromal Cells Regulation. Cells 2018, 7, 36.
- Ikeda, Y.; Taniguchi, K.; Nagase, N.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Reactive oxygen species may influence on the crossroads of stemness, senescence, and carcinogenesis in a cell via the roles of APRO family proteins. Explor. Med. 2021, 2, 443–454.
- Zhang, L.; Qian, Y.; Li, J.; Zhou, X.; Xu, H.; Yan, J.; Xiang, J.; Yuan, X.; Sun, B.; Sisodia, S.S.; et al. BAD-mediated neuronal apoptosis and neuroinflammation contribute to Alzheimer’s disease pathology. iScience 2021, 24, 102942.
- Acosta, S.; Jernberg, J.; Sanberg, C.D.; Sanberg, P.R.; Small, B.J.; Gemma, C.; Bickford, P.C. NT-020, a natural therapeutic approach to optimize spatial memory performance and increase neural progenitor cell proliferation and decrease inflammation in the aged rat. Rejuvenation Res. 2010, 13, 581–588.




