Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Izabela Kołodziejczyk | -- | 1490 | 2023-08-08 08:37:24 | | | |
| 2 | Conner Chen | Meta information modification | 1490 | 2023-08-10 04:22:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kołodziejczyk, I.; Tomczyk, P.; Kaźmierczak, A. Basic Differences between Cell Cycle and Endocycle. Encyclopedia. Available online: https://encyclopedia.pub/entry/47770 (accessed on 07 February 2026).
Kołodziejczyk I, Tomczyk P, Kaźmierczak A. Basic Differences between Cell Cycle and Endocycle. Encyclopedia. Available at: https://encyclopedia.pub/entry/47770. Accessed February 07, 2026.
Kołodziejczyk, Izabela, Przemysław Tomczyk, Andrzej Kaźmierczak. "Basic Differences between Cell Cycle and Endocycle" Encyclopedia, https://encyclopedia.pub/entry/47770 (accessed February 07, 2026).
Kołodziejczyk, I., Tomczyk, P., & Kaźmierczak, A. (2023, August 08). Basic Differences between Cell Cycle and Endocycle. In Encyclopedia. https://encyclopedia.pub/entry/47770
Kołodziejczyk, Izabela, et al. "Basic Differences between Cell Cycle and Endocycle." Encyclopedia. Web. 08 August, 2023.
Copy Citation
The standard cell cycle is divided into two periods: (1) the interphase, with the phases G1, S, and G2 and (2) cell division, either mitosis or meiosis. Initially, each new cell is in the G1 (Gap 1) phase. Then, the content of genetic material in the cell nucleus amounts to 2C, i.e., it reaches the basic value in vegetative cells.
endoreplication
endocycles
polyploids
1. Classic Cell Cycle
The standard cell cycle is divided into two periods: (1) the interphase, with the phases G1, S, and G2 and (2) cell division, either mitosis or meiosis. Initially, each new cell is in the G1 (Gap 1) phase [1][2]. Then, the content of genetic material in the cell nucleus amounts to 2C, i.e., it reaches the basic value in vegetative cells [3][4]. In generative cells, it is 1C [5][6]. As mentioned above, the DNA content of the cell nucleus is reflected in the size of the entire cell; therefore, in the first phase of the cell cycle, the cell volume is low.
The next step in the classical cell cycle is the S phase (synthesis), which means successive cell growth and one round of DNA replication. The amount of genetic material is gradually increased as the original DNA strand is copied, the C value goes from 2 to 4 in somatic cells, and from 1 to 2 in reproductive cells [7].
The S phase is the gateway to understanding what happens in the non-classical type of cell cycle—the process of endoreplication described here (Figure 1).
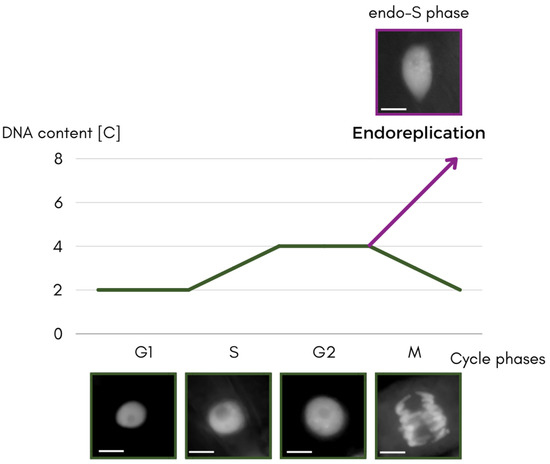
Figure 1. Scheme of the separating the paths of the classical cycle and the endocycle, through the cycle phases with DNA content expressed in universal units [C] of the cell nucleus changes. Nuclei of the apical part of roots of Vicia faba ssp. minor seedlings stained with 4′,6-diamidino-2-phenylindole (DAPI) and converted to grayscale. Scale bar is 10 μm.
In the phase ending the classical cycle interphase, G2 (Gap 2), the cell reaches its final size, adequate to the content of the cell nucleus (Figure 2) [3]. Now, after one round of replication, the amount of DNA is 4C in vegetative cells and 2C in generative cells [6].
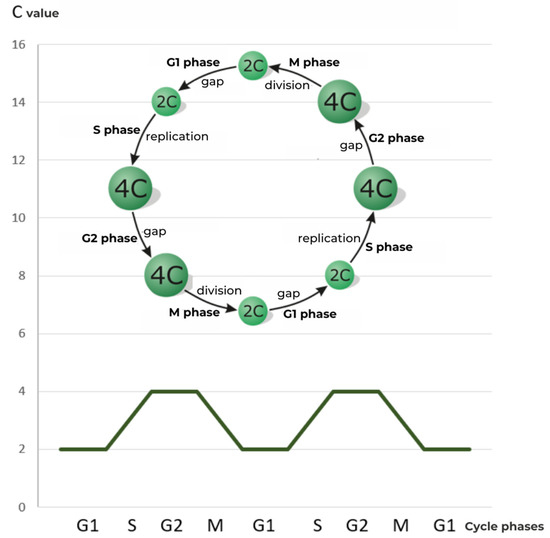
Figure 2. Scheme of the cell division cycle, with G1, S, G2, M phases, and relative DNA content expressed in universal units [C].
During the classical cycle ending with division, the value of C, characteristic of the G2 phase, decreases to the value of C, which is typical of the G1 phase. Then, the cycle begins anew [5]. There are cases where unreduced gametes (e.g., diploid instead of haploid) are formed during meiosis; when combined, they create a new organism that is immediately polyploid [6][8]. However, it is a different type of polyploidization known as species polyploidy or auto-polyploidy [9][10][11][12], which will not be discussed extensively here, unlike the organo- or tissue-specific endopolyploidy, which occurs during the lifetime of an individual, resulting from endoreplication.
2. Foundations of Endoreplication
Young plant cells are characterized by high proliferation. After some time, those that lie outside the meristems begin to differentiate, which leads to the formation of tissues [5][13].
Differentiation (Figure 3) is associated with numerous changes within the cell in terms of its morphology, function, and biochemical arsenal [3][14][15][16]. The reason for such transformations is the need to shape an appropriate pool of matrices for the production of cellular components [17][18][19]. Behind the acquisition of the former is a different expression of the desired genes, and in the case of endoreplication, increasing their availability by copying the entire genome, not once, as in the case of the S phase of the classic cell cycle, but multiple times [1][20][21][22].
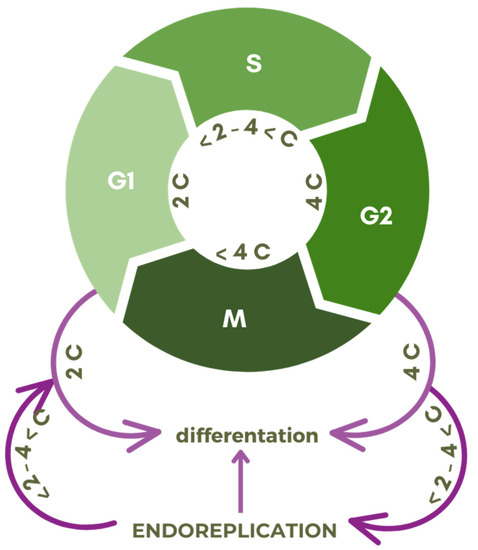
Figure 3. Scheme of transition points of the cell division cycle with G1, S, G2, M phases, and relative DNA content expressed in universal units (C) to the endocycle (endoreplication).
Endoreplication, called the endocycle, is a succession of G and S phases during which the cell grows and increases the level of its genetic material, which is expressed in the multiplication of the C value [3][4].
Endocycles are therefore described as division-free cycles, since the latter would normally reduce the C value by half. This does not occur in the case of endoreplication. Therefore, the cell subjected to it becomes polyploid and has a cell nucleus with an increased DNA content, often many times over, as compared to its basic content (Figure 4).
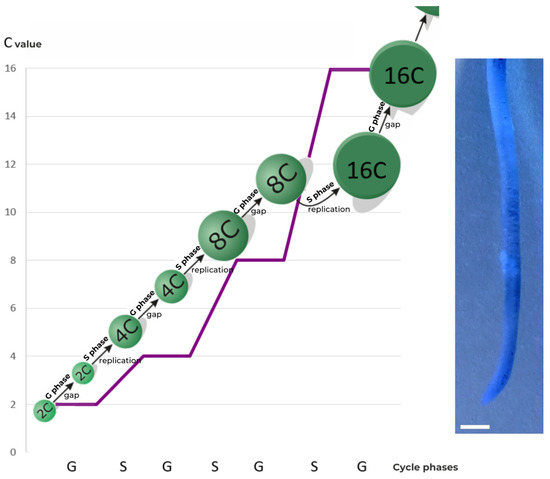
Figure 4. Scheme of the endocycle phases and relative DNA content expressed in universal units [C]. Image shows the apical part of root of Vicia faba ssp. minor seedlings, converted using PicosmosTools 2.6.0.1 software (first free version published in Softonic on September 29, 2015, by “Free Time”, England). Scale bar is 2 mm.
3. Endocycle Types
Endocycles come in several forms, which can be distinguished by their different courses and the obtained C value. As the papers [1][23][24][25] report, these forms include endoreduplication, endomitosis, incomplete replication, and amplification.
Endoreduplication
The occurrence of endoreduplication in cells can be recognized by two symptoms. First, in the interphase, there is an increase in the volume of chromocenters, but there is no increase in their basal number. Second, after the interphase, the cell produces chromosomes that are ostensibly similar to those formed in the classical cycle. The chromosomes even align in the equatorial plane during endoreduplication, but they never separate. This gives rise to polythene chromosomes, i.e., structures consisting of many strands of DNA. They can be composed of multiple, even hundreds of, sister chromatids, which depends on the number of rounds of replication within endocycles. During endoreduplication, no karyokinesis or cytokinesis occurs, the spindle does not function, and no phragmoplast is formed [23].
In low polyploid cells, endoreduplication may be preceded by other types of endocycles. The occurrence of endoreduplication has been described in the seed endosperm and suspensor of many species. And it is, along with endomitosis, the most common cause of polyploidization.
Endomitosis
Endomitosis is a form of endocycle that closely resembles the standard cell cycle. In its course, DNA multiplication takes place, which is visible in the interphase: the number of chromocenters is doubled but their volume is not enlarged. Their number increases exponentially, corresponding to the number of replication rounds performed.
When genetic material condenses, chromosomes analogous to mitotic ones become visible. They line up within equatorial plate, as in the metaphase, and due to the action of the karyokinetic spindle, sister chromatids are separated as in the anaphase. However, they do not reach the poles of the cell as in the telophase, and the karyokinesis is only provisional and not accompanied by cytokinesis [1]. Other sources say that the karyokinetic spindle does not arise during endomitosis [26], although such a course of events would cover to some extent the similarity of endomitosis to mitosis.
In each case, one nuclear envelope is finally rebuilt, inside which all chromosomes are locked. A cell is therefore equipped with a multiplied number of chromosomes, centromeres, and nucleoli but still has one cell nucleus.
The aforementioned similarities to the division occurring in the classical cell cycle allowed scientists to distinguish four phases of endomitosis: endoprophase, endometaphase, endoanaphase, and ostensible endotelophase (ostensible due to the fact that this phase is the most divergent from its mitotic counterpart). Therefore, endomitosis could be compared to a cell cycle in which mitosis occurs but is not completed by division [1][25].
Incomplete Replication
During incomplete replication, genetic material is duplicated, but in an incomplete volume. Hence, the phenomenon is also referred to as underreplication. This means that the cell nucleus, after the process of incomplete replication, reaches the content of genetic material always slightly below a full multiple of C [2][24].
It depends on the needs of the plant, which goes through various stages of development during its life [2]. The functions performed by cells often require an enhanced expression of only part of the genes present in the genome, while others are redundant at this time. Incomplete replication is economical in the sense that it does not copy DNA strand fragments that will not be ultimately used. Another significant saving is the fact that during the synthesis of deoxyribonucleic acid, taking place as part of incomplete replication, the area of heterochromatin is very frequently omitted [24].
What is observed in this process are fragmentary polythene chromosomes. Incomplete replication may start upon the cell entering the endocycle, or it may occur after some degree of ploidy has been achieved.
Amplification
Amplification, or overreplication, is analogous to the process described above; however, it consists of the additional synthesis of only selected fragments of DNA strands to increase the chances of plant survival in unfavorable environmental conditions or the expression of selected genes that are needed, for example, at some period of development [2][27]. During amplification, the replication rounds include short fragments of genetic material or single genes [24].
In cell nuclei subjected to cytometric tests, the C-value is always slightly above polyploidy when amplified. However, it is difficult to show this unequivocally because the same values may also mean that the cell undergoes a replication process or a different type of endocycle [24][27]. Hence, to demonstrate that the genetic material has indeed been amplified, preliminary studies should be confirmed via analyses carried out at the molecular level.
References
- Lee, H.O.; Davidson, J.M.; Duronio, R.J. Endoreplication: Polyploidy with purpose. Genes Dev. 2009, 23, 2461–2477.
- Nagl, W. DNA endoreduplication and polyteny understood as evolutionary strategies. Nature 1976, 261, 614–615.
- Gregory, T.R. Coincidence, coevolution, or causation? DNA content, cell size, and the C-value enigma. Biol. Rev. 2001, 76, 65–101.
- Chen, Z.J. Genetic and Epigenetic Mechanisms for Gene Expression and Phenotypic Variation in Plant Polyploids. Annu. Rev. Plant Biol. 2007, 58, 377–406.
- Boudolf, V.; Vlieghe, K.; Beemster, G.T.; Magyar, Z.; Acosta, J.A.T.; Maes, S.; Van Der Schueren, E.; Inzé, D.; De Veylder, L. The Plant-Specific Cyclin-Dependent Kinase CDKB1;1 and Transcription Factor E2Fa-DPa Control the Balance of Mitotically Dividing and Endoreduplicating Cells in Arabidopsis. Plant Cell 2004, 16, 2683–2692.
- De Storme, N.; Copenhaver, G.P.; Geelen, D. Production of Diploid Male Gametes in Arabidopsis by Cold-Induced Destabi-lization of Postmeiotic Radial Microtubule Arrays. Plant Physiol. 2012, 160, 1808–1826.
- Kitagawa, M.; Jackson, D. Control of Meristem Size. Annu. Rev. Plant Biol. 2019, 70, 269–291.
- Fox, D.T.; Soltis, D.E.; Soltis, P.S.; Ashman, T.-L.; Van de Peer, Y. Polyploidy: A Biological Force From Cells to Ecosystems. Trends Cell Biol. 2020, 30, 688–694.
- Frawley, L.E.; Orr-Weaver, T.L. Polyploidy. Curr. Biol. 2015, 25, R353–R358.
- Tate, J.A.; Soltis, D.E.; Soltis, P.S.; Gregory, T.R. The Evolution of the Genome; Academic Press: Cambridge, MA, USA, 2005; Volume 2005, pp. 371–426.
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846.
- Bardil, A.; Tayalé, A.; Parisod, C. Evolutionary dynamics of retrotransposons following autopolyploidy in the Buckler Mustard species complex. Plant J. 2015, 82, 621–631.
- Bell, S.P.; Dutta, A. DNA Replication in Eukaryotic Cells. Annu. Rev. Biochem. 2002, 71, 333–374.
- Cebolla, A.; Vinardell, J.M.; Kiss, E.; Oláh, B.; Roudier, F.; Kondorosi, A.; Kondorosi, E. The mitotic inhibitor ccs52 is required for endoreduplication and ploidy-dependent cell enlargement in plants. EMBO J. 1999, 18, 4476–4484.
- Jovtchev, G.; Schubert, V.; Meister, A.; Barow, M.; Schubert, I. Nuclear DNA content and nuclear and cell volume are positively correlated in angiosperms. Cytogenet. Genome Res. 2006, 114, 77–82.
- Beaulieu, J.M.; Leitch, I.J.; Patel, S.; Pendharkar, A.; Knight, C.A. Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol. 2008, 179, 975–986.
- Butterfass, T. A nucleotypic control of chloroplast reproduction. Protoplasma 1983, 118, 71–74.
- Umeda, M.; Ikeuchi, M.; Ishikawa, M.; Ito, T.; Nishihama, R.; Kyozuka, J.; Torii, K.U.; Satake, A.; Goshima, G.; Sakakibara, H. Plant stem cell research is uncovering the secrets of longevity and persistent growth. Plant J. 2021, 106, 326–335.
- Sabelli, P.A. Replicate and die for your own good: Endoreduplication and cell death in the cereal endosperm. J. Cereal Sci. 2012, 56, 9–20.
- Bourdon, M.; Pirrello, J.; Cheniclet, C.; Coriton, O.; Bourge, M.; Brown, S.; Moïse, A.; Peypelut, M.; Rouyère, V.; Renaudin, J.-P.; et al. Evidence for karyoplasmic homeostasis during endoreduplication and a ploidy-dependent increase in gene transcription during tomato fruit growth. Development 2012, 139, 3817–3826.
- Chao, D.-Y.; Dilkes, B.; Luo, H.; Douglas, A.; Yakubova, E.; Lahner, B.; Salt, D.E. Polyploids Exhibit Higher Potassium Uptake and Salinity Tolerance in Arabidopsis. Science 2013, 341, 658–659.
- Hannweg, K.; Steyn, W.; Bertling, I. In vitro-induced tetraploids of Plectranthus esculentus are nematode-tolerant and have enhanced nutritional value. Euphytica 2016, 207, 343–351.
- Schubert, V.; Klatte, M.; Pecinka, A.; Meister, A.; Jasencakova, Z.; Schubert, I. Sister Chromatids Are Often Incompletely Aligned in Meristematic and Endopolyploid Interphase Nuclei of Arabidopsis thaliana. Genetics 2006, 172, 467–475.
- Biskup, A.; Izmailow, R. Endosperm Development in Seeds of Echium vulgare L. from Polluted Sites. Acta Biol. Cracoviensia Ser. Bot. 2004, 46, 39–44.
- Oksala, T.; Therman, E. Endomitosis in Tapetal Cells of Eremurus (Liliaceae). Am. J. Bot. 1977, 64, 866–872.
- D’amato, F. Role of Polyploidy in Reproductive Organs and Tissues. In Embryology of Angiosperms; Springer: Berlin/Heidelberg, Germany, 1984; pp. 519–566.
- Nagl, W. Gene Amplification and Related Events. In Somaclonal Variation in Crop Improvement I.; Bajaj, Y.P.S., Ed.; Bio-Technology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 1990; pp. 153–201.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
10 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




