
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Brandon Lucke-Wold | -- | 2774 | 2023-08-09 22:18:45 | | | |
| 2 | Peter Tang | + 1 word(s) | 2775 | 2023-08-10 03:27:54 | | |
Video Upload Options
Spinal cord injury (SCI) is a profoundly debilitating yet common central nervous system condition resulting in significant morbidity and mortality rates. Major causes of SCI encompass traumatic incidences such as motor vehicle accidents, falls, and sports injuries. Treatment strategies for SCI aim to improve and enhance neurologic functionality. The ability for neural stem cells (NSCs) to differentiate into diverse neural and glial cell precursors has stimulated the investigation of stem cell scaffolds as potential therapeutics for SCI. Various scaffolding modalities including composite materials, natural polymers, synthetic polymers, and hydrogels have been explored.
1. Introduction
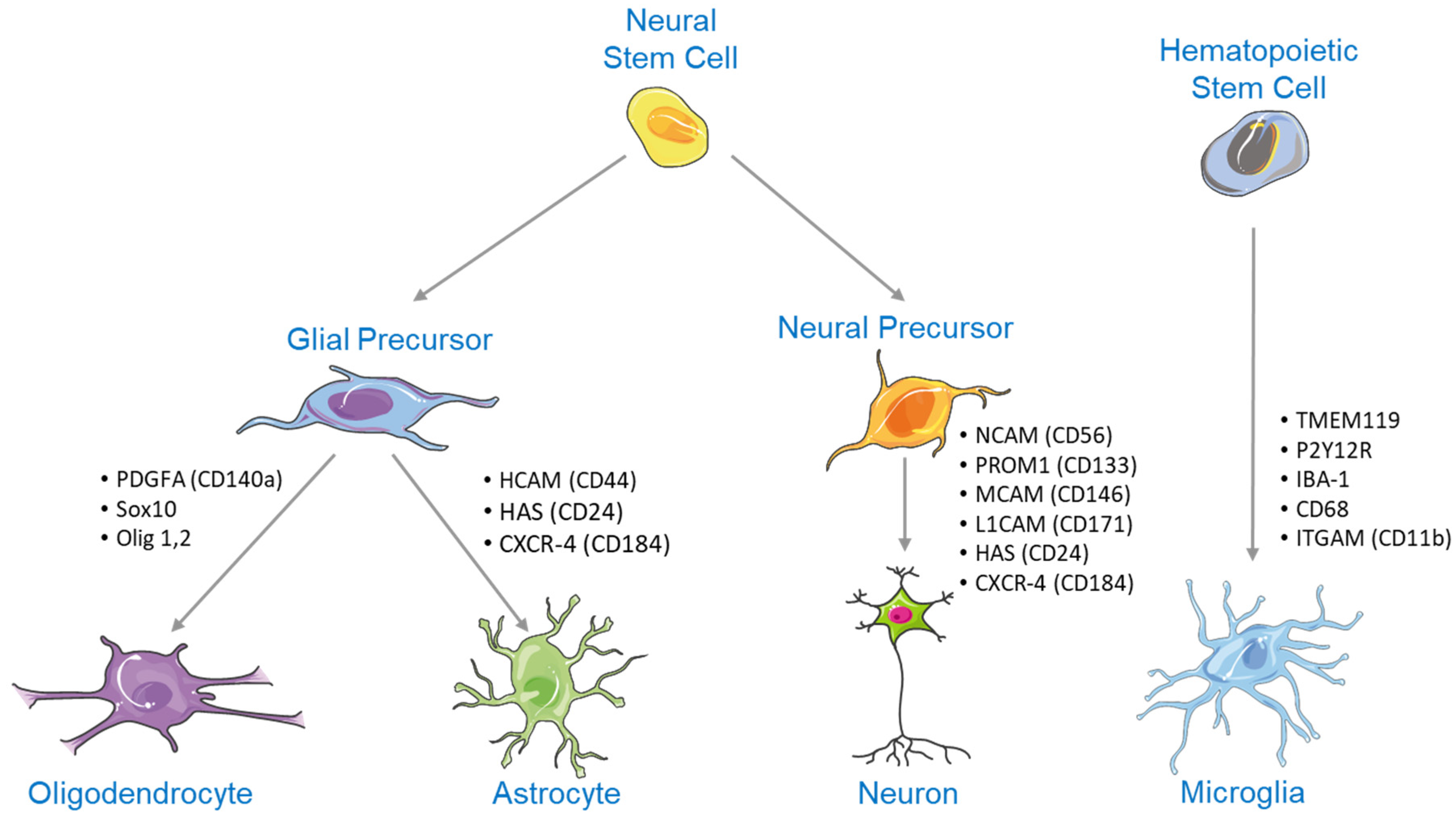
2. NSC Differentiation
3. Substrates Indicated for Axonal Regrowth Post-Injury
4. Overview of Stem Cell Scaffolding
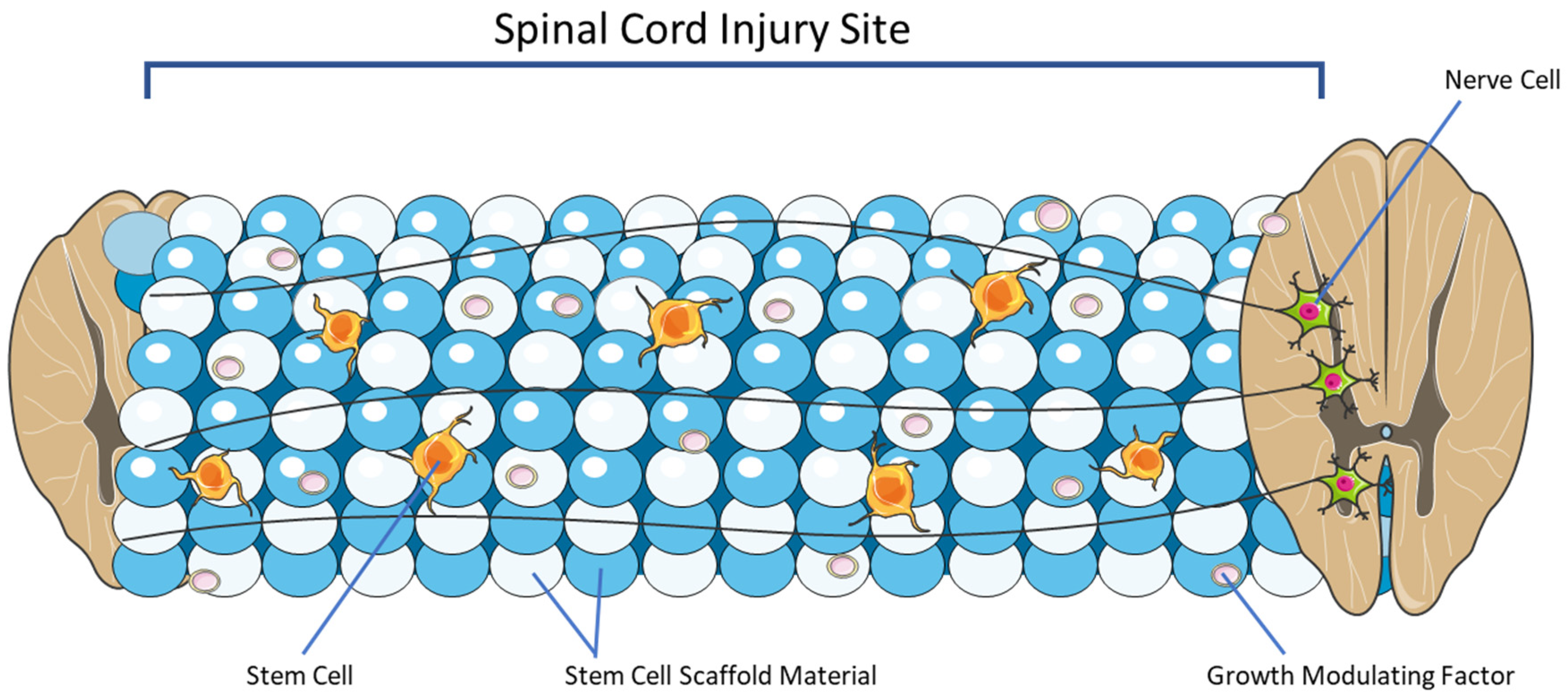
5. Emerging Pre-Clinical Studies and Their Applications for Clinical Adoption
|
Source |
Subject |
Stem Cell Type |
Scaffold Material |
Outcome |
|---|---|---|---|---|
|
Kourgiantaki et al. [35] |
C57/BL6 mice |
NSPCs |
Collagen |
Improved axonal elongation, neural regeneration at SCI lesions, enhanced NSPC differentiation, and functional integration of the regenerated cells into the preexisting neural network |
|
Liu et al. [37] |
Sprague-Dawley rats |
NSCs |
Collagen |
Improved hindlimb motor function, nerve regeneration, and neural cell extension |
|
Deng et al. [36] |
Sprague-Dawley rats and beagle canines |
MSCs |
Collagen |
Increased motor scores, reduced SCI lesions |
|
Deng et al. [36] |
Humans |
MSCs |
Collagen |
Emergence of novel nerve fiber growth, improved electrophysiological activity of neurons adjacent to the SCI lesion, increased daily life scores, increased American Spinal Injury Association scores, improved bladder and bowel functioning |
|
Tang et al. [42] |
Humans |
Bone marrow mononuclear cells and MSCs |
Collagen |
Improved bowel and bladder sensation, improved voluntary walking activity, enhanced finger mobility |
|
Liu et al. [34] |
Sprague-Dawley rats |
NSPCs |
Collagen modified with N-cadherin |
Increased NSPC recruitment to SCI lesion, improved locomotor activity |
|
Chen et al. [41] |
Sprague-Dawley rats |
MSCs |
Collagen modified with silk |
Improved nerve fiber regeneration, enhanced remyelination, establishment of novel synaptic connections at the SCI lesion |
|
Deng et al. [38] |
Beagle canines |
MSCs |
Collagen modified with heparan sulfate |
Improved locomotor activity, improved urodynamic parameters, modulation of cytokines |
|
Source |
Subject |
Stem Cell Type |
Scaffold Material |
Outcome |
|---|---|---|---|---|
|
Wang et al. [52] |
Sprague-Dawley rats |
NSCs |
Matrigel |
Slight neural recovery and improved motor function |
|
Li et al. [50] |
Sprague-Dawley rats |
MSCs |
Hyaluronic acid hydrogel with manganese dioxide nanoparticles |
Enhanced MSC growth and differentiation, restoration of locomotor function |
|
Abdolahi et al. [48] |
Sprague-Dawley rats |
NSCs |
PuraMatrix peptide hydrogel |
Enhance NSC survival and differentiation, reduced SCI lesion volume, improved neurologic functioning |
|
Yang et al. [51] |
C57/BL6 mice |
NSPCs |
Hydrogel enhanced with agarose, gelatin, and polypyrrole |
Enhanced NSPC differentiation, reduced SCI lesion volume |
|
He et al. [46] |
Sprague-Dawley rats |
MenSCs |
DSCG/GelMA hydrogel |
Improved motor function, reduced inflammation, enhanced MenSC differentiation |
|
Cai et al. [47] |
Sprague-Dawley rats |
NSCs |
GelMA-MXene hydrogel |
Improved motor function, reduced inflammation, enhanced NSC differentiation |
|
Shen et al. [49] |
C57/BL6 mice |
NSCs |
IL-10-enhanced hydrogel |
Enhanced NSC differentiation, neural regeneration, and axonal regrowth |
References
- Barbiellini Amidei, C.; Salmaso, L.; Bellio, S.; Saia, M. Epidemiology of traumatic spinal cord injury: A large population-based study. Spinal Cord 2022, 60, 812–819.
- Aarabi, B.; Albrecht, J.S.; Simard, J.M.; Chryssikos, T.; Schwartzbauer, G.; Sansur, C.A.; Crandall, K.; Gertner, M.; Howie, B.; Wessell, A.; et al. Trends in Demographics and Markers of Injury Severity in Traumatic Cervical Spinal Cord Injury. J. Neurotrauma 2021, 38, 756–764.
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282.
- Sterner, R.C.; Sterner, R.M. Immune response following traumatic spinal cord injury: Pathophysiology and therapies. Front. Immunol. 2022, 13, 1084101.
- Gumy, L.F.; Tan, C.L.; Fawcett, J.W. The role of local protein synthesis and degradation in axon regeneration. Exp. Neurol. 2010, 223, 28–37.
- Hendricks, B.K.; Shi, R. Mechanisms of neuronal membrane sealing following mechanical trauma. Neurosci. Bull. 2014, 30, 627–644.
- Hur, E.M.; Saijilafu; Zhou, F.Q. Growing the growth cone: Remodeling the cytoskeleton to promote axon regeneration. Trends Neurosci. 2012, 35, 164–174.
- Guthrie, S. Neurotrophic factors: Are they axon guidance molecules? Adv. Exp. Med. Biol. 2007, 621, 81–94.
- Fiani, B.; Arshad, M.A.; Shaikh, E.S.; Baig, A.; Farooqui, M.; Ayub, M.A.; Zafar, A.; Quadri, S.A. Current updates on various treatment approaches in the early management of acute spinal cord injury. Rev. Neurosci. 2021, 32, 513–530.
- Hsieh, Y.L.; Tay, J.; Hsu, S.H.; Chen, W.T.; Fang, Y.D.; Liew, C.Q.; Chou, E.H.; Wolfshohl, J.; d’Etienne, J.; Wang, C.H.; et al. Early versus Late Surgical Decompression for Traumatic Spinal Cord Injury on Neurological Recovery: A Systematic Review and Meta-Analysis. J. Neurotrauma 2021, 38, 2927–2936.
- Badhiwala, J.H.; Wilson, J.R.; Witiw, C.D.; Harrop, J.S.; Vaccaro, A.R.; Aarabi, B.; Grossman, R.G.; Geisler, F.H.; Fehlings, M.G. The influence of timing of surgical decompression for acute spinal cord injury: A pooled analysis of individual patient data. Lancet Neurol. 2021, 20, 117–126.
- Aarabi, B.; Akhtar-Danesh, N.; Chryssikos, T.; Shanmuganathan, K.; Schwartzbauer, G.T.; Simard, J.M.; Olexa, J.; Sansur, C.A.; Crandall, K.M.; Mushlin, H.; et al. Efficacy of Ultra-Early (<12 h), Early (12–24 h), and Late (>24–138.5 h) Surgery with Magnetic Resonance Imaging-Confirmed Decompression in American Spinal Injury Association Impairment Scale Grades A, B, and C Cervical Spinal Cord Injury. J. Neurotrauma 2020, 37, 448–457.
- Zarepour, A.; Hooshmand, S.; Gokmen, A.; Zarrabi, A.; Mostafavi, E. Spinal Cord Injury Management through the Combination of Stem Cells and Implantable 3D Bioprinted Platforms. Cells 2021, 10, 3189.
- Blando, S.; Anchesi, I.; Mazzon, E.; Gugliandolo, A. Can a Scaffold Enriched with Mesenchymal Stem Cells Be a Good Treatment for Spinal Cord Injury? Int. J. Mol. Sci. 2022, 23, 7545.
- Dorazco-Valdes, J. Estudio comparativo entre los aspectos clinicos, electroencefalograficos y la prueba del dibujo de la figura humana en el nino epileptico . J. Neurol. Sci. 1968, 6, 373–380.
- Valdoz, J.C.; Johnson, B.C.; Jacobs, D.J.; Franks, N.A.; Dodson, E.L.; Sanders, C.; Cribbs, C.G.; Van Ry, P.M. The ECM: To Scaffold, or Not to Scaffold, That Is the Question. Int. J. Mol. Sci. 2021, 22, 12690.
- Tang, Y.; Yu, P.; Cheng, L. Current progress in the derivation and therapeutic application of neural stem cells. Cell Death Dis. 2017, 8, e3108.
- Kaminska, A.; Radoszkiewicz, K.; Rybkowska, P.; Wedzinska, A.; Sarnowska, A. Interaction of Neural Stem Cells (NSCs) and Mesenchymal Stem Cells (MSCs) as a Promising Approach in Brain Study and Nerve Regeneration. Cells 2022, 11, 1464.
- de Vasconcelos, P.; Lacerda, J.F. Hematopoietic Stem Cell Transplantation for Neurological Disorders: A Focus on Inborn Errors of Metabolism. Front. Cell. Neurosci. 2022, 16, 895511.
- De Gioia, R.; Biella, F.; Citterio, G.; Rizzo, F.; Abati, E.; Nizzardo, M.; Bresolin, N.; Comi, G.P.; Corti, S. Neural Stem Cell Transplantation for Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 3103.
- Navarro Quiroz, E.; Navarro Quiroz, R.; Ahmad, M.; Gomez Escorcia, L.; Villarreal, J.L.; Fernandez Ponce, C.; Aroca Martinez, G. Cell Signaling in Neuronal Stem Cells. Cells 2018, 7, 75.
- Huebner, E.A.; Strittmatter, S.M. Axon regeneration in the peripheral and central nervous systems. In Results and Problems in Cell Differentiation; Springer: Berlin/Heidelberg, Germany, 2009; Volume 48, pp. 339–351.
- Abdollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A. The triad of nanotechnology, cell signalling, and scaffold implantation for the successful repair of damaged organs: An overview on soft-tissue engineering. J. Control. Release 2021, 332, 460–492.
- Golland, B.; Tipper, J.L.; Hall, R.M.; Tronci, G.; Russell, S.J. A Biomimetic Nonwoven-Reinforced Hydrogel for Spinal Cord Injury Repair. Polymers 2022, 14, 4376.
- Gao, C.; Li, Y.; Liu, X.; Huang, J.; Zhang, Z. 3D bioprinted conductive spinal cord biomimetic scaffolds for promoting neuronal differentiation of neural stem cells and repairing of spinal cord injury. Chem. Eng. J. 2023, 451, 138788.
- Zeng, X.; Zeng, Y.S.; Ma, Y.H.; Lu, L.Y.; Du, B.L.; Zhang, W.; Li, Y.; Chan, W.Y. Bone marrow mesenchymal stem cells in a three-dimensional gelatin sponge scaffold attenuate inflammation, promote angiogenesis, and reduce cavity formation in experimental spinal cord injury. Cell Transpl. 2011, 20, 1881–1899.
- Bruzauskaite, I.; Bironaite, D.; Bagdonas, E.; Bernotiene, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes-different cell effects. Cytotechnology 2016, 68, 355–369.
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491.
- Jia, G.; Huang, H.; Niu, J.; Chen, C.; Weng, J.; Yu, F.; Wang, D.; Kang, B.; Wang, T.; Yuan, G.; et al. Exploring the interconnectivity of biomimetic hierarchical porous Mg scaffolds for bone tissue engineering: Effects of pore size distribution on mechanical properties, degradation behavior and cell migration ability. J. Magnes. Alloy. 2021, 9, 1954–1966.
- Zhao, H.; Li, L.; Ding, S.; Liu, C.; Ai, J. Effect of porous structure and pore size on mechanical strength of 3D-printed comby scaffolds. Mater. Lett. 2018, 223, 21–24.
- Kim, H.Y.; Kim, H.N.; Lee, S.J.; Song, J.E.; Kwon, S.Y.; Chung, J.W.; Lee, D.; Khang, G. Effect of pore sizes of PLGA scaffolds on mechanical properties and cell behaviour for nucleus pulposus regeneration in vivo. J. Tissue Eng. Regen. Med. 2017, 11, 44–57.
- Liu, H.; Feng, Y.; Che, S.; Guan, L.; Yang, X.; Zhao, Y.; Fang, L.; Zvyagin, A.V.; Lin, Q. An Electroconductive Hydrogel Scaffold with Injectability and Biodegradability to Manipulate Neural Stem Cells for Enhancing Spinal Cord Injury Repair. Biomacromolecules 2023, 24, 86–97.
- Mneimneh, A.T.; Mehanna, M.M. Collagen-based scaffolds: An auspicious tool to support repair, recovery, and regeneration post spinal cord injury. Int. J. Pharm. 2021, 601, 120559.
- Liu, W.; Xu, B.; Xue, W.; Yang, B.; Fan, Y.; Chen, B.; Xiao, Z.; Xue, X.; Sun, Z.; Shu, M.; et al. A functional scaffold to promote the migration and neuronal differentiation of neural stem/progenitor cells for spinal cord injury repair. Biomaterials 2020, 243, 119941.
- Kourgiantaki, A.; Tzeranis, D.S.; Karali, K.; Georgelou, K.; Bampoula, E.; Psilodimitrakopoulos, S.; Yannas, I.V.; Stratakis, E.; Sidiropoulou, K.; Charalampopoulos, I.; et al. Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. NPJ Regen. Med. 2020, 5, 12.
- Deng, W.S.; Ma, K.; Liang, B.; Liu, X.Y.; Xu, H.Y.; Zhang, J.; Shi, H.Y.; Sun, H.T.; Chen, X.Y.; Zhang, S. Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regen. Res. 2020, 15, 1686–1700.
- Liu, S.; Xie, Y.Y.; Wang, L.D.; Tai, C.X.; Chen, D.; Mu, D.; Cui, Y.Y.; Wang, B. A multi-channel collagen scaffold loaded with neural stem cells for the repair of spinal cord injury. Neural Regen. Res. 2021, 16, 2284–2292.
- Deng, W.S.; Yang, K.; Liang, B.; Liu, Y.F.; Chen, X.Y.; Zhang, S. Collagen/heparin sulfate scaffold combined with mesenchymal stem cells treatment for canines with spinal cord injury: A pilot feasibility study. J. Orthop. Surg. 2021, 29, 23094990211012293.
- Deng, W.S.; Liu, X.Y.; Ma, K.; Liang, B.; Liu, Y.F.; Wang, R.J.; Chen, X.Y.; Zhang, S. Recovery of motor function in rats with complete spinal cord injury following implantation of collagen/silk fibroin scaffold combined with human umbilical cord-mesenchymal stem cells. Rev. Assoc. Med. Bras. 2021, 67, 1342–1348.
- Tang, F.; Tang, J.; Zhao, Y.; Zhang, J.; Xiao, Z.; Chen, B.; Han, G.; Yin, N.; Jiang, X.; Zhao, C.; et al. Long-term clinical observation of patients with acute and chronic complete spinal cord injury after transplantation of NeuroRegen scaffold. Sci. China Life Sci. 2022, 65, 909–926.
- Chen, C.; Xu, H.H.; Liu, X.Y.; Zhang, Y.S.; Zhong, L.; Wang, Y.W.; Xu, L.; Wei, P.; Chen, Y.X.; Liu, P.; et al. 3D printed collagen/silk fibroin scaffolds carrying the secretome of human umbilical mesenchymal stem cells ameliorated neurological dysfunction after spinal cord injury in rats. Regen. Biomater. 2022, 9, rbac014.
- Ortiz, A.C.; Fideles, S.O.M.; Pomini, K.T.; Bellini, M.Z.; Pereira, E.; Reis, C.H.B.; Pilon, J.P.G.; de Marchi, M.A.; Trazzi, B.F.M.; da Silva, W.S.; et al. Potential of Fibrin Glue and Mesenchymal Stem Cells (MSCs) to Regenerate Nerve Injuries: A Systematic Review. Cells 2022, 11, 221.
- Kirschenbaum, B.; Goldman, S.A. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc. Natl. Acad. Sci. USA 1995, 92, 210–214.
- DeBari, M.K.; King, C.I.; Altgold, T.A.; Abbott, R.D. Silk Fibroin as a Green Material. ACS Biomater. Sci. Eng. 2021, 7, 3530–3544.
- Shriver, Z.; Capila, I.; Venkataraman, G.; Sasisekharan, R. Heparin and heparan sulfate: Analyzing structure and microheterogeneity. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 207, pp. 159–176.
- He, W.; Zhang, X.; Li, X.; Ju, D.; Mao, T.; Lu, Y.; Gu, Y.; Qi, L.; Wang, Q.; Wu, Q.; et al. A decellularized spinal cord extracellular matrix-gel/GelMA hydrogel three-dimensional composite scaffold promotes recovery from spinal cord injury via synergism with human menstrual blood-derived stem cells. J. Mater. Chem. B 2022, 10, 5753–5764.
- Cai, J.; Zhang, H.; Hu, Y.; Huang, Z.; Wang, Y.; Xia, Y.; Chen, X.; Guo, J.; Cheng, H.; Xia, L.; et al. GelMA-MXene hydrogel nerve conduits with microgrooves for spinal cord injury repair. J. Nanobiotechnol. 2022, 20, 460.
- Abdolahi, S.; Aligholi, H.; Khodakaram-Tafti, A.; Khaleghi Ghadiri, M.; Stummer, W.; Gorji, A. Improvement of Rat Spinal Cord Injury Following Lentiviral Vector-Transduced Neural Stem/Progenitor Cells Derived from Human Epileptic Brain Tissue Transplantation with a Self-assembling Peptide Scaffold. Mol. Neurobiol. 2021, 58, 2481–2493.
- Shen, H.; Xu, B.; Yang, C.; Xue, W.; You, Z.; Wu, X.; Ma, D.; Shao, D.; Leong, K.; Dai, J. A DAMP-scavenging, IL-10-releasing hydrogel promotes neural regeneration and motor function recovery after spinal cord injury. Biomaterials 2022, 280, 121279.
- Li, L.; Xiao, B.; Mu, J.; Zhang, Y.; Zhang, C.; Cao, H.; Chen, R.; Patra, H.K.; Yang, B.; Feng, S.; et al. A MnO(2) Nanoparticle-Dotted Hydrogel Promotes Spinal Cord Repair via Regulating Reactive Oxygen Species Microenvironment and Synergizing with Mesenchymal Stem Cells. ACS Nano 2019, 13, 14283–14293.
- Yang, B.; Liang, C.; Chen, D.; Cheng, F.; Zhang, Y.; Wang, S.; Shu, J.; Huang, X.; Wang, J.; Xia, K.; et al. A conductive supramolecular hydrogel creates ideal endogenous niches to promote spinal cord injury repair. Bioact. Mater. 2022, 15, 103–119.
- Wang, J.; Chu, R.; Ni, N.; Nan, G. The effect of Matrigel as scaffold material for neural stem cell transplantation for treating spinal cord injury. Sci. Rep. 2020, 10, 2576.
- Liu, S.; Yang, H.; Chen, D.; Xie, Y.; Tai, C.; Wang, L.; Wang, P.; Wang, B. Three-dimensional bioprinting sodium alginate/gelatin scaffold combined with neural stem cells and oligodendrocytes markedly promoting nerve regeneration after spinal cord injury. Regen. Biomater. 2022, 9, rbac038.
- Koffler, J.; Zhu, W.; Qu, X.; Platoshyn, O.; Dulin, J.N.; Brock, J.; Graham, L.; Lu, P.; Sakamoto, J.; Marsala, M.; et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019, 25, 263–269.
- Shao, R.; Li, C.; Chen, Y.; Zhang, L.; Yang, H.; Zhang, Z.; Yue, J.; Gao, W.; Zhu, H.; Pan, H.; et al. LncRNA-GAS5 promotes spinal cord repair and the inhibition of neuronal apoptosis via the transplantation of 3D printed scaffold loaded with induced pluripotent stem cell-derived neural stem cells. Ann. Transl. Med. 2021, 9, 931.
- Liu, X.; Song, S.; Chen, Z.; Gao, C.; Li, Y.; Luo, Y.; Huang, J.; Zhang, Z. Release of O-GlcNAc transferase inhibitor promotes neuronal differentiation of neural stem cells in 3D bioprinted supramolecular hydrogel scaffold for spinal cord injury repair. Acta Biomater. 2022, 151, 148–162.




