
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sara FAHDE | -- | 3801 | 2023-08-17 17:09:54 | | | |
| 2 | Lindsay Dong | + 2 word(s) | 3803 | 2023-08-21 03:59:54 | | |
Video Upload Options
Rhizobia can associate with non-legume roots, which ultimately leads to the stimulation of growth through diverse direct and indirect mechanisms. For example, rhizobia can enhance growth through phytohormones production, the improvement of plant nutrient uptake, such as the solubilization of precipitated phosphorus, the production of siderophores to address iron needs, and also the reduction of ethylene levels through the aminocyclopropane-1-carboxylate (ACC) deaminase enzyme to cope with drought stress. Additionally, rhizobia can improve, indirectly, non-legume growth through biocontrol of pathogens and the induction of systemic resistance in the host plant. It can also increase root adherence to soil by releasing exopolysaccharides, which regulate water and soil nutrient movement.
1. Direct Mechanism
1.1. N2 Fixation
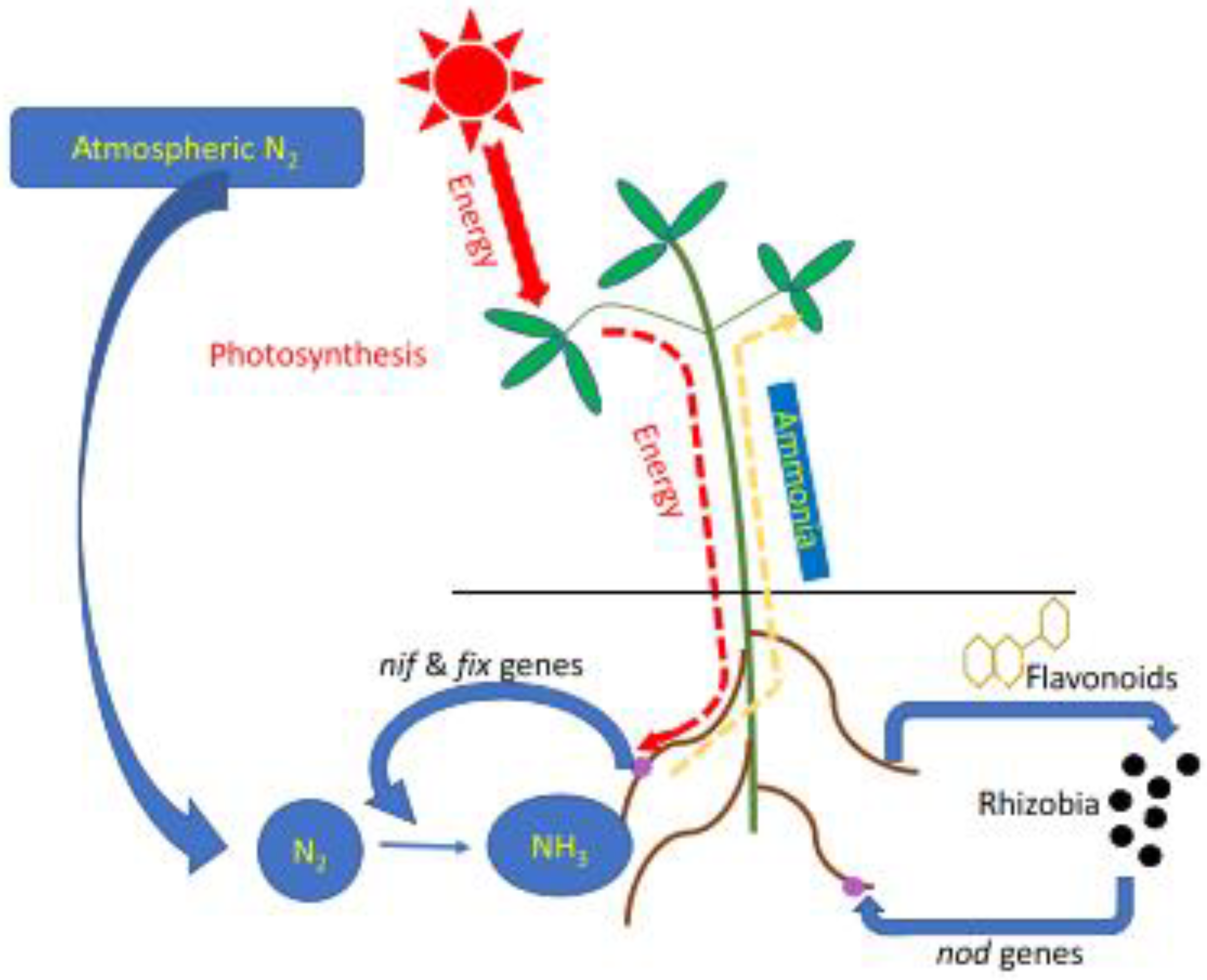
1.2. Phosphate Solubilization
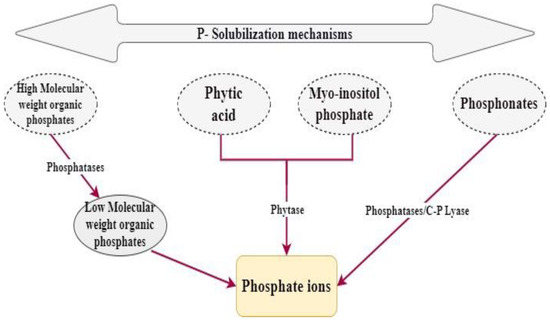
-
Inorganic P solubilization mechanisms
-
Organic Acid ProductionThe production of organic acid is considered as the initial mechanism to be used in order to solubilize inorganic phosphorus [14]. This phenomenon depends mostly on the soil’s pH.Phosphate Solubilizing Microbes (PSM) produce, during their growth, some organic acids that have the potential to decrease the soil’s pH. This acidification enables the solubilization of rock phosphate [14].It has been established that the acids produced by PSM are mainly glycolic (monocarbocyclic hydroxy acids), 2-keto gluconic (monocarboxylic), acetic acids, malic (dicarboxylic hydroxy acids), oxalic acid, citric acid, and succinic acid (dicarboxylic acid). However, in the midst of all these acids, gluconic acid has been found to be the lead acid to be used in the P solubilization mechanism [14][15].
-
Inorganic Acid ProductionInorganic acids do not appear to be as effective as organic acids for the solubilization of P. Nitrifying and sulfur-oxidizing bacteria generate inorganic acids during the oxidation of nitrogenous or inorganic sulfur compounds. These inorganic acids then interact with insoluble phosphate compounds, transforming them into soluble variants [14].
-
ChelationFulvic, humic, and 2-keto gluconic acids are some known acids that play the role of chelators of substances, such as aluminum, calcium, and iron cations, which facilitate the inorganic phosphorus solubilization. These acids are liberated all along the processes of plant debris degradation by microorganisms [14].Besides the previously mentioned inorganic P solubilization mechanisms, there are other mechanisms, such as mineral P solubilization through proton (H+) extrusion. This process effectively lowers the pH of the environment without requiring the release of acids [13]. Furthermore, microorganisms that produce exopolysaccharides have the ability to form complexes with metals, resulting in the solubilization of metal phosphates [14].
-
-
Organic P Solubilization Mechanisms
1.3. Phytohormones Production/Regulation (Plant Growth Regulators)
-
Auxins
-
Cytokinins
-
Gibberellins
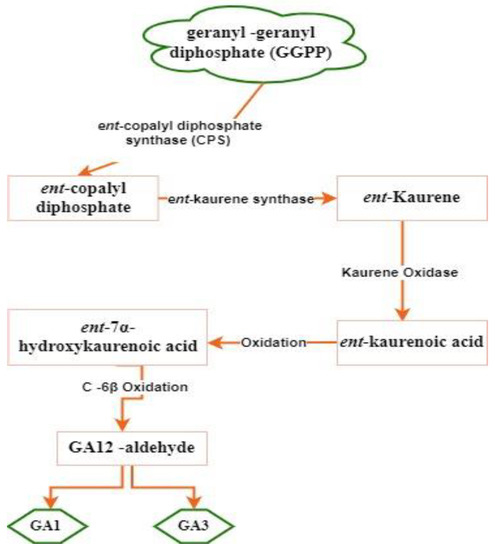
-
Abscisic acid
-
Ethylene
1.4. Siderophores Production
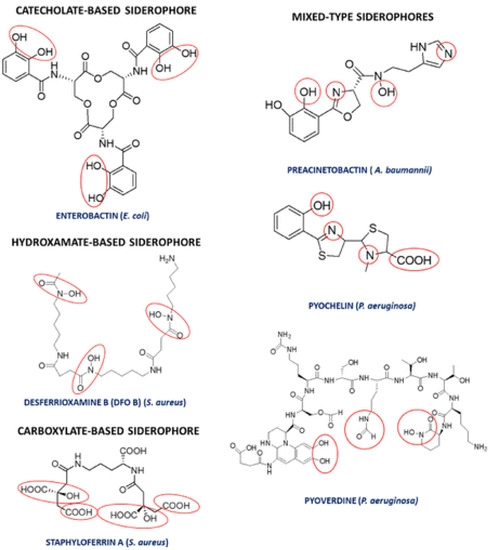
2. Indirect Mechanism
2.1. Antibiotics Synthesis
2.2. Induction of Systemic Resistance
-
Systemic Acquired Resistance (SAR)
-
Induced Systemic Resistance (ISR)
2.3. Production of Cell Wall-Degrading Enzymes
2.4. Production of Hydrogen Cyanide (HCN)
References
- Lindström, K.; Mousavi, S.A. Effectiveness of nitrogen fixation in rhizobia. Microb. Biotechnol. 2020, 13, 1314–1335.
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech 2015, 5, 355–377.
- Laslo, É.; Mara, G. Is PGPR an Alternative for NPK Fertilizers in Sustainable Agriculture? In Microbial Interventions in Agriculture and Environment; Singh, D.P., Gupta, V.K., Prabha, R., Eds.; Springer: Singapore, 2019; pp. 51–62.
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total. Environ. 2020, 743, 140682.
- Aroca, R.; Ruiz-Lozano, J. Induction of Plant Tolerance to Semi-arid Environments by Beneficial Soil Microorganisms—A Review. In Climate Change, Intercropping, Pest Control and Beneficial Microorganisms; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 121–135.
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598.
- Antoun, H.; Beauchamp, C.J.; Goussard, N.; Chabot, R.; Lalande, R. Potential of Rhizobium and Bradyrhizobium species as plant growth promoting rhizobacteria on non-legumes: Effect on radishes (Raphanus sativus L.). In Molecular Microbial Ecology of the Soil; Hardarson, G., Broughton, W.J., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 57–67.
- Vargas, L.K.; Volpiano, C.G.; Lisboa, B.B.; Giongo, A.; Beneduzi, A.; Passaglia, L.M.P. Potential of Rhizobia as Plant Growth-Promoting Rhizobacteria. In Microbes for Legume Improvement; Zaidi, A., Khan, M.S., Musarrat, J., Eds.; Springer International Publishing: Cham, Germany, 2017; pp. 153–174.
- Tahir, M.; Sarwar, M.A. Plant Growth Promoting Rhizobacteria (PGPR): A Budding Complement of Synthetic Fertilizers for Improving Crop Production. Group 2013, 19, 79–87.
- Sharma, D.; Gahtyari, N.C.; Chhabra, R.; Kumar, D. Role of Microbes in Improving Plant Growth and Soil Health for Sustainable Agriculture. In Advances in Plant Microbiome and Sustainable Agriculture; Yadav, A.N., Rastegari, A.A., Yadav, N., Kour, D., Eds.; Microorganisms for Sustainability; Springer: Singapore, 2020; Volume 19, pp. 207–256.
- Tailor, A.J.; Joshi, B.H. Harnessing Plant Growth Promoting Rhizobacteria Beyond Nature: A Review. J. Plant Nutr. 2014, 37, 1534–1571.
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2023, 255, 571–586.
- Swarnalakshmi, K.; Yadav, V.; Tyagi, D.; Dhar, D.W.; Kannepalli, A.; Kumar, S. Significance of Plant Growth Promoting Rhizobacteria in Grain Legumes: Growth Promotion and Crop Production. Plants 2020, 9, 1596.
- Prabhu, N.; Borkar, S.; Garg, S. Phosphate solubilization by microorganisms. In Advances in Biological Science Research; Elsevier: Amsterdam, The Netherlands, 2019; pp. 161–176.
- Wang, Q.; Liu, J.; Zhu, H. Genetic and Molecular Mechanisms Underlying Symbiotic Specificity in Legume-Rhizobium Interactions. Front. Plant Sci. 2018, 9, 313.
- Sijilmassi, B.; Filali-Maltouf, A.; Fahde, S.; Ennahli, Y.; Boughribil, S.; Kumar, S.; Amri, A. In-Vitro Plant Growth Promotion of Rhizobium Strains Isolated from Lentil Root Nodules under Abiotic Stresses. Agronomy 2020, 10, 1006.
- Defez, R.; Andreozzi, A.; Romano, S.; Pocsfalvi, G.; Fiume, I.; Esposito, R.; Angelini, C.; Bianco, C. Bacterial IAA-Delivery into Medicago Root Nodules Triggers a Balanced Stimulation of C and N Metabolism Leading to a Biomass Increase. Microorganisms 2019, 7, 403.
- Ghosh, S.; Ghosh, P.; Maiti, T.K. Production and Metabolism of Indole Acetic Acid (IAA) by Root Nodule Bacteria (Rhizobium): A Review. Appl. Microbiol. 2011, 5, 523–540.
- Silini, A.R.; Parolini, O.; Huppertz, B.; Lang, I. Soluble Factors of Amnion-Derived Cells in Treatment of Inflammatory and Fibrotic Pathologies. Curr. Stem Cell Res. Ther. 2013, 8, 6–14.
- Malik, J.A. (Ed.) Handbook of Research on Microbial Remediation and Microbial Biotechnology for Sustainable Soil. In Advances in Environmental Engineering and Green Technologies; IGI Global: Pennsylvania, PA, USA, 2021.
- Hayat, R.; Ahmed, I.; Sheirdil, R.A. An Overview of Plant Growth Promoting Rhizobacteria (PGPR) for Sustainable Agriculture. In Crop Production for Agricultural Improvement; Ashraf, M., Öztürk, M., Ahmad, M.S.A., Aksoy, A., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 557–579.
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500.
- Jaiswal, S.K.; Mohammed, M.; Ibny, F.Y.I.; Dakora, F.D. Rhizobia as a Source of Plant Growth-Promoting Molecules: Potential Applications and Possible Operational Mechanisms. Front. Sustain. Food Syst. 2021, 4, 619676.
- Jeyanthi, V.; Kanimozhi, S. Plant Growth Promoting Rhizobacteria (PGPR)-Prospective and Mechanisms: A Review. J. Pure Appl. Microbiol. 2018, 12, 733–749.
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448.
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573.
- Gray, E.J.; Smith, D.L. Intracellular and extracellular PGPR: Commonalities and distinctions in the plant–bacterium signaling processes. Soil. Biol. Biochem. 2005, 37, 395–412.
- Pahari, A.; Pradhan, A.; Nayak, S.; Mishra, B. Plant Growth Promoting Rhizobacteria (Pgpr): Prospects and Application. In Frontiers in Soil and Environmental Microbiology, 1st ed.; Nayak, S.K., Mishra, B.B., Eds.; CRC Press: Boca Raton, FL, USA, 2020; Volume 56, pp. 47–56.
- Medeot, D.B.; Paulucci, N.S.; Albornoz, A.I.; Fumero, M.V.; Bueno, M.A.; Garcia, M.B.; Woelke, M.R.; Okon, Y.; Dardanelli, M.S. Plant Growth Promoting Rhizobacteria Improving the Legume–Rhizobia Symbiosis. In Microbes for Legume Improvement; Khan, M.S., Musarrat, J., Zaidi, A., Eds.; Springer: Vienna, Austria, 2010; pp. 473–494.
- Tatsukami, Y.; Ueda, M. Rhizobial gibberellin negatively regulates host nodule number. Sci. Rep. 2016, 6, 27998.
- Dobbelaere, S.; Vanderleyden, J.; Okon, Y. Plant Growth-Promoting Effects of Diazotrophs in the Rhizosphere. Crit. Rev. Plant Sci. 2003, 22, 107–149.
- Boiero, L.; Perrig, D.; Masciarelli, O.; Penna, C.; Cassán, F.; Luna, V. Phytohormone production by three strains of Bradyrhizobium japonicum and possible physiological and technological implications. Appl. Microbiol. Biotechnol. 2007, 74, 874–880.
- Wani, S.P.; Gopalakrishnan, S. Plant Growth-Promoting Microbes for Sustainable Agriculture. In Plant Growth Promoting Rhizobacteria (PGPR): Prospects for Sustainable Agriculture; Sayyed, R.Z., Reddy, M.S., Antonius, S., Eds.; Springer: Singapore, 2019; pp. 19–45.
- Kircheva, N.; Dudev, T. Gallium as an Antibacterial Agent: A DFT/SMD Study of the Ga3+/Fe3+ Competition for Binding Bacterial Siderophores. Inorg. Chem. 2020, 59, 6242–6254.
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial Plant Biostimulants: A Sustainable Way towards Improving Growth, Productivity, and Health of Crops. Sustainability 2021, 13, 2856.
- Albelda-Berenguer, M.; Monachon, M.; Joseph, E. Siderophores: From Natural Roles to Potential Applications. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 193–225.
- Roy, N.; Chakrabartty, P.K. Effect of Aluminum on the Production of Siderophore by Rhizobium sp. (Cicer arietinum). Curr. Microbiol. 2000, 41, 5–10.
- Rogers, N.J.; Carson, K.C.; Glenn, A.R.; Dilworth, M.J.; Poole, R.K. Alleviation of aluminum toxicity to Rhizobium leguminosarum bv. viciae by the hydroxamate siderophore vicibactin. Biometals 2001, 14, 59–66.
- O'Hara, G.W.; Goss, T.J.; Dilworth, M.J.; Glenn, A.R. Maintenance of Intracellular pH and Acid Tolerance in Rhizobium meliloti. Appl. Environ. Microbiol. 1989, 55, 1870–1876.
- Karthika, S.; Varghese, S.; Jisha, M.S. Exploring the efficacy of antagonistic rhizobacteria as native biocontrol agents against tomato plant diseases. 3 Biotech 2020, 10, 320.
- Kenawy, A.; Dailin, D.J.; Abo-Zaid, G.A.; Malek, R.A.; Ambehabati, K.K.; Zakaria, K.H.N.; Sayyed, R.Z.; El Enshasy, H.A. Biosynthesis of Antibiotics by PGPR and Their Roles in Biocontrol of Plant Diseases. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Sayyed, R.Z., Ed.; Microorganisms for Sustainability; Springer: Singapore, 2019; Volume 13, pp. 1–35.
- Breil, B.; Borneman, J.; Triplett, E.W. A newly discovered gene, tfuA, involved in the production of the ribosomally synthesized peptide antibiotic trifolitoxin. J. Bacteriol. 1996, 178, 4150–4156.
- Chakraborty, U.; Purkayastha, R.P. Role of rhizobitoxine in protecting soybean roots from Macrophomina phaseolina infection. Can. J. Microbiol. 1984, 30, 285–289.
- Díaz-Valle, A.; López-Calleja, A.C.; Alvarez-Venegas, R. Enhancement of Pathogen Resistance in Common Bean Plants by Inoculation with Rhizobium etli. Front. Plant Sci. 2019, 10, 1317.
- Elbadry, M.; Taha, R.M.; Eldougdoug, K.A.; Gamal-Eldin, H. Induction of systemic resistance in faba bean (Vicia faba L.) to bean yellow mosaic potyvirus (BYMV) via seed bacterization with plant growth promoting rhizobacteria. J. Plant Dis. Prot. 2006, 113, 247–251.
- Dutta, S.; Mishra, A.; Kumar, B.D. Induction of systemic resistance against fusarial wilt in pigeon pea through interaction of plant growth promoting rhizobacteria and rhizobia. Soil Biol. Biochem. 2008, 40, 452–461.
- Kumar, H.; Bajpai, V.K.; Dubey, R.; Maheshwari, D.; Kang, S.C. Wilt disease management and enhancement of growth and yield of Cajanus cajan (L) var. Manak by bacterial combinations amended with chemical fertilizer. Crop. Prot. 2010, 29, 591–598.
- Deshwal, V.K.; Dubey, R.C.; Maheshwari, D.K. Isolation of plant growth-promoting strains of Bradyrhizobium (Arachis) sp. with biocontrol potential against Macrophomina phaseolina causing charcoal rot of peanut. Curr. Sci. 2023, 84, 3.
- Abd El-Rahman, A.F.; Shaheen, H.A.; Abd El-Aziz, R.M.; Ibrahim, D.S.S. Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne incognita. Egypt. J. Biol. Pest Control 2019, 29, 41.
- Jan, B.; Sajad, S.; Reshi, Z.A.; Mohiddin, F.A. Plant Growth Promoting Rhizobacteria (PGPR): Eco-Friendly Approach for Sustainable Agriculture. In Plant-Microbe Dynamics: Recent Advances for Sustainable Agriculture, 1st ed.; Pirzadah, T.B., Malik, B., Hakeem, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 185–200.
- Mishra, J.; Arora, N.K. Secondary metabolites of fluorescent pseudomonads in biocontrol of phytopathogens for sustainable agriculture. Appl. Soil Ecol. 2018, 125, 35–45.




