Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Laura Clara Grandi | -- | 3976 | 2023-08-09 12:17:40 | | | |
| 2 | Rita Xu | Meta information modification | 3976 | 2023-08-10 03:37:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Grandi, L.C.; Bruni, S. Mirror-like Responses in Neurological and Psychiatric Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/47831 (accessed on 07 February 2026).
Grandi LC, Bruni S. Mirror-like Responses in Neurological and Psychiatric Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/47831. Accessed February 07, 2026.
Grandi, Laura Clara, Stefania Bruni. "Mirror-like Responses in Neurological and Psychiatric Diseases" Encyclopedia, https://encyclopedia.pub/entry/47831 (accessed February 07, 2026).
Grandi, L.C., & Bruni, S. (2023, August 09). Mirror-like Responses in Neurological and Psychiatric Diseases. In Encyclopedia. https://encyclopedia.pub/entry/47831
Grandi, Laura Clara and Stefania Bruni. "Mirror-like Responses in Neurological and Psychiatric Diseases." Encyclopedia. Web. 09 August, 2023.
Copy Citation
What is the significance of a touch encoded by slow-conducted unmyelinated C-tactile (CT) fibers? It is the so-called affiliative touch, which has a fundamental social impact. In humans, it has been demonstrated that the affiliative valence of this kind of touch is encoded by a dedicated central network, not involved in the encoding of discriminative touch, namely, the “social brain”. Moreover, CT-related touch has significant consequences on the human autonomic system, not present in the case of discriminative touch, which does not involve CT fibers as the modulation of vagal tone. In addition, CT-related touch provokes central effects as well.
social touch
CT fibers
mirror neurons
social brain
touch therapy
1. Introduction
Both humans and nonhuman primates exchange emotionally and socially relevant signals through touch [1]. Social touch helps create and maintain bonds among individuals of the same species and groups. For example, grooming is critical for building lasting and reassuring social bonds in nonhuman primates. About “grooming”, it is well established that there is a social function behind this behavior, compared with the more obvious one of cleaning, which explains why individuals spend more time grooming others than is necessary. This is even more significant if researchers consider that during this period, the level of vigilance of both groomers and groomed individuals is lower than it should be, implying that they may not react immediately against predators in the event of an attack [2][3][4].
Similarly, in humans, touch has a crucial role in interactions with the external world: It is the first sense to develop during ontogeny, [5], and it plays an early and pivotal role in social interactions [6]. Indeed, decreased exposure to social touch during development, either due to its unavailability (e.g., as in the case of preterm infants placed in incubators or of infants of mothers with postpartum depression) or to atypical touch perception (e.g., as might be in autism) has serious consequences for subsequent brain and cognitive development [7], such as a reduction in grey matter in adults [8][9].
In the last decade, it has been demonstrated that C-tactile (CT) unmyelinated afferents contribute to pleasant touch and provide the neurobiological substrate for interpersonal touch transmission [10][11]. These conclusions are supported by the data collected with different approaches, including brain imaging studies, studies carried out in animals, healthy human subjects, patients with a deficit in the CT system, analyses of autonomic and central effects elicited through CT activation, and behavioral studies. A growing body of literature supports the idea that the CT system belongs to the interoceptive system, which is known to be involved in encoding the emotional components of the touch. In humans, the “social brain” has been proposed as the neural network underlying the coding of CT-related touch. This circuit involves specific brain regions, including the posterior insular cortex, a critical cortical target for CT afferents. Interestingly, the same area seems involved in coding the observed caresses, but only if the touch is given at the optimal speed to activate the CT fibers [12]. However, investigating neural mechanisms for vicarious touch requires extending the study of the relevant neural network to include the putative mirror neuron system [7].
Considering the social role of the CT system, pleasant touch in psychiatric disorders characterized by a deficit in social behavior has also been explored. From the analysis of the literature, it can be inferred that there are a few disorders relevant to both mirror neurons and CT systems, such as autism spectrum disorder. Beyond this, a growing body of evidence suggests that a decreased activation of the mirror neuron system may be involved in the pathophysiology of psychiatric diseases characterized by social impairments, such as schizophrenia and psychopathy [13]. On the other hand, other psychiatric disorders mainly may involve the CT-fiber system [14] (e.g., anorexia nervosa, anxiety, and post-traumatic stress disorders).
2. CT Fibers: The Discovery
Neurophysiological and neuroanatomical studies have demonstrated that discriminative and affiliative types of touch are encoded in two different ways. Traditionally, research on touch has mostly focused on the class of receptors responsible for transducing information about pressure/vibration, temperature, itch, and pain, i.e., the discriminative system. In comparison, the affective system has been particularly studied in recent decades.
CT fibers were first identified in cats in 1939, and they have been found in different species and body sites ever since (i.e., mice, guinea pigs, rats, pigs, and nonhuman primates) [15][16][17][18][19]. As they were identified in humans only in 1988, for a long time, it was believed that these fibers had disappeared in humans for an evolutionary reason. Subsequently, they were found in the infraorbital nerve, the hairy side of the arm, and the legs [20][21]. Therefore, the idea that CT fibers were absent in humans due to being unnecessary, was overruled and then replaced by research on the skin and the importance of emotional valence of touch, as well as by evidence that some disorders (e.g., depression) have deficits in this social system. Researchers will deepen the discussion on this topic in the “Social Touch and Its Implication for Neurologic and Psychiatric Disorders” section. A critical year is 2010, when Morrison and colleagues [22] proposed the hypothesis “skin as a social organ”, which gives CT fibers the role of transmission and processing the social dimension of specific touch. Olausson [23] elaborated on the social touch hypothesis during the same period.
3. Properties of CT Fibers
Animal and human studies conducted with von Frey monofilaments have established the CT fibers’ physiological properties. Their receptive fields are round/oval and consist of one to nine small responsive spots distributed over an area up to 35 mm2 [20], and they respond to innocuous dynamic stimulation across the hairy skin surface [24][25]. The stimulus must be characterized by a force in the range of 0.22–2.5 mN and a velocity of 1–10 cm/s. Velocities lower than 1 cm/s or higher than 10 cm/s lead to a decrease in their firing frequency. This typical response to stimuli has the characteristic upside-down U-firing shape. Once activated, the fibers produce high-frequency trains of action potentials (50–100 impulses/s), with a peak rate of 100 impulses/s. A crucial feature to be considered is that CT fibers are unmyelinated, meaning they have slow conduction velocity (0.6–1.3 m/s) [24]. This aspect cannot be correlated with the discriminative aspect of the touch because researchers need fast speed in order to discriminate the features of the touched object. Indeed, Aβ fibers are myelinated and have these functions. By contrast, CT fibers are not myelinated.
4. Effects of CT Fibers on the Autonomous Nervous System
At the peripheral skin level, it has been demonstrated that CT stimulation evokes a sympathetic skin response. In studies in which skin resistance changes were registered with a constant current electrodermal recording device, using Ag–AgCl electrodes placed on the glabrous skin of the nondominant hand, CT activation was shown to trigger sympathetic arousal [26][27]. Moreover, it has been shown that the stimulation of CT fibers also modulates the level of stress hormones and positively modulates the parasympathetic system. The vagal tone is the activity of the vagus nerve, which is a fundamental component of the parasympathetic branch of the autonomic nervous system. The vagus nerve regulates the automatic systems, and therefore different situations, e.g., anxiety or stress. The vagal tone can be measured by heart rate variability, i.e., the balance between sympathetic and parasympathetic activity in the body in a resting state but also different conditions [28][29].
In humans, it has been demonstrated that among self-stroking, stroking, and being stroked by a partner, just the last condition has a significant autonomic effect, such as the decrement in heart rate [30], even though all of them are stimuli rated as pleasant. In this experiment, the stroke was applied with speed and pressure optimal for the modulation of CT fibers. No visual feedback was provided. Thus, participants could not see each other during the task. An interesting conclusion of the authors is that the absence of the role of the autonomic system during stroking could be due to the absence of visual feedback and because of the absence of CT fibers on the glabrous side of the palm. Moreover, both self-stroking and stroking are perceived as less pleasant than being stroked based on (1) the mechanisms to distinguish self-touch and externally produced touch and (2) the mechanism according to which researchers correctly predict the pleasantness of the self-touch [30].
A great effect of affective touch is a reduction in pain in healthy subjects [31] and in some diseases, such as chronic pain. Chronic pain is defined as pain for 3 consecutive months even after treatment [32]. Nowadays, the treatment combines pharmacological and physical rehabilitation, but the combination is often insufficient to resolve the problem. New therapies are necessary. Larissa L. Meijer and colleagues [33] reported the effect of affective touch on a 73-year-old woman with a chronic pain diagnosis. Affective touch was applied with a speed of around 3 cm/s, i.e., the optimal speed to modulate CT fibers, and nonaffective touch was applied with a speed of 18 cm/s, outside the range to modulate CT fibers. The patient reported that from the third to the seventh day, the pain disappeared. Importantly, this was not reported during the nonaffective touch treatment.
Another effect of affective touch is the modulation of hormones and neuropeptides. In chimpanzees, grooming with a bond partner increase the urinary secretion of oxytocin [34], supporting the fact that the affective touch can contribute to the production of such neuropeptide. On the other hand, several findings demonstrate that hormones and neuropeptides can regulate the production and perception of the social touch. It is known that oxytocin is one critical player in affiliative behaviors. Since 1980, research has highlighted the potent effect of this neuropeptide on social interactions in both humans and animals. Drago and colleagues [35] demonstrated that the intracerebroventricular injection of oxytocin induces an increment in grooming in rats. The authors have hypothesized that oxytocin increased affiliative behavior through central mechanisms, in particular through dopamine and opioid systems. Indeed, the opiate receptor antagonist naloxone and the dopamine antagonist haloperidol decreased or suppressed the grooming behavior, respectively. In a study involving female bonobos, oxytocin administration increased grooming behaviors, even if the authors of the study underline that interindividual differences are likely [36]. In humans, the administration of oxytocin may regulate the social evaluations of others after being touched [37]. In the same study, the authors demonstrated an interaction between touch and facial expressions, with angry faces negatively affecting the rating of touch pleasantness [37]. Another study demonstrated that the intranasal application of oxytocin to humans at a resting state induces an increment in heart rate variability, an index of the vagal tone [38].
Finally, another crucial aspect of social touch is the self/other touch distinction since it can be dysfunctional in some neurological diseases. Indeed, differences in the cortical and spinal modulation during self- and other touch have been investigated. Interestingly, researchers have found important differences in the modulation of the insula and the anterior cingulate cortex during these two conditions [39].
5. Social Touch and Mirror Neurons
In the last few years, several studies have tried to reveal neural mechanisms for perceiving and understanding social interactions [40]. Understanding the conspecific’s experiences is crucial for social behavior, and according to the mirror neuron theory, this understanding is accomplished by an internal simulation of other’s experiences we are observing [41].
Mirror neurons, discovered originally in monkey brains and later in human brains, were first described in a seminal paper in 1992 as a class of monkey premotor cells discharging during action execution and observation [42]. Rozzi and colleagues [43] recorded these neurons also from the cortex of the inferior parietal lobule of macaques. Subsequently, mirror-like neurons were found in different brain areas and animal species (rodents, birds, bats).
After the discovery of mirror neurons for body actions, it has been advanced the hypothesis that an analogous mechanism could be involved in the observation of tactile stimulations. Goldman and Gallese were the first to hypothesize a somatosensory mirror mechanism, which would allow observers to map the observed tactile stimulations onto their somatosensory system [44]. Subsequently, attesting to the social importance of touch, mirror neuron-type responses to observed touch have been reported within the same neural regions activating when the touch is experienced first-hand [45]. Several studies suggest that the posterior portion of the human secondary somatosensory cortex plays a key role in mirror-touch synesthesia [46]. Imaging studies (with functional magnetic resonance imaging) showed that observing another person being touched activates brain areas, such as the primary and secondary somatosensory areas and premotor cortex, which are normally activated when an individual’s body is touched [45].
Functionally, the somatosensory cortex has been linked to empathic ability [47], the recognition of emotional expressions [48], and the affective valence and intensity of the observed social touch, such as caressing and slapping someone else’s hand. Intriguingly, observing these last two stimuli more strongly activates primary and secondary somatosensory areas than the observation of a simple contact without affective connotation [49].
The insula cortex is also a region of interest in affective mechanisms [50]. Evidence on affective touch in humans suggests that the insular cortex could be an interface between exteroceptive and interoceptive perceptions of social touch during skin-to-skin communication. Morrison et al. [12] showed that the responses to dynamic stroking touch can be “velocity-tuned” and “socially specific”. They found that the posterior insula cortex was most activated for CT optimal velocity social stroking rather than non-CT optimal velocities or nonsocial dynamic touch. Evidence supports that individual differences in vicarious responses to touch exist due to personality traits or cognitive state [51][52]. Furthermore, the link between tactile experience and vicarious responding is tangible in patients carrying a heritable mutation, resulting in reduced C-fiber density. This, in turn, implies that these patients not only evaluate the directly experienced CT-related optimal touch as less pleasant [53] but also feel a smaller sense of gratification from observing the same touch compared with control subjects [11].
Empirical evidence shows that the social touch can affect the way we mentally represent our body, and it does this in a somatotopic manner [54]. In addition, the observation of tactile stimulation delivered to a virtual hand is associated with changes in brain activity related to vicarious somatosensation [55]. This last study shows how changes in body ownership can affect the neural activity of brain structures underlying vicarious somatosensation. It is noteworthy that, among those brain regions that changed their neural activity during the vicarious somatosensation, there was also the insula, which not only is the target of CT fibers but is also known to be involved in the processing of somatosensory aspects of altered body ownership [55].
Finally, it seems fair to say that the mirror mechanism allows a translation of the perceived action, regardless of whether we have seen, heard, or felt it, into the identical motor representation of the given action goal, resulting in an embodied link between two individuals [56]. The motor resonance mechanism of mirror neurons most likely represents the neural correlate of understanding others’ actions and intentions, which has been described as “embodied simulation” [57][58][59][60]. However, actions are not the unique experiences characterizing interpersonal relationships, which instead imply also sharing affective states, such as emotions and sensations. It is well known that the same brain structures involved in the experience of emotions and sensations are also activated when the same emotions and sensations are recognized in others. Several “mirroring” mechanisms exist in human brains and, thanks to the “intentional consonance” [61], these mechanisms allow us to experience intersubjective relationships and empathize with others.
Overall, several studies have described mirror neurons and mirror-like responses not only during the execution/observation of actions but also for many other kinds of stimuli, such as tactile stimulations, showing that merely viewing touch involves the observers’ somatosensory cortices. There is a large body of evidence that indicates that information about others’ actions, emotions, sensations, and communicative messages are mapped onto the same beholder’s neural substrates devoted to those first-person processes [62]. Therefore, it would appear that the mirror mechanism allows for a basic widespread remapping of other-related information belonging to a large variety of domains (especially social cognition and social behavior) onto primarily self-related brain structures. Notably, a few of these structures, which are involved in the processing of emotions, belong to the mirror network, and they are also targets of CT fibers.
6. Social Touch and Mirror Neurons
In the last few years, several studies have tried to reveal neural mechanisms for perceiving and understanding social interactions [40]. Understanding the conspecific’s experiences is crucial for social behavior, and according to the mirror neuron theory, this understanding is accomplished by an internal simulation of other’s experiences we are observing [41].
Overall, several studies have described mirror neurons and mirror-like responses not only during the execution/observation of actions but also for many other kinds of stimuli, such as tactile stimulations, showing that merely viewing touch involves the observers’ somatosensory cortices. There is a large body of evidence that indicates that information about others’ actions, emotions, sensations, and communicative messages are mapped onto the same beholder’s neural substrates devoted to those first-person processes [62]. Therefore, it would appear that the mirror mechanism allows for a basic widespread remapping of other-related information belonging to a large variety of domains (especially social cognition and social behavior) onto primarily self-related brain structures. Notably, a few of these structures, which are involved in the processing of emotions, belong to the mirror network, and they are also targets of CT fibers.
7. From Social Brain to Social Behavior
Neuroanatomical studies have been performed on areas where neurons with mirror properties have been found. Through those studies, researchers can identify two different mirror neuron networks: sensorimotor and emotional (Figure 1 A).
The network underlying affective touch (Figure 1B) includes the thalamus, the primary somatosensory cortex, the insular cortex, the medial prefrontal cortex, and the amygdala. Interestingly, the insular cortex is the cortical node of both mirror neurons and affective touch systems. Additionally, it is known that, in neurotypical individuals, CT optimal touch activates social brain networks, and they include, as researchers already underlined, the bilateral insula together with the parietal operculum, the orbitofrontal cortex, the primary somatosensory cortex, the posterior superior temporal sulcus, and the inferior frontal gyrus [63][64][65][66].
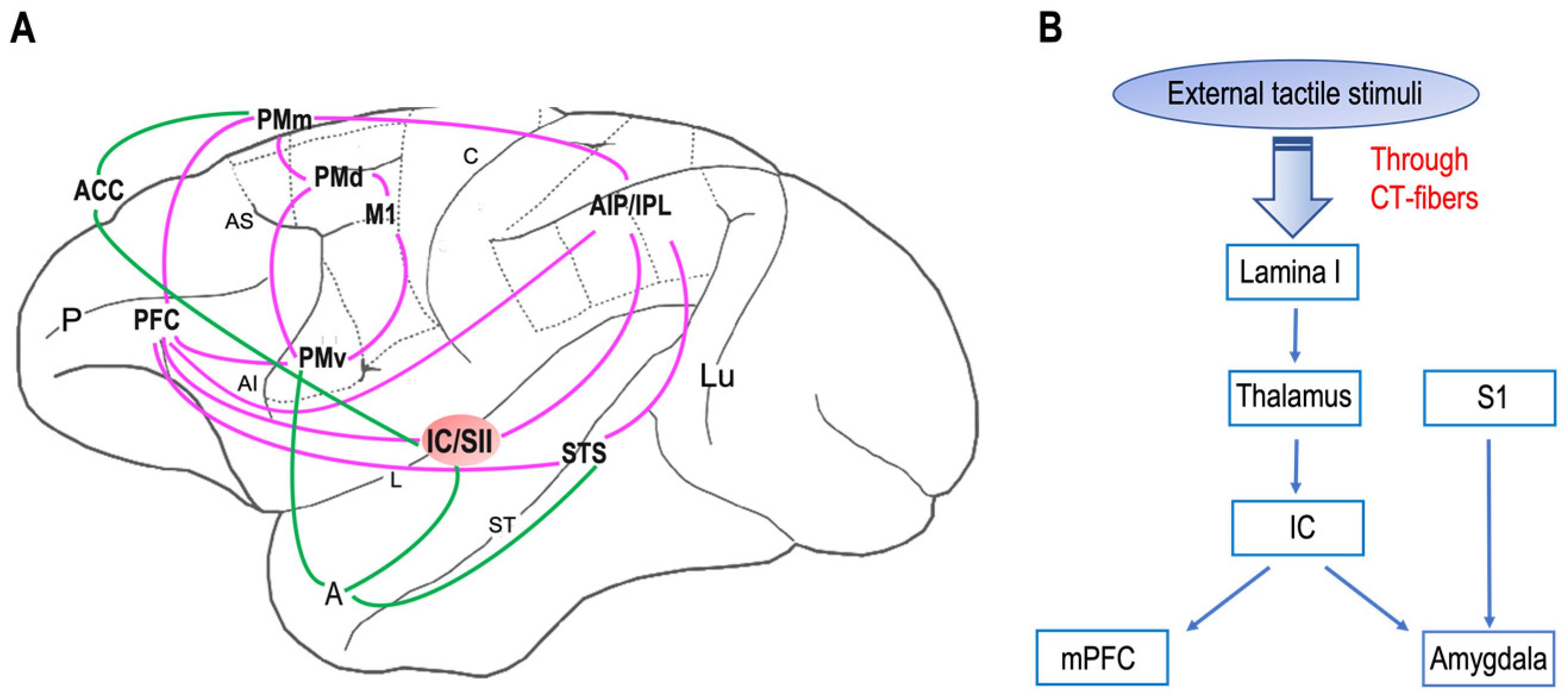
Figure 1. Neural networks of mirror neuron system and affective touch: (A) Organization of primate sensorimotor (purple) and emotional (green) mirror neuron networks based on macaque neuroanatomical studies on areas in which neurons with mirror properties have been found. The sensorimotor network includes, beyond the ventral premotor cortex (PMv) and the inferior parietal lobule (IPL), the primary motor cortex (M1), the dorsal premotor cortex (PMd), the mesial premotor cortex (PMm), the prefrontal cortex (PFC), the anterior intraparietal area (AIP), and the secondary somatosensory cortex (SII). The emotional network includes the anterior cingulate cortex (ACC), amygdala (A), and insula (IC). Abbreviations: P, principal sulcus; AI, arcuate inferior sulcus; arcuate superior sulcus; L, lateral sulcus; C, central sulcus; ST, superior temporal sulcus; Lu, lunatu. (B) The circuit of affective touch includes external tactile stimuli, which produce mechanical energy. This mechanical energy, received at the somatosensory nerve endings in the skin, is transduced and transmitted via CT fibers up through lamina I and to thalamic nuclei (i.e., ventromedial posterior nucleus, ventral posterior inferior nucleus) and processed at the insula (IC), before reaching the social centers in the cortex (e.g., medial prefrontal cortex, mPFC) and amygdala. Abbreviations: S1, primary somatosensory cortex.
8. Social Touch and Its Implication for Neurologic and Psychiatric Disorders
A strong body of evidence supports the finding that social touch is relevant to several neurological and psychiatric disorders since CT fibers’ dysfunction appears involved in these pathologies. For example, it has been demonstrated that, in patients displaying a congenitally reduced density of unmyelinated sensory fibers, the pIC does not activate when a slow and gentle touch is applied. This is most likely due to poor CT afferent inputs to the cortex [12].
Anorexia nervosa is an eating disorder characterized by abnormally low body weight, an intense fear of gaining weight, and a distorted perception of the body (overestimation of body size and shape). People with anorexia place a high value on controlling their weight and shape, using extreme efforts that tend to significantly interfere with their lives. Importantly, these patients have deficits in interoceptive processing and their internal physiological condition interpretation, creating and maintaining a mismatch between internal and external bodily sensations. Some studies have reported that anorexia nervosa patients perceive touch that activates CT fibers as less pleasant than control healthy individuals [67].
Croy and colleagues [67] investigated this topic in patients with one or more psychiatric disorders (somatoform disorders, post-traumatic stress disorders, anxiety, somatoform disorders, and mood and affective disorders). Patients reported significantly fewer interpersonal touch episodes compared with a healthy control group, regardless of whether they lived alone or not. Moreover, patients differed from the control group in terms of touch pleasantness but not regarding its awareness.
Finally, there are a few clinical conditions (e.g., autism spectrum disorder) related not only to CT fibers but also to the mirror neuron system.
Altogether, the data available supporting the fact that subjects with autism spectrum disorder have a typical theory of mind [68], suggest that the social impairments displayed by these people are more related to the inability to “experience” the socioemotional attributes of affective touch rather than the inability to “interpret” the experiences of others [69].
9. Touch as Therapy?
Human studies have shown that affiliative touch and massage could be useful in several clinical pictures. Recently, a meta-analysis on the effect of therapeutic massage on symptoms of Parkinson’s disease provided evidence that therapeutic massage could alleviate the typical motor symptoms of this illness, improving motor functions even though it did not ameliorate the quality of daily life of patients in comparison to healthy subjects [70]. In the reviewed studies in this meta-analysis, the applied massages were traditional Chinese Tuina, a common massage technique targeting the limbs of patients; acupressure; and Thai massage.
Recently, Tsuji and colleagues analyzed the salivary oxytocin concentration in children with a diagnosis of autism spectrum disorder when receiving a massage from their mothers [71].
Lately, mechanical affective touch therapy has been proposed as a useful treatment for controlling symptoms of anxiety disorders. The applied stimulation through mechanical affective touch therapy has CT fibers as its target. Electroencephalography studies revealed that the treatment also led to increased occipital theta and alpha oscillatory activity [72].
Preterm newborns represent another patient population for whom CT-related touch is increasingly used as a treatment. There is evidence that tactile stimulation improves the immune system in premature newborns [73].
The International Workshop on Kangaroo Mother Care, 2009, recommends the implementation of continuous kangaroo mother care as the gold standard pervading all medical and nursing care, based on empirical studies and clinical guidelines.
Altogether, these studies emphasize how several touch techniques (i.e., psychoactive massage therapy) have been introduced into different fields, from the treatment of psychiatric disorders (depression, anxiety, or psychosomatic disorders) to the treatment of chronic pain, such as in patients with cancer [74].
That said, researchers believe there are different motivations to encourage future investigations in academic medicine, clinical psychology, and basic research to take steps to make touch therapy attractive and accepted as an affective-oriented assessment and intervention, resulting in a more precise and standardized classification of clinical conditions affecting emotional processing.
10. Conclusions
Because of the critical role of social touch in human well-being and the evidence supporting its use as a treatment for both newborns and adults, more detailed studies are necessary. Indeed, investigating the correlation between each disorder and the corresponding CT dysfunction and/or distorted perception of affiliative touch should help to develop new treatments based on massage and CT-fiber stimulation in association with pharmacological therapies.
Ultimately, it would be useful to develop new therapies based on the protocols targeting CT fibers in order to alleviate symptoms in several clinical conditions. These treatments should improve not only behavioral symptoms, as seen in autistic children, but also the autonomic state. Importantly, new investigations might underline the possible effect of CT stimulation in pathological conditions in terms of circuit modulation in the “social brain”.
References
- Hertenstein, M.J.; Verkamp, J.M.; Kerestes, A.M.; Holmes, R.M. The communicative functions of touch in humans, nonhuman primates, and rats: A review and synthesis of the empirical research. Genet. Soc. Gen. Psychol. Monogr. 2006, 132, 5–94. Available online: https://www.depauw.edu/learn/lab/publications/documents/touch/2006_Touch_The%2520communicative_functions_of_touch_in_humans.pdf (accessed on 20 March 2023).
- Boccia, M.L. Comparison of the physical characteristics of grooming in two species of macaques (Macaca nemestrina and M. radiata). J. Comp. Psychol. 1989, 103, 177–183.
- Boccia, M.L.; Reite, M.; Laudenslager, M. On the physiology of grooming in a pigtail macaque. Physiol. Behav. 1989, 45, 667–670.
- Dunbar, R.I. The social role of touch in humans and primates: Behavioural function and neurobiological mechanisms. Neurosci. Biobehav. Rev. 2010, 34, 260–268.
- Gallace, A.; Spence, C. The science of interpersonal touch: An overview. Neurosci. Biobehav. Rev. 2010, 34, 246–259.
- Castiello, U.; Becchio, C.; Zoia, S.; Nelini, C.; Sartori, L.; Blason, L.; D’Ottavio, G.; Bulgheroni, M.; Gallese, V. Wired to be social: The ontogeny of human interaction. PLoS ONE 2010, 5, e13199.
- Gliga, T.; Farroni, T.; Cascio, C.J. Social touch: A new vista for developmental cognitive neuroscience? Dev. Cogn. Neurosci. 2019, 35, 1–4.
- Nelson, C.A.; Fox, N.A.; Zeanah, C.H. Romania’s Abandoned Children. Deprivation, Brain Development, and the Struggle for Recovery; Harvard University Press: Cambridge, UK, 2014.
- Cascio, C.J.; Cascio, D.; Moore, F. McGlone Social touch and human development Dev. Cogn. Neurosci. 2019, 35, 5–11.
- Kirsch, L.P.; Krahé, C.; Blom, N.; Crucianelli, L.; Moro, V.; Jenkinson, P.M.; Fotopoulou, A. Reading the mind in the touch: Neurophysiological specificity in the communication of emotions by touch. Neuropsychologia. 2018, 116, 136–149.
- Croy, I.; Luong, A.; Triscoli, C.; Hofmann, E.; Olausson, H.; Sailer, U. Interpersonal stroking touch is targeted to C tactile afferent activation. Behav. Brain Res. 2016, 297, 37–40.
- Morrison, I.; Bjornsdotter, M.; Olausson, H. Vicarious responses to social touch in posterior insular cortex are tuned to pleasant caressing speeds. J. Neurosci. 2011, 31, 554–9562.
- Jeon, H.; Lee, S.H. From Neurons to Social Beings: Short Review of the Mirror Neuron System Research and Its Socio-Psychological and Psychiatric Implications. Clin. Psychopharmacol. Neurosci. 2018, 16, 18–31.
- Croy, I.; Geide, H.; Paulus, M.; Weidner, K.; Olausson, H. Affective touch awareness in mental health and disease relates to autistic traits—An explorative neurophysiological investigation. Psychiatry Res. 2016, 245, 491–496.
- Douglas, W.W.; Ritchie, J.M. On the Frequency of Firing of Mammalian Nonmedullated Nerve Fibers. J. Physiol. 1957, 139, 400–407.
- Bessou, P.; Burgess, P.R.; Perl, E.R.; Taylor, C.B. Dynamic Properties of Mechanoreceptors with Unmyelinated (C) Fibers. J. Neurophysiol. 1971, 34, 116–131.
- Iggo, A.; Kornhuber, H.H. A Quantitative Study of C-Mechanoreceptors in Hairy Skin of the Cat. J. Physiol. 1977, 271, 549–565.
- Kumazawa, T.; Perl, E.R. Excitation of Marginal and Substantia Gelatinosa Neurons in the Primate Spinal Cord: Indications of Their Place in Dorsal Horn Functional Organization. J. Comp. Neurol. 1978, 177, 417–434.
- Johansson, R.S.; Trulsson, M.; Olsson, K.A.; Abbs, J.H. Mechanoreceptive afferent activity in the infraorbital nerve in man during speech and chewing movements. Exp. Brain Res. 1988, 72, 209–214.
- Nordin, M. Low-threshold mechanoreceptive and nociceptive units with unmyelinated (C) fibers in the human supraorbital nerve. J. Physiol. 1990, 426, 229–240.
- Vallbo, A.B.; Olausson, H.; Wessberg, J.; Kakuda, N. Receptive field characteristics of tactile units with myelinated afferents in hairy skin of human subjects. J. Physiol. 1995, 483, 783–79595.
- Morrison, I.; Löken, L.S.; Olausson, H. The skin as a social organ. Exp. Brain Res. 2010, 204, 305–314.
- Olausson, H.; Wessberg, J.; Morrison, I.; McGlone, F.; Vallbo, A.B. The Neurophysiology of Unmyelinated Tactile Afferents. Neurosci. Biobehav. Rev. 2010, 34, 185–191.
- Vallbo, A.B.; Olausson, H.; Wessberg, J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J. Neurophysiol. 1999, 81, 2753–2763.
- Wiklund Fernström, K.; Wessberg, J.; Vallbo, A. Temperature response of unmyelinated low-threshold mechanoreceptors (CT) in human hairy skin. Soc. Neurosci. Abstr. 2003, 29, 585.8.
- Olausson, H.; Cole, J.; Vallbo, A.B.; McGlone, F.; Elam, M.; Kramer, H.H.; Rylander, K.; Wessberg, J.; Elam, M.; Bushnell, M.C. Unmyelinated tactile afferents have opposite effects on insular and somatosensory cortical processing. Neurosci. Lett. 2008, 436, 128–132.
- Olausson, H.; Cole, J.; Rylander, K.; McGlone, F.; Lamarre, Y.; Gunnar Wallin, B.; Krämer, H.; Wessberg, J.; Elam, M.; Bushnell, M.C.; et al. Functional role of unmyelinated tactile afferents in human hairy skin: Sympathetic response and perceptual localization. Exp. Brain Res. 2008, 184, 135–140.
- Pomeranz, B.; Macaulay, R.J.; Caudill, M.A.; Kutz, I.; Adam, D.; Gordon, D.; Kilborn, K.M.; Barger, A.C.; Shannon, D.C.; Cohen, R.J.; et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am. J. Physiol. 1985, 248, H151–H153.
- Porges, S.W. Cardiac vagal tone: A physiological index of stress. Neurosci. Biobehav. Rev. 1995, 19, 225–233.
- Triscoli, C.; Croy, I.; Olausson, H.; Sailer, U. Touch between romantic partners: Being stroked is more pleasant than stroking and decelerates heart rate. Physiol. Behav. 2017, 177, 169–175.
- Savallampi, M.; Maallo, A.M.S.; Shaikh, S.; McGlone, F.; Bariguian-Revel, F.J.; Olausson, H.; Boehme, R. Social Touch Reduces Pain Perception—An Fmri Study of Cortical Mechanisms. Brain Sci. 2023, 13, 393.
- Merskey, H.; Bogduk, N. Classification of Chronic Pain, 2nd ed.; IASP Press: Seattle, WA, USA, 1994; p. 1.
- Meijer, L.L.; Ruis, C.; van der Smagt, M.J.; Dijkerman, H.C. Chronic pain relief after receiving affective touch: A single case report. J. Neuropsychol. 2023, 1–6.
- Pedersen, C.A.; Caldwell, J.D.; Drago, F.; Noonan, L.R.; Peterson, G.; Hood, L.E.; Prange, A.J., Jr. Grooming behavioral effects of oxytocin. Pharmacology, ontogeny, and comparisons with other nonapeptides. Ann. N. Y. Acad. Sci. 1988, 525, 245–256.
- Drago, F.; Pedersen, C.A.; Caldwell, J.D.; Prange, A.J., Jr. Oxytocin potently enhances novelty-induced grooming behavior in the rat. Brain Res. 1986, 368, 287–295.
- Brooks, J.; Kano, F.; Yeow, H.; Morimura, N.; Yamamoto, S. Testing the effect of oxytocin on social grooming in bonobos. Am. J. Primatol. 2022, 84, e23444.
- Ellingsen, D.M.; Wessberg, J.; Chelnokova, O.; Olausson, H.; Laeng, B.; Leknes, S. In touch with your emotions: Oxytocin and touch change social impressions while others’ facial expressions can alter touch. Psychoneuroendocrinology 2014, 39, 11–20.
- Kemp, A.H.; Quintana, D.S.; Kuhnert, R.L.; Griffiths, K.; Hickie, I.B.; Guastella, A.J. Oxytocin increases heart rate variability in humans at rest: Implications for social approach-related motivation and capacity for social engagement. PLoS ONE 2012, 7, e44014.
- Boehme, R.; Hauser, S.; Gerling, G.J.; Heilig, M.; Olausson, H. Distinction of self-produced touch and social touch at cortical and spinal cord levels. Proc. Natl. Acad. Sci. USA 2019, 116, 2290–2299.
- Cacioppo, J.T.; Decety, J. Social neuroscience: Challenges and opportunities in the study of complex behavior. Ann. N. Y. Acad. Sci. 2011, 1224, 162–173.
- Rizzolatti, G.; Fogassi, L.; Gallese, V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2001, 2, 661–670.
- di Pellegrino, G.; Fadiga, L.; Fogassi, L.; Gallese, V.; Rizzolatti, G. Understanding motor events: A neurophysiological study. Exp. Brain Res. 1992, 91, 176–180.
- Rozzi, S.; Ferrari, P.F.; Bonini, L.; Rizzolatti, G.; Fogassi, L. Functional organization of inferior parietal lobule convexity in the macaque monkey: Electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. Eur. J. Neurosci. 2008, 28, 1569–1588.
- Goldman, A.; Gallese, V. Reply to Schulkin. Trends Cogn. Sci. 2000, 4, 255–256.
- Keysers, C.; Wicker, B.; Gazzola, V.; Anton, J.L.; Fogassi, L.; Gallese, V. A touching sight: SII/PV activation during the observation and experience of touch. Neuron 2004, 42, 335–346.
- Holle, H.; Banissy, M.J.; Ward, J. Functional and structural brain differences associated with mirror-touch synaesthesia. Neuroimage 2013, 83, 1041–1050.
- Gallese, V. The manifold nature of interpersonal relations: The quest for a common mechanism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 517–528.
- Pitcher, D.; Garrido, L.; Walsh, V.; Duchaine, B.C. Transcranial magnetic stimulation disrupts the perception and embodiment of facial expressions. J. Neurosci. 2008, 28, 8929–8933.
- Ebisch, S.J.; Ferri, F.; Salone, A.; Perrucci, M.G.; D’Amico, L.; Ferro, F.M.; Romani, G.L.; Gallese, V. Differential involvement of somatosensory and interoceptive cortices during the observation of affective touch. J. Cogn. Neurosci. 2011, 23, 1808–1822.
- Ishida, H.; Fornia, L.; Grandi, L.C.; Umiltà, M.A.; Gallese, V. Somato-motor haptic processing in posterior inner perisylvian region (SII/pIC) of the macaque monkey. PLoS ONE 2013, 30, e69931.
- Schaefer, M.; Heinze, H.J.; Rotte, M. Touch and personality: Extraversion predicts somatosensory brain response. Neuroimage 2012, 62, 432–438.
- McCabe, C.; Rolls, E.T.; Bilderbeck, A.; McGlone, F. Cognitive influences on the affective representation of touch and the sight of touch in the human brain. Soc. Cogn. Affect. Neurosci. 2008, 3, 97–108.
- Macefield, V.G.; Norcliffe-Kaufmann, L.; Löken, L.; Axelrod, F.B.; Kaufmann, H. Disturbances in affective touch in hereditary sensory & autonomic neuropathy type III. Int. J. Psychophysiol. 2014, 93, 56–61.
- Martinez, V.R.; Giovanola, Y.; Ionta, S. Social Touch Somatotopically Affects Mental Body Representations. Neuroscience 2022, 494, 178–186.
- Pamplona, G.S.P.; Salgado, J.A.D.; Staempfli, P.; Seifritz, E.; Gassert, R.; Ionta, S. Illusory Body Ownership Affects the Cortical Response to Vicarious Somatosensation. Cereb. Cortex 2022, 32, 312–328.
- Gallese, V.; Rochat, M.J.; Berchio, C. The mirror mechanism and its potential role in autism spectrum disorder. Dev. Med. Child. Neurol. 2013, 55, 15–22.
- Gallese, V. The roots of empathy: The shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology 2003, 36, 171–180.
- Gallese, V. Embodied simulation: From neurons to phenomenal experience. Phenomenol. Cogn. Sci. 2005, 4, 23–48.
- Gallese, V. Embodied simulation theory: Imagination and narrative. Neuropsychoanalysis 2011, 13, 196–200.
- Gallese, V.; Sinigaglia, C. What is so special about embodied simulation? Trends Cogn. Sci. 2011, 15, 512–519.
- Gallese, V. Intentional attunement: A neurophysiological perspective on social cognition and its disruption in autism. Brain Res. 2006, 1079, 15–24.
- Bonini, L.; Rotunno, C.; Arcuri, E.; Gallese, V. Mirror neurons 30 years later: Implications and applications. Trends Cogn. Sci. 2022, 26, 767–781.
- Davidovic, M.; Starck, G.; Olausson, H. Processing of affective and emotionally neutral tactile stimuli in the insular cortex. Dev. Cogn. Neurosci. 2019, 35, 94–103.
- Hagberg, E.E.; Ackerley, R.; Lundqvist, D.; Schneiderman, J.; Jousmäki, V.; Wessberg, J. Spatio-temporal profile of brain activity during gentle touch investigated with magnetoencephalography. NeuroImage 2019, 201, 116024.
- Lee Masson, H.; de Beeck, H.O.; Boets, B. Reduced task-dependent modulation of functional network architecture for positive versus negative affective touch processing in autism spectrum disorders. NeuroImage 2020, 219, 117009.
- Perini, I.; Gustafsson, P.A.; Igelström, K.; Jasiunaite-Jokubaviciene, B.; Kämpe, R.; Mayo, L.M.; Molander, J.; Olausson, H.; Zetterqvist, M.; Heilig, M. Altered relationship between subjective perception and central representation of touch hedonics in adolescents with autism-spectrum disorder. Transl. Psychiatry 2021, 11, 224.
- Crucianelli, L.; Cardi, V.; Treasure, J.; Jenkinson, P.M.; Fotopoulou, A. The perception of affective touch in anorexia nervosa. Psychiatry Res. 2016, 239, 72–78.
- Gernsbacher, M.A.; Yergeau, M. Empirical failures of the claim that autistic people lack a theory of mind. Arch. Sci. Psychol. 2019, 7, 102.
- Haggarty, C.J.; Trotter, P.D.; McGlone, F.; Walker, S.C. Children’s vicarious ratings of social touch are tuned to the velocity but not the location of a caress. PLoS ONE 2021, 16, e0256303.
- Kang, Z.; Xing, H.; Lin, Q.; Meng, F.; Gong, L. Effectiveness of therapeutic massage for improving motor symptoms in Parkinson’s disease: A systematic review and meta-analysis. Front. Neurol. 2022, 13, 915232.
- Tsuji, S.; Yuhi, T.; Furuhara, K.; Ohta, S.; Shimizu, Y.; Higashida, H. Salivary oxytocin concentrations in seven boys with autism spectrum disorder received massage from their mothers: A pilot study. Front. Psychiatry 2015, 6, 58.
- Carpenter, L.L.; Kronenberg, E.F.; Tirrell, E.; Kokdere, F.; Beck, Q.M.; Temereanca, S.; Fukuda, A.M.; Garikapati, S.; Hagberg, S. Mechanical Affective Touch Therapy for Anxiety Disorders: Feasibility, Clinical Outcomes, and Electroencephalography Bi-omarkers From an Open-Label Trial. Front. Psychiatry 2022, 13, 877574.
- Ang, J.Y.; Lua, J.L.; Mathur, A.; Thomas, R.; Asmar, B.I.; Savasan, S.; Buck, S.; Long, M.; Shankaran, S. A randomized placebo-controlled trial of massage therapy on the immune system of preterm infants. Pediatrics 2012, 130, e1549–e1558.
- Cassileth, B.R.; Vickers, A.J. Massage therapy for symptom control: Outcome study at a major cancer center. J. Pain Symptom Manag. 2004, 28, 244–249.
More
Information
Subjects:
Behavioral Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
652
Revisions:
2 times
(View History)
Update Date:
10 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




