1.2. Phosphate Solubilization
After nitrogen, phosphorus is considered as the second limiting macronutrient for plant growth and development
[9][60]. It has the main role in plant’s molecular biology, and it does not just enter in the formation of macromolecules, such as DNA, RNA, ATP, but also in cell division, the formation of new tissue, and energy transfers by plants
[10][61].
Despite its abundant presence in soils, phosphorus accessibility is relatively limited due to the fact that the majority of phosphorus in soils exists in insoluble forms (inorganic bound, fixed or labile, or organic bound)
[11][62].
It has been found by Vargas et al.
[8][24] that only less than 5% of soil phosphorus is available for uptake by plants
[8][24]. The only two chemical forms of (P) that can be absorbed by plants are monobasic (HPO
4−) and dibasic (HPO
42−) ions
[12][21].
One of the most important PGPR traits is their ability to convert phosphates from insoluble to soluble forms. Microbes that are considered as the most efficient phosphate solubilizers are rhizobia, including
R. leguminosarum,
R. meliloti,
M. mediterraneum,
Bradyrhizobium sp., and
B. japonicum [2][54], along with
Bacillus,
Pseudomonas, and some fungi, such as
Aspergillus and
Penicillium [13][63].
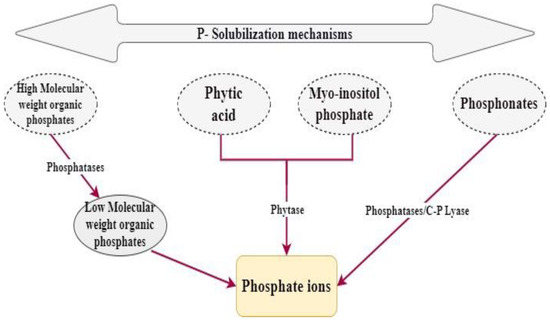
Numerous mechanisms of action are involved in the P solubilization activity of PGPR (
Figure 2), and these depend on the type of P available on soil, regarding whether it is organic or inorganic P.
Figure 2.
Mechanism of phosphate solubilization by microorganisms.
Organic P immobilization can be achieved by multiple microbial enzymes, such as phytase, phosphohydrolase, and phosphonatase. These enzymes provoke the lysis of many organic O compounds present freely in soil. Hence, the release of phosphate ions that are ready for the assimilation occurs
[14][64].
1.3. Phytohormones Production/Regulation (Plant Growth Regulators)
Plant hormones, also known as phytohormones, are natural organic compounds produced by plants, and they play the role of chemical messengers that influence plant growth and its interaction with the environment.
It has been proved by many studies that phytohormone production by several bacteria is one of the most interesting and important mechanisms of plant growth promotion by bacteria
[16][17][18][49,65,66].
There are five main groups of hormones: auxins, gibberellins, cytokinins, ethylene, and abscisic acid
[19][67].
Auxins are powerful molecules that are consistently synthesized by plants
[4][56]. They are known as key regulators of cell division stimulation and elongation
[8][24], as well as abiotic stress control
[4][56].
It is well known that PGPR play major role in the enhancement of the plant physiology, differentiation, expansion, and cell division by their ability to produce auxins and, particularly, Indole Acetic Acid (IAA)
[4][56]. Over 80% of rhizosphere bacteria, such as
Azospirillum,
Pseudomonas,
Klebsiella,
Rhizobium,
Mesorhizobium,
Bradyrhizobium,
Paenibacillus, and
Bacillus, actively produce and release auxins
[8][20][24,68].
IAA produced by PGPR is a signaling molecule included in the bacteria’s physiology, with a main role in direct plant growth promotion
[10][61], which is performed by controlling the root initiation and morphogenesis, cell elongation and differentiation, flowering, apical dominance, and many more plant development processes
[21][16].
The IAA produced by rhizobacteria has the biggest effect on the roots. It was observed that the post-inoculation by PGPR improved the plant’s nutrition and soil exchanges
[22][20]. The amount of IAA produced by rhizobia depends on the plant flavonoids and phenolic acids released in the rhizosphere, such as protocatechuic acid, 4-hydroxybenzaldehyde, and p-coumaric acid
[23][37].
Rhizobia producing IAA might include several pathways of IAA biosynthesis
[22][20]. Therefore, the bacterial effect on the plant is mainly influenced according to the pathway chosen
[24][30]. It is known that the main precursor for IAA production is tryptophan. Tryptophan-dependent pathways are identified as five different routes, such as indo-3-pyruvate (IPyA), tryptophan side-chain oxidase (TSO), indole-3-acetamide (IAM), which is related to pathogenic bacteria, tryptamine (TAM), and indole-3-acetonitrile (IAN)
[13][63].
Tryptophan-independent pathways are still less known. However, it has been reported that
Azospirillum brasilense is capable of producing 10% of IAA using tryptophan-dependent pathways, while 90% of IAA is produced without including the tryptophan as precursor
[22][20]. Additionally, in spite of the fact that the tryptophan production was blocked, maize mutants showed a high level of IAA produced, which confirmed the tryptophan-independent path
[8][24].
The production of IAA by rhizobia is regulated by specific genes. The biosynthesis of IAA in rhizobia primarily involves the indole-3-pyruvate pathway (IPyA), which has been identified in
Bradyrhizobium,
Rhizobium, and
Azospirillum [18][25][66,69].
The genes encoding for the two enzymes are, respectively,
iaaM and
iaaH, and they were identified in
Rhizobium sp. and
Bradyrhizobium sp.
[18][66].
Cytokinins stimulate cell division and enlargement, shoot and root elongation, root hair formation, leaf expansion, and chlorophyll accumulation during plant development
[26][70]. Moreover, recent studies have found that, in addition to the plant growth promotion and nutrient optimization by cytokinins, they can also be involved in plant defense responses and in delaying leaf senescence
[27][71].
Cytokinins are purine derivatives, and the most abundant CKs are adenine-type, with a replacement in the N6 position, either with an isoprenoid or with an aromatic side chain
[22][20]. Some of the well-known CKs are trans-zeatin (6-(4-hydroxy-3-methyl-trans-2-butenylamino) purine), i6Ade (6-(3-methyl-2-butenylamino) purine), dihydrozeatin (6-(4-hydroxy-3-methyl-butylamino) purine), and cis-zeatin (6-(4-hydroxy3-methyl-cis-2-butenylamino) purine)
[28][72].
Compared to the auxins, cytokinins are usually present in small amounts, and their production by PGPR was less identified due to the limitations of their quantification methods
[29][73].
In spite of their identification restrictions, it has been found that CKs can be released by almost 90% of the rhizospheric microorganisms cultured in vitro
[2][54]. When tested in-vitro, several plants associated microorganisms, e.g.,
Rhizobium sp.,
Azotobacter sp.,
Pantoea agglomerans,
Rhodospirillum rubrum,
Pseudomonas fluorescens,
Bacillus subtilis, and
Paenibacillus polymyxa, showed the production of cytokinin, together with some other growth promoting substances
[24][30].
Gibberellins encompass a vast category of phytohormones, consisting of 136 distinct molecules. Among these, one hundred and twenty-eight are derived from plants, seven are derived from fungi, while a mere four gibberellins (GA1, GA3, GA4, GA20) have been attributed to bacterial sources
[22][20].
Just as with the auxins and cytokinins, gibberellins are a group of phytohormones that is associated with many plant mechanisms, namely, seed germination, flowering, seed dormancy regulation, ripening of fruit, root growth promotion, and abundance of root hair. However, to date, there is no known function for gibberellins in fungi and bacteria
[4][56].
Gibberellins are composed of a complex tetracarbocyclic diterpenes molecules that consist of a skeleton of 19–20 carbon atoms as a common structure
[22][20].
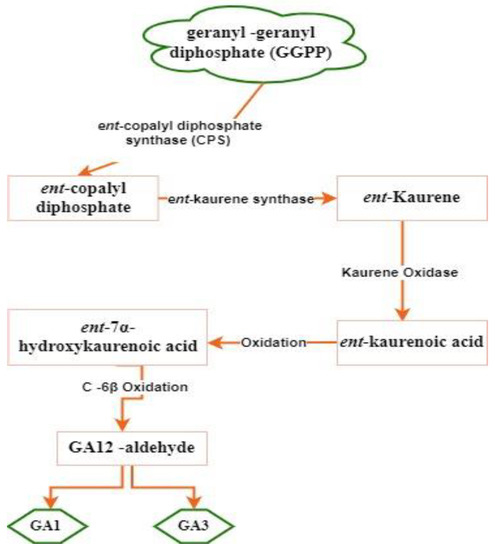
The exact mechanism of gibberellic acid (GA) synthesis within nodules is complex and can vary, depending on the specific host plant and associated symbiotic microorganisms. In general, the synthesis of GA within nodules involves a series of enzymatic reactions (
Figure 3).
Figure 3.
GA synthesis mechanism in nodules of
Mesorhizobium loti through an enzymatic synthesis pathway [30]. through an enzymatic synthesis pathway [77].
Abscisic acid is a terpene hormone class that consists of three isoprene units, also called sesquiterpenes. Abscisic acid acts especially as a plant growth inhibitor. For instance, ABA plays an essential role in senescence processes, inducing seed dormancy, stomatal closure, and stimulating proteins storage inside the seeds during dormancy
[2][54]. The importance of ABA in the rhizosphere appeared under abiotic stress, such as drought stress and freezing temperatures. Its production is accentuated under these conditions in order to regulate plant capacity to survive in harsh environment
[4][56].
The production of abscisic acid is carried out in several parts of the plant, primarily in the leaves, stems, seeds and fruits, as well as partially in the chloroplast
[4][56]. However, its production seems to be different from one plant organ to another and during certain specific phases (ABA).
Abscisic acid has been detected using TLC or radio-immunoassay as a product of many PGPRs, mainly
Rhizobium sp.,
B. japonicum,
Azospirillum sp.,
Achromobacter xylosoxidans,
Bacillus pumilus, and
Lysinibacillus halotolerans [31][32][80,81].
Just as with all other plant regulators, ethylene is also considered as an essential phytohormone for plant growth and development. Out of all phytohormones, ethylene is the only one in the gaseous state
[11][62].
At low concentrations, ethylene plays a role in plant growth by regulating many physiological responses in plant, including seed germination stimulation, adventitious roots and root hair promotion, as well as breaking seed dormancy
[4][56]. However, high ethylene concentrations provoke the inhibition of the root elongation process, premature senescence, and abscission. Moreover, the nodules’ formation process in leguminous plants, along with symbiotic N
2 fixation, are inhibited
[21][16].
Ethylene could be produced by many bacterial species along with the aminocyclopropane-1-carboxylate (ACC) deaminase, which is the direct precursor of ethylene biosynthesis in plants, and consequently, plant growth promoting rhizobacteria play a key role in adjusting/lowering the levels of ethylene in plants
[29][73].
1.4. Siderophores Production
Iron is a crucial micronutrient for all life forms and also is abundant in the lithosphere, being the fourth most common element in earth crust by weight
[4][56]. In plants, iron is indispensable for chlorophyll synthesis and biosynthesis, it is involved in DNA synthesis, and it has a key role in electron transport, redox reaction, detoxification of oxygen radicals, and many more biochemical processes
[24][30].
Despite its huge abundance, iron is not accessible to plants due to its presence under insoluble forms of hydroxides [Fe(OH)
3] and oxyhydroxides [FeO(OH)]
[33][83], while the plant root tends to absorb iron under its reduced form (ferrous Fe
2+)
[11][62].
Siderophores released by several rhizobacteria play key roles in overcoming this situation and increase the iron accessibility for plants. Siderophores are of low molecular weight (400–1000 Da), water soluble, and iron chelating molecules with high affinity for the ferric form Fe
3⁺ (Kd = 10
−20–10
−50)
[27][71]. These siderophores have a high affinity, which facilitates the sequestration and transportation of iron into the cells and, thus, enhances plant growth
[2][54]. Microbial siderophores, which are regulated by a specific
sid gene
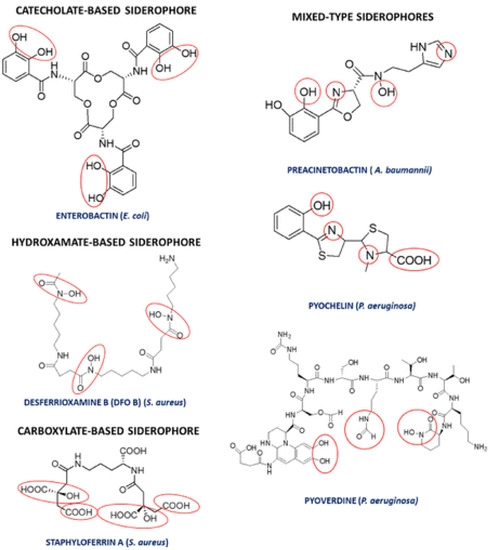
[13][63], belong mainly to four classes, viz. carboxylates, hydroxamates, phenol catecholates, and pyoverdines, depending on their structure, iron correlating functional groups, and types of ligands
[24][30]. Moreover, there are some siderophores called mixed siderophores, which result from a combination of structures of two main classes, as depicted in
Figure 4 [20][68].
Figure 4. Classification of siderophores based on their chemical structures. The metal-binding subunits are circled in red. Reprinted with permission from Kircheva, Nikoleta, and Todor Dudev. “Gallium as an antibacterial agent: a DFT/SMD study of the Ga
3+/Fe
3+ competition for binding bacterial siderophores”. Inorganic Chemistry 59.9 (2020): 6242–6254. Copyright 2023 American Chemical Society
[34][84].
Rhizobial siderophores have been considered mainly as chelators that enhance iron nutrition and plant growth. Siderophore-producing bacteria are identified as efficient biocontrol agents (BCAs), and they have been involved indirectly in the inhibition of pathogen development through their Fe
3⁺ ion-binding ability, which limits iron availability to plant pathogens and fungi that are not able to assimilate the iron–siderophore complex, thus preventing their growth
[22][35][20,85].
Moreover, siderophores can also improve plant growth in contaminated soils. In the iron uptake process, several heavy metals, such as aluminum, cadmium, copper, lead, and zinc, as well as with radionuclides, including uranium, can interfere and cause a toxic effect
[36][87]. Siderophores have binding ability and form a complex siderophore–metal, which increases the concentration of soluble metal. Consequently, bacterial siderophores help to alleviate the stresses imposed on plants by heavy metal-contaminated soils
[24][30].
Roy and Chakrabartty
[37][88] studied the production of siderophores by
Rhizobium sp. under high concentration of Al
3+. Apart from enhancing iron availability, siderophores produced by rhizobia also have the capability to form complexes with Al
3+, thereby mitigating its toxicity. Rogers et al.
[38][89] observed analogous findings that demonstrated the efficacy of vicibactin, a hydroxamate siderophore, produced by
R. leguminosarum bv.
viciae, in alleviating aluminum toxicity. The complex has the potential to be transported into the bacterial cytoplasm. Nevertheless, it is unlikely to cause toxicity within the intracellular environment, as aluminum cannot be released from the complex through reduction. Furthermore, the complex accumulates as a non-toxic molecule, and, even if it is released, Al
3+ will precipitate as Al(OH)
3 at the slightly alkaline pH of the cytoplasm
[38][39][89,90].
2. Indirect Mechanism
2.1. Antibiotics Synthesis
PGPRs play a vital role in plant protection. One of the fundamental methods of PGPR as biocontrol agents is the production of antibiotics. Antibiotics are antagonistic compounds produced by microorganisms against phytopathogens
[40][91].
Antibiotics synthetized by PGPR comprise a diverse group of low-molecular-weight organic substances that negatively impact the growth or metabolic activities of other microorganisms. They are also considered to have antiviral, cytotoxic, insecticidal, anthelmintic, and phytotoxic effects and are produced due to the interaction between microorganisms in order to survive under competition or predation
[35][85].
For biological control, the widely known antibiotics are 2,4 diacetylphologlucinol (DAPG), phenazine, pyoluteorin, pyrrolnitrin, tropolone, tensin, oomycin A, cyclic lipopeptides (all of which are diffusible), and hydrogen cyanide (HCN, which is volatile)
[40][91].
Numerous microorganisms are able to produce different extracellular metabolites with inhibitory actions even at low concentrations, in particular, PGPR (
Bacillus sp.) participate in the suppression of phytopathogenic microorganisms by producing several antibiotics, such as mycosubtilin, bacillomycin D surfactin, and fengycin, while antibiotics produced by fluorescent
Pseudomonas include pyoluteorin, phenazines, viscosin, and massetolide A
[41][92].
Many studies have proved the role of antibiotics produced by rhizobia in phytopathogen control.
R. leguminosarun bv.
trifolii T24 has been reported to release the peptide antibiotic trifolitoxin (TFX)
[42][93]. In another study, Chakraborty and Purkayastha
[43][94] showed the effective suppression of
M. phaseolina infecting soybean by the direct action of antibiotic rhizobitoxine produced by
Bradyrhizobium japonicum.
Rhizobium sp. strains ORN 24 and ORN 83 were considered as bacteriocin producers, with antagonistic activities against
Pseudomonas savastanoi, which is responsible for olive knot disease
[8][24]. Moreover, the growth and yield of
Brassica campestris were observed to be enhanced by the presence of
Mesorhizobium loti MP6, a bacterial strain isolated from the root nodules of
Mimosa pudica. Moreover,
Mesorhizobium loti MP6 isolate demonstrated significant antagonistic properties against
Sclerotinia sclerotiorum, a pathogen known for inducing white rot in Brassica campestris. Importantly, a prolonged incubation period resulted in a remarkable 75% inhibition of
S. sclerotiorum growth.
Antibiotic production is closely related to the metabolic status of the cell, which is also linked to nutrient availability, as well as environmental stimuli, including minerals, pH, temperature, and trace elements, particularly zinc levels (Zn), which may influence the genetic stability of bacteria, which can impact their capacity to synthetize secondary metabolites
[11][62]. The mechanism of action of bacterial antibiotics is to cause membrane damages by inhibiting the synthesis of pathogen cell walls, which consequently influence the cell membrane structures
[24][30]. For instance,
Rhizobium spp.,
Azospirillum spp.,
Klebsiella pneumoniae,
Yersinia spp., and
Frankia spp. were found to possess a pectinolytic ability
[4][56].
In addition to the use of microbial antagonists against phytopathogens in agricultural crops as an alternative to chemical pesticides
[22][20], certain antibiotics that are synthesized by PGPR are now being investigated for their potential applications in experimental pharmaceuticals. This emerging area of research holds promise in discovering new compounds to combat the issues arising from multidrug-resistant pathogenic bacteria
[11][62].
2.2. Induction of Systemic Resistance
Including their plant growth promoting ability, PGPR are also capable of enhancing the defensive system in their host plant against a wide range of phytopathogens—for instance, fungi, pathogenic bacteria, viruses, or, also, in some cases, insects and nematodes, naturally existing in soil
[11][35][62,85].
Plant-induced resistance is classified into two major phenomena, Systemic Acquired Resistance (SAR) and Induced Systemic Resistance (ISR), which can be distinguished based on the nature of the stimulant, as well as the regulatory mechanism implicated
[24][30].
Systemic Acquired Resistance (SAR) is a defense mechanism that can be induced by the presence of a broad range of either pathogenic or non-pathogenic microorganisms or even chemicals accumulated in the rhizosphere, including salicylic acid (SA), 2,6-dichloro-isonicotinic acid (INA), and S-methyl ester (BTH)
[10][61]. Various genes encoding for pathogenic-related proteins (chitinase and glucanase) are involved in salicylic acid signal transduction
[24][30].
Instead of requiring the pathogenic-related proteins or salicylic acid accumulation, ISR depends on jasmonic acid (JA) and ethylene signaling pathways
[24][30]. Similar to SAR, the ISR defense mechanism is also induced against several types of elicitors. However, it does not generate noticeable symptoms on the PGPR host plant
[11][62].
Many studies have reported the ability to induce systemic resistance in plants by rhizobial species, such as
R. etli, R. leguminosarum bv.
phaseoli, and
R. leguminosarum bv.
trifolii [2][54]. Díaz-Valle et al.
[44][18] studied the inoculation of common bean with
Rhizobium etli which as a result stimulated the plant resistance to infection by
Pseudomonas syringae pv. phaseolicola through the activation of defense related genes.
Elbadry et al.
[45][95] inoculated Faba bean (
Vicia faba L.) with
Pseudomonas fluorescens FB11 and
Rhizobium leguminosarum bv.
viceae FBG05 to study their systemic resistance induction to bean yellow mosaic potyvirus (BYMV). A significant drop in virus concentration, as well as a remarkable decrease in the Percent Disease Incident (PDI), were proved in the inoculated plants. The association of PGPR strains with
Rhizobium evaluated by Dutta et al.
[46][96] showed an optimistic result. When inoculated with a mixture of PGPR
B. cereus or
P. aeruginosa and
Rhizobium, pigeon pea (
Cajanus cajan) showed a high resistance level when exposed to the pathogenic
Fusarium udum compared to the individual elicitor and the non-inoculated control.
2.3. Production of Cell Wall-Degrading Enzymes
.2.3. Production of Cell Wall-Degrading Enzymes
Among the biocontrol mechanisms most used against soil borne pathogens, there is the production of cell wall-degrading enzymes
[22][20]. The release of lytic enzymes, such as β-1,3-glucanase, proteases, chitinases, lipase, or cellulase, result in the suppression of pathogens’ growth and activities by degrading their cell wall
[33][83].
A variety of PGPR are known for their ability to produce cell wall-degrading enzymes. For instance,
Paenibacillus spp. and
Streptomyces spp. strains were able to inhibit the development of
Fusarium oxysporum through the production of β-1,3-glucanase
[4][56]. Moreover, it is known that the pectinolytic activity is generally related to phytopathogenic bacteria. However, some non-pathogenic
Rhizobium species were also found to degrade pectin
[6][58]. Furthermore, Kumar et al.
[47][97] reported that
Sinorhizobium fredii KCC5 and
Pseudomonas fluorescens LPK2 were able to produce β-1,3-glucanase and chitinase, which caused the growth inhibition of
Fusarium udum.
2.4. Production of Hydrogen Cyanide (HCN)
Hydrogen cyanide is a secondary metabolite produced by many microorganisms, and it is a volatile compound that is known for its antimicrobial role and disease inhibition
[48][98]. In association with glycine (main precursor of HCN), HCN synthetase enzyme forms HCN. The latter most likely acts as an inhibitor of electron transport, which eventually causes the suppression of the energy supply chain and consequently affects microorganisms’ growth
[49][99].
Several bacterial genera have shown cyanogenesis ability (cyanide production), for instance,
Rhizobium,
Bacillus,
Alcaligenes,
Pseudomonas, and
Aeromonas [50][76]. However, fluorescent
Pseudomonas is preponderantly identified as an HCN producer
[51][100].