
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Christopher Lucchesi | -- | 1559 | 2023-08-07 20:21:37 | | | |
| 2 | Wendy Huang | + 14 word(s) | 1573 | 2023-08-08 08:42:49 | | |
Video Upload Options
The bladder is a hollow organ located in the lower abdominal region. The bladder is contiguous with the ureters above, through which urine flows from the kidneys, and the urethra below, which dispels urine from the body. There are four parts to the bladder, the apex or dome, body, fundus, and neck. The apex is the anterosuperior part of the bladder that points towards the abdominal wall. The fundus, or base, is the posteroinferior part of the bladder. The fundus lies on the inferior aspect of the proximal wall while the apex lies on the anterior aspect of the wall, extending towards the pubic symphysis. The body of the bladder is the large area situated between the apex and the fundus. The neck of the bladder is the constricted part of the bladder that leads to the urethra. The upper part of the bladder consists of the apex and body which are above the ureteric orifices. The lower part consists of the fundus, neck, and trigone. The trigone is an inverted triangular-shaped area of space that is made entirely of smooth muscle. Its superolateral angles are formed by the ureteric orifices. The neck lies at the base of the trigone and it is the most inferior point of the bladder.
1. Introduction
2. Stages of Bladder Cancer
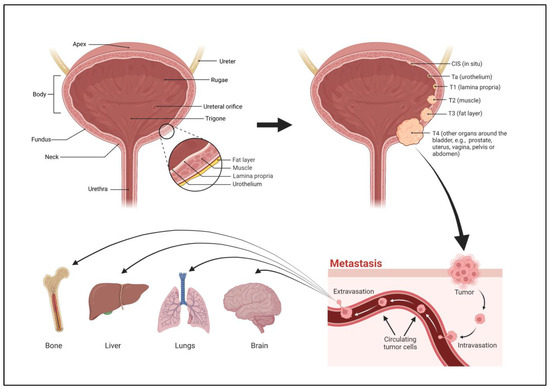
3. Grades of Bladder Cancer
4. Types of Bladder Cancer
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33.
- Martin, P.; Hooker, M.; Aktar, M.; Stockton, M. How many workers are employed in California agriculture? Calif. Agric. 2016, 71, 30–34.
- Isharwal, S.; Konety, B. Non-muscle invasive bladder cancer risk stratification. Indian J. Urol. 2015, 31, 289–296.
- Bilim, V.; Kuroki, H.; Shirono, Y.; Murata, M.; Hiruma, K.; Tomita, Y. Advanced Bladder Cancer: Changing the Treatment Landscape. J. Pers. Med. 2022, 12, 1745.
- Park, J.C.; Citrin, D.E.; Agarwal, P.K.; Apolo, A.B. Multimodal management of muscle-invasive bladder cancer. Curr. Probl. Cancer 2014, 38, 80–108.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Halaseh, S.A.; Halaseh, S.; Alali, Y.; Ashour, M.E.; Alharayzah, M.J. A Review of the Etiology and Epidemiology of Bladder Cancer: All You Need to Know. Cureus 2022, 14, e27330.
- Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Cogliano, V. Carcinogenicity of some aromatic amines, organic dyes, and related exposures. Lancet Oncol. 2008, 9, 322–323.
- Cumberbatch, M.G.K.; Jubber, I.; Black, P.C.; Esperto, F.; Figueroa, J.D.; Kamat, A.M.; Kiemeney, L.; Lotan, Y.; Pang, K.; Silverman, D.T.; et al. Epidemiology of Bladder Cancer: A Systematic Review and Contemporary Update of Risk Factors in 2018. Eur. Urol. 2018, 74, 784–795.
- Rosiello, G.; Palumbo, C.; Deuker, M.; Stolzenbach, L.F.; Martin, T.; Tian, Z.; Gallina, A.; Montorsi, F.; Black, P.; Kassouf, W.; et al. Racial differences in the distribution of bladder cancer metastases: A population-based analysis. Cent. Eur. J. Urol. 2020, 73, 407–415.
- Wang, Y.; Chang, Q.; Li, Y. Racial differences in Urinary Bladder Cancer in the United States. Sci. Rep. 2018, 8, 12521.
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15.
- Sui, X.; Lei, L.; Chen, L.; Xie, T.; Li, X. Inflammatory microenvironment in the initiation and progression of bladder cancer. Oncotarget 2017, 8, 93279–93294.
- Manley, K.V.; Hubbard, R.; Swallow, D.; Finch, W.; Wood, S.J.; Biers, S.M. Risk factors for development of primary bladder squamous cell carcinoma. Ind. Mark. Manag. 2017, 99, 155–160.
- Mostafa, M.H.; Sheweita, S.A.; O’connor, P.J. Relationship between Schistosomiasis and Bladder Cancer. Clin. Microbiol. Rev. 1999, 12, 97–111.
- Sharma, P.K.; Pandey, P.K.; Vijay, M.K.; Bera, M.K.; Singh, J.P.; Saha, K. Squamous cell carcinoma in exstrophy of the bladder. Korean J. Urol. 2013, 54, 555–557.
- Talar-Williams, C.; Hijazi, Y.M.; Walther, M.M.; Linehan, W.M.; Hallahan, C.W.; Lubensky, I.; Kerr, G.S.; Hoffman, G.S.; Fauci, A.S.; Sneller, M.C. Cyclophosphamide-Induced Cystitis and Bladder Cancer in Patients with Wegener Granulomatosis. Ann. Intern. Med. 1996, 124, 477–484.
- Suriano, F.; Altobelli, E.; Sergi, F.; Buscarini, M. Bladder cancer after radiotherapy for prostate cancer. Rev. Urol. 2013, 15, 108–112.
- Bhat, S.; Sathyanarayanaprasad, M.; Paul, F. Primary squamous cell carcinoma of bladder exstrophy in an adult. Indian J. Urol. 2015, 31, 142–143.
- Miyazaki, J.; Nishiyama, H. Epidemiology of urothelial carcinoma. Int. J. Urol. 2017, 24, 730–734.
- Yin, P.N.; Kc, K.; Wei, S.; Yu, Q.; Li, R.; Haake, A.R.; Miyamoto, H.; Cui, F. Histopathological distinction of non-invasive and invasive bladder cancers using machine learning approaches. BMC Med. Inform. Decis. Mak. 2020, 20, 162.
- Adamczyk, P.; Pobłocki, P.; Kadlubowski, M.; Ostrowski, A.; Wróbel, A.; Mikołajczak, W.; Adamowicz, J.; Drewa, T.; Juszczak, K. A Comprehensive Approach to Clinical Staging of Bladder Cancer. J. Clin. Med. 2022, 11, 761.
- Blanca, A.; Lopez-Beltran, A.; Lopez-Porcheron, K.; Gomez-Gomez, E.; Cimadamore, A.; Bilé-Silva, A.; Gogna, R.; Montironi, R.; Cheng, L. Risk Classification of Bladder Cancer by Gene Expression and Molecular Subtype. Cancers 2023, 15, 2149.
- Lopez-Beltran, A. Bladder cancer: Clinical and pathological profile. Scand. J. Urol. Nephrol. 2008, 42, 95–109.
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.-L.; et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014, 25, 152–165.
- Apollo, A.; Ortenzi, V.; Scatena, C.; Zavaglia, K.; Aretini, P.; Lessi, F.; Franceschi, S.; Tomei, S.; Sepich, C.A.; Viacava, P.; et al. Molecular characterization of low grade and high grade bladder cancer. PLoS ONE 2019, 14, e0210635.
- Kirkali, Z.; Chan, T.; Manoharan, M.; Algaba, F.; Busch, C.; Cheng, L.; Kiemeney, L.; Kriegmair, M.; Montironi, R.; Murphy, W.M.; et al. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology 2005, 66, 4–34.
- Neuzillet, Y.; Paoletti, X.; Ouerhani, S.; Mongiat-Artus, P.; Soliman, H.; de The, H.; Sibony, M.; Denoux, Y.; Molinie, V.; Herault, A.; et al. A meta-analysis of the relationship between FGFR3 and TP53 mutations in bladder cancer. PLoS ONE 2012, 7, e48993.
- Kaseb, H.; Aeddula, N.R. Bladder Cancer; StatPearls: Tampa, FL, USA, 2023.
- Minato, A.; Noguchi, H.; Tomisaki, I.; Fukuda, A.; Kubo, T.; Nakayama, T.; Fujimoto, N. Clinical Significance of Squamous Differentiation in Urothelial Carcinoma of the Bladder. Cancer Control 2018, 25, 1073274818800269.
- Amin, M.B. Histological variants of urothelial carcinoma: Diagnostic, therapeutic and prognostic implications. Mod. Pathol. 2009, 22 (Suppl. S2), S96–S118.
- Makise, N.; Morikawa, T.; Kawai, T.; Nakagawa, T.; Kume, H.; Homma, Y.; Fukayama, M. Squamous differentiation and prognosis in upper urinary tract urothelial carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 7203–7209.
- Warrick, J.I. Clinical Significance of Histologic Variants of Bladder Cancer. J. Natl. Compr. Cancer Netw. 2017, 15, 1268–1274.
- Martin, J.W.; Carballido, E.M.; Ahmed, A.; Farhan, B.; Dutta, R.; Smith, C.; Youssef, R.F. Squamous cell carcinoma of the urinary bladder: Systematic review of clinical characteristics and therapeutic approaches. Arab. J. Urol. 2016, 14, 183–191.
- Ho, C.-H.; Sung, K.-C.; Lim, S.-W.; Liao, C.-H.; Liang, F.-W.; Wang, J.-J.; Wu, C.-C. Chronic Indwelling Urinary Catheter Increase the Risk of Bladder Cancer, Even in Patients Without Spinal Cord Injury. Medicine 2015, 94, e1736.
- PDQ Supportive and Palliative Care Editorial Board. PDQ Cancer Information Summaries. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK65883/ (accessed on 9 May 2023).
- Bandini, M.; Pederzoli, F.; Madison, R.; Briganti, A.; Ross, J.S.; Niegisch, G.; Yu, E.Y.; Bamias, A.; Agarwal, N.; Sridhar, S.S.; et al. Unfavorable Cancer-specific Survival After Neoadjuvant Chemotherapy and Radical Cystectomy in Patients with Bladder Cancer and Squamous Cell Variant: A Multi-institutional Study. Clin. Genitourin. Cancer 2020, 18, e543–e556.
- Dadhania, V.; Czerniak, B.; Guo, C.C. Adenocarcinoma of the urinary bladder. Am. J. Clin. Exp. Urol. 2015, 3, 51–63.
- Ismaili, N. A rare bladder cancer—Small cell carcinoma: Review and update. Orphanet J. Rare Dis. 2011, 6, 75.




