
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | EVANDO ARAÚJO | -- | 4516 | 2023-08-07 15:34:10 | | | |
| 2 | Sirius Huang | Meta information modification | 4516 | 2023-08-08 02:42:51 | | |
Video Upload Options
The improper disposal of toxic and carcinogenic organic substances resulting from the manufacture of dyes, drugs and pesticides can contaminate aquatic environments and potable water resources and cause serious damage to animal and human health and to the ecosystem. In this sense, heterogeneous photocatalysis stand out as one effective and cost-effective water depollution technique. The use of metal oxide nanocomposites (MON), from the mixture of two or more oxides or between these oxides and other functional semiconductor materials, have gained increasing attention from researchers and industrial developers as a potential alternative to produce efficient and environmentally friendly photocatalysts for the remediation of water contamination by organic compounds.
1. Introduction
2. Metal Oxides and Photocatalysis
2.1. Fundamentals of Metal Oxides
2.2. Electronic Structure
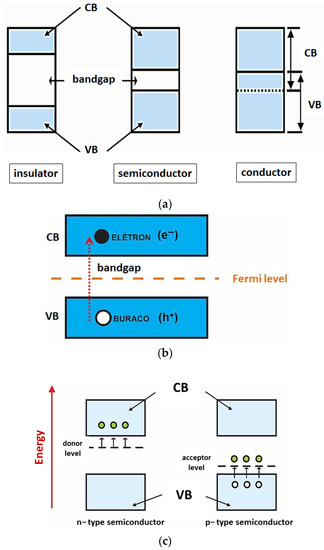
2.3. Metal Oxide-Based Photocatalysis
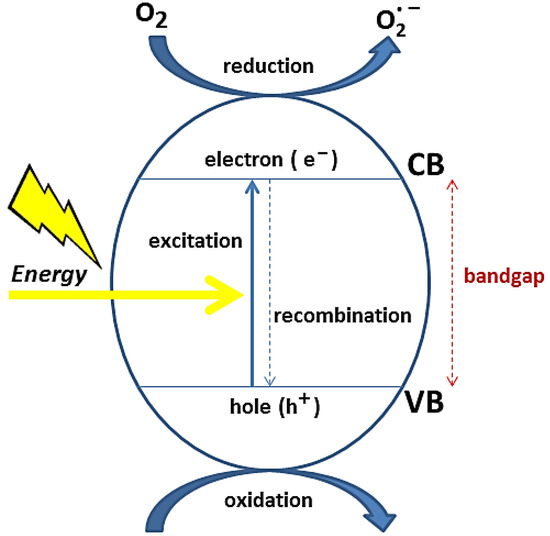
Charge Transfer in MON-Based Photocatalysts by Surface Tailoring
References
- Akhtar, N.; Ishak, M.I.S.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660.
- Breida, M.; Younssi, S.A.; Ouammou, M.; Bouhria, M.; Hafsi, M. Pollution of Water Sources from Agricultural and Industrial Effluents: Special Attention to NO3−, Cr(VI), and Cu(II). In Water Chemistry; IntechOpen: London, UK, 2019.
- Chubarenko, I.; Bagaev, A.; Zobkov, M.; Esiukova, E. On some physical and dynamical properties of microplastic particles in marine environment. Mar. Pollut. Bull. 2016, 108, 105–112.
- Lin, L.; Yang, H.; Xu, X. Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246.
- Rezai, B.; Allahkarami, E. Wastewater Treatment Processes-Techniques, Technologies, Challenges Faced, and Alternative Solutions. In Soft Computing Techniques in Solid Waste and Wastewater Management; Karri, R.R., Ravindran, G., Dehghani, M.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 35–53.
- Zhao, W.; Chen, I.-W.; Huang, F. Toward large-scale water treatment using nanomaterials. Nano Today 2019, 27, 11–27.
- Kotia, A. Cost-Effective Water Purification-Method. Encyclopedia. Available online: https://encyclopedia.pub/entry/6246 (accessed on 29 May 2023).
- Khan, S.T.; Malik, A. Engineered nanomaterials for water decontamination and purification: From lab to products. J. Hazard. Mater. 2018, 363, 295–308.
- de Jesus, R.A.; de Assis, G.C.; de Oliveira, R.J.; Bilal, M.; Bharagava, R.N.; Iqbal, H.M.; Ferreira, L.F.R.; Figueiredo, R.T. Metal oxide nanoparticles for environmental remediation. In Micro and Nano Technologies, Biodegradation and Biodeterioration at the Nanoscale; Hafiz, M.N.I., Bilal, M., Nguyen, T.A., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 529–560.
- Pattnaik, A.; Sahu, J.; Poonia, A.K.; Ghosh, P. Current perspective of nano-engineered metal oxide based photocatalysts in advanced oxidation processes for degradation of organic pollutants in wastewater. Chem. Eng. Res. Des. 2023, 190, 667–686.
- Lu, F.; Didier, A. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coordination Chemistry Reviews 2020, 408, 213180.
- Shanaah, H.H.; Alzaimoor, E.F.H.; Rashdan, S.; Abdalhafith, A.A.; Kamel, A.H. Photocatalytic Degradation and Adsorptive Removal of Emerging Organic Pesticides Using Metal Oxide and Their Composites: Recent Trends and Future Perspectives. Sustainability 2023, 15, 7336.
- Pereira, M.F.G.; Nascimento, M.M.; Cardoso, P.H.N.; Oliveira, C.Y.B.; Tavares, G.F.; Araújo, E.S. Preparation, Microstructural Characterization and Photocatalysis Tests of V5+-Doped TiO2/WO3 Nanocomposites Supported on Electrospun Membranes. Inorganics 2022, 10, 143.
- Jamjoum, H.A.A.; Umar, K.; Adnan, R.; Razali, M.R.; Ibrahim, M.N.M. Synthesis, Characterization, and Photocatalytic Activities of Graphene Oxide/metal Oxides Nanocomposites: A Review. Front. Chem. 2021, 9, 752276.
- Patil, S.R. Metal oxide-based composites as photocatalysts. In Metal Oxides, Advances in Metal Oxides and Their Composites for Emerging Applications; Delekar, S.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 633–672.
- Böer, K.W.; Pohl, U.W. Bands and Bandgaps in Solids. In Semiconductor Physics; Springer: Singapore, 2023; pp. 257–317.
- He, H. 2-Metal oxide semiconductors and conductors. In Metal Oxides, Solution Processed Metal Oxide Thin Films for Electronic Applications; Zheng, C., Ghenadii, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 7–30.
- Védrine, J.C. Metal Oxides in Heterogeneous Oxidation Catalysis: State of the Art and Challenges for a More Sustainable World. Chemsuschem 2019, 12, 577–588.
- Melak, F.; Bogale, B.; Asere, T.G.; Yai, T. Photocatalytic degradation of methylene blue dye using cuprous oxide/graphene nanocomposite. Curr. Nanomater. 2022, 8, 182–193.
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217.
- Sagar, D.; Delekar, S.D. (Eds.) Advances in Metal Oxides and Their Composites for Emerging Applications; Elsevier: Amsterdam, The Netherlands, 2022; 746p, ISBN 9780323857062.
- Samriti; Rajput, V.; Gupta, R.K.; Prakash, J. Engineering metal oxide semiconductor nanostructures for enhanced charge transfer: Fundamentals and emerging SERS applications. J. Mater. Chem. C 2021, 10, 73–95.
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694.
- Wang, Q.; Rhimi, B.; Wang, H.; Wang, C. Efficient photocatalytic degradation of gaseous toluene over F-doped TiO2/exfoliated bentonite. Appl. Surf. Sci. 2020, 530, 147286.
- Pham, T.-H.; Jung, S.H.; Kim, T. Enhanced photodegradation of toxic volatile organic pollutants using Ni-doped graphitic carbon nitride under natural solar light. Sol. Energy 2021, 224, 18–26.
- Abo-Dief, H.M.; Hussein, O.K.; Ihsan, A.; El-Bahy, S.M.; Raslan, A.M.; Shahid, M.; Warsi, M.F. Ternary metal oxide WO3.NiO.ZnO nanoparticles and their composite with CNTs for organic dye photocatalytic degradation. Ceram. Int. 2022, 48, 22228–22236.
- Perera, M.; Wijenayaka, L.A.; Siriwardana, K.; Dahanayake, D.; de Silva, K.M.N. Gold nanoparticle decorated titania for sustainable environmental remediation: Green synthesis, enhanced surface adsorption and synergistic photocatalysis. RSC Adv. 2020, 10, 29594–29602.
- Li, S.S. Energy Band Theory. In Semiconductor Physical Electronics; Li, S.S., Ed.; Springer: New York, NY, USA, 2006.
- Callaway, J. Quantum Theory of the Solid State: Pt. A; Academic Press: Cambridge, UK, 2013.
- Ge, L.; Han, C.; Liu, J. Novel visible light-induced g-C3N4/Bi2WO6 composite photocatalysts for efficient degradation of methyl orange. Appl. Catal. B Environ. 2011, 108–109, 100–107.
- Li, D.; Li, R.; Zeng, F.; Yan, W.; Deng, M.; Cai, S. The photoexcited electron transfer and photocatalytic mechanism of g-C3N4/TiO2 heterojunctions: Time-domain ab initio analysis. Appl. Surf. Sci. 2023, 614, 156104.
- He, X.; Xiao, Z.; Katase, T.; Ide, K.; Hosono, H.; Kamiya, T. Intrinsic and Extrinsic Defects in Layered Nitride Semiconductor SrTiN2. J. Phys. Chem. C 2019, 123, 19307–19314.
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloys Compd. 2020, 828, 154281.
- Yang, H. A short review on heterojunction photocatalysts: Carrier transfer behavior and photocatalytic mechanisms. Mater. Res. Bull. 2021, 142, 111406.
- Singh, A.K.; Singh, A.K.; Sinha, S.R.P. Fermi-Level Modulation of Chemical Vapor Deposition-Grown Monolayer Graphene via Nanoparticles to Macromolecular Dopants. ACS Omega 2021, 7, 744–751.
- Siu, C. Semiconductor Physics. Electronic Devices, Circuits, and Applications; Springer International Publishing: Cham, Switzerland, 2022; pp. 35–39.
- Sagadevan, S.; Marlinda, A.; Johan, M.R.; Umar, A.; Fouad, H.; Alothman, O.Y.; Khaled, U.; Akhtar, M.; Shahid, M. Reduced graphene/nanostructured cobalt oxide nanocomposite for enhanced electrochemical performance of supercapacitor applications. J. Colloid Interface Sci. 2020, 558, 68–77.
- Sharma, S.; Sudhakara, P.; Omran, A.A.B.; Singh, J.; Ilyas, R.A. Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications. Polymers 2021, 13, 2898.
- Kotowska, U.; Karpińska, J.; Kiejza, D.; Ratkiewicz, A.; Piekutin, J.; Makarova, K.; Olchowik-Grabarek, E. Oxidation of contaminants of emerging concern by combination of peracetic acid with iron ions and various types of light radiation–Optimization, kinetics, removal efficiency and mechanism investigation. J. Mol. Liq. 2023, 369, 120859.
- Coronado, J.M. A Historical Introduction to Photocatalysis. In Design of Advanced Photocatalytic Materials for Energy and Environmental Applications. Green Energy and Technology; Coronado, J., Fresno, F., Hernández-Alonso, M., Portela, R., Eds.; Springer: London, UK, 2013.
- Zhu, D.; Zhou, Q. Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review. Environ. Nanotechnology, Monit. Manag. 2019, 12, 100255.
- Friehs, E.; AlSalka, Y.; Jonczyk, R.; Lavrentieva, A.; Jochums, A.; Walter, J.-G.; Stahl, F.; Scheper, T.; Bahnemann, D. Toxicity, phototoxicity and biocidal activity of nanoparticles employed in photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2016, 29, 1–28.
- Bora, L.V.; Mewada, R.K. Visible/solar light active photocatalysts for organic effluent treatment: Fundamentals, mechanisms and parametric review. Renew. Sustain. Energy Rev. 2017, 76, 1393–1421.
- Karim, A.V.; Shriwastav, A. Degradation of amoxicillin with sono, photo, and sonophotocatalytic oxidation under low-frequency ultrasound and visible light. Environ. Res. 2021, 200, 111515.
- Bharathi, P.; Harish, S.; Archana, J.; Navaneethan, M.; Ponnusamy, S.; Muthamizhchelvan, C.; Shimomura, M.; Hayakawa, Y. Enhanced charge transfer and separation of hierarchical CuO/ZnO composites: The synergistic effect of photocatalysis for the mineralization of organic pollutant in water. Appl. Surf. Sci. 2019, 484, 884–891.
- Menon, N.G.; Tatiparti, S.S.V.; Mukherji, S. Synthesis, characterization and photocatalytic activity evaluation of TiO2–ZnO nanocomposites: Elucidating effect of varying Ti:Zn molar ratio. Colloids Surf. A Physicochem. Eng. Asp. 2018, 565, 47–58.
- Alam, U.; Shah, T.A.; Khan, A.; Muneer, M. One-pot ultrasonic assisted sol-gel synthesis of spindle-like Nd and V codoped ZnO for efficient photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2018, 212, 427–437.
- Natarajan, T.S.; Mozhiarasi, V.; Tayade, R.J. Nitrogen Doped Titanium Dioxide (N-TiO2): Synopsis of Synthesis Methodologies, Doping Mechanisms, Property Evaluation and Visible Light Photocatalytic Applications. Photochemistry 2021, 1, 371–410.
- Huang, J.; Dou, L.; Li, J.; Zhong, J.; Li, M.; Wang, T. Excellent visible light responsive photocatalytic behavior of N-doped TiO2 toward decontamination of organic pollutants. J. Hazard. Mater. 2020, 403, 123857.
- Olana, M.H.; Sabir, F.K.; Bekele, E.T.; Gonfa, B.A. Citrus sinensis and Musa acuminata Peel Waste Extract Mediated Synthesis of TiO2/rGO Nanocomposites for Photocatalytic Degradation of Methylene Blue under Visible Light Irradiation. Bioinorg. Chem. Appl. 2022, 2022, 5978707.
- Sivakami, A.; Sarankumar, R.; Sudhagar, P. Graphene–Metal Oxides Nanocomposite Heterojunction as an Efficient Photocatalyst for Energy and Environmental Applications. In Heterojunction Photocatalytic Materials; Jenny Stanford Publishing: New York, NY, USA, 2022; pp. 227–255.
- Kusiak-Nejman, E.; Wanag, A.; Kapica-Kozar, J.; Kowalczyk, Ł.; Zgrzebnicki, M.; Tryba, B.; Przepiórski, J.; Morawski, A.W. Methylene blue decomposition on TiO2/reduced graphene oxide hybrid photocatalysts obtained by a two-step hydrothermal and calcination synthesis. Catal. Today 2019, 357, 630–637.
- Usman, A.K.; Cursaru, D.-L.; Brănoiu, G.; Şomoghi, R.; Manta, A.-M.; Matei, D.; Mihai, S. A Modified Sol–Gel Synthesis of Anatase -TiO2/Au Hybrid Nanocomposites for Enhanced Photodegradation of Organic Contaminants. Gels 2022, 8, 728.
- Tahir, M.B.; Farman, S.; Rafique, M.; Shakil, M.; Khan, M.I.; Ijaz, M.; Mubeen, I.; Ashraf, M.; Riaz, K.N. Photocatalytic performance of hybrid WO3/TiO2 nanomaterials for the degradation of methylene blue under visible light irradiation. Int. J. Environ. Anal. Chem. 2021, 101, 1448–1460.
- Younas, U.; Ahmad, A.; Islam, A.; Ali, F.; Pervaiz, M.; Saleem, A.; Waseem, M.; Aljuwayid, A.M.; Habila, M.A.; Naqvi, S.R. Fabrication of a novel nanocomposite (TiO2/WO3/V2O5) by hydrothermal method as catalyst for hazardous waste treatment. Fuel 2023, 349, 128668.
- Han, X.; Kuang, Q.; Jin, M.; Xie, Z.; Zheng, L. Synthesis of Titania Nanosheets with a High Percentage of Exposed (001) Facets and Related Photocatalytic Properties. J. Am. Chem. Soc. 2009, 131, 3152–3153.
- Dai, Y.; Cobley, C.M.; Zeng, J.; Sun, Y.; Xia, Y. Synthesis of Anatase TiO2 Nanocrystals with Exposed Facets. Nano Lett. 2009, 9, 2455–2459.
- Liu, M.; Piao, L.; Zhao, L.; Ju, S.; Yan, Z.; He, T.; Zhou, C.; Wang, W. Anatase TiO2 single crystals with exposed and facets: Facile synthesis and enhanced photocatalysis. Chem. Commun. 2010, 46, 1664–1666.
- Yu, J.; Qi, L.; Jaroniec, M. Hydrogen Production by Photocatalytic Water Splitting over Pt/TiO2 Nanosheets with Exposed (001) Facets. J. Phys. Chem. C 2010, 114, 13118–13125.
- Sosnowchik, B.D.; Chiamori, H.C.; Ding, Y.; Ha, J.-Y.; Wang, Z.L.; Lin, L. Titanium dioxide nanoswords with highly reactive, photocatalytic facets. Nanotechnology 2010, 21, 485601.
- Batzill, M. Fundamental aspects of surface engineering of transition metal oxide photocatalysts. Energy Environ. Sci. 2011, 4, 3275–3286.
- Sydorchuk, V.; Levytska, S.; Shcherban, N.; Khalameida, S. Transition metal oxides supported onto silica gel as visible light-driven photocatalysts. Res. Chem. Intermed. 2020, 46, 3997–4015.
- Bandaranayake, S.; Hruska, E.; Londo, S.; Biswas, S.; Baker, L.R. Small Polarons and Surface Defects in Metal Oxide Photocatalysts Studied Using XUV Reflection–Absorption Spectroscopy. J. Phys. Chem. C 2020, 124, 22853–22870.
- Koutavarapu, R.; Tamtam, M.R.; Rao, M.; Peera, S.G.; Shim, J. Recent progress in transition metal oxide/sulfide quantum dots-based nanocomposites for the removal of toxic organic pollutants. Chemosphere 2021, 272, 129849.
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582.
- Thompson, T.L.; Yates, J.T. TiO2-based Photocatalysis: Surface Defects, Oxygen and Charge Transfer. Top. Catal. 2005, 35, 197–210.
- Hotsenpiller, P.A.M.; Bolt, J.D.; Farneth, W.E.; Lowekamp, J.B.; Rohrer, G.S. Orientation Dependence of Photochemical Reactions on TiO2 Surfaces. J. Phys. Chem. B 1998, 102, 3216–3226.
- Giocondi, J.L.; Salvador, P.A.; Rohrer, G.S. The origin of photochemical anisotropy in SrTiO3. Top. Catal. 2007, 44, 529–533.
- Giocondi, J.L.; Rohrer, G.S. Spatial Separation of Photochemical Oxidation and Reduction Reactions on the Surface of Ferroelectric BaTiO3. J. Phys. Chem. B 2001, 105, 8275–8277.
- Giocondi, J.L.; Rohrer, G.S. The Influence of the Dipolar Field Effect on the Photochemical Reactivity of Sr2Nb2O7 and BaTiO3 Microcrystals. Top. Catal. 2008, 49, 18–23.
- Kalinin, S.V.; Bonnell, D.A.; Alvarez, T.; Lei, X.; Hu, Z.; Ferris, J.H.; Zhang, Q.; Dunn, S. Atomic Polarization and Local Reactivity on Ferroelectric Surfaces: A New Route toward Complex Nanostructures. Nano Lett. 2002, 2, 589–593.
- Bhardwaj, A.; Burbure, N.V.; Rohrer, G.S. Enhanced Photochemical Reactivity at the Ferroelectric Phase Transition in Ba1−xSrxTiO3. J. Am. Ceram. Soc. 2010, 93, 4129–4134.
- Bullard, J.W.; Cima, M.J. Orientation Dependence of the Isoelectric Point of TiO2 (Rutile) Surfaces. Langmuir 2006, 22, 10264–10271.
- Beck, T.; Klust, A.; Batzill, M.; Diebold, U.; Di Valentin, C.; Selloni, A. Surface Structure of TiO2(011)-(2×1). Phys. Rev. Lett. 2004, 93, 036104.
- Tao, J.; Luttrell, T.; Batzill, M. A two-dimensional phase of TiO2 with a reduced bandgap. Nat. Chem. 2011, 3, 296–300.
- Lahiri, J.; Batzill, M. Surface Functionalization of ZnO Photocatalysts with Monolayer ZnS. J. Phys. Chem. C 2008, 112, 4304–4307.
- Lahiri, J.; Senanayake, S.; Batzill, M. Soft X-ray photoemission of clean and sulfur-covered polar ZnO surfaces: A view of the stabilization of polar oxide surfaces. Phys. Rev. B 2008, 78, 155414.




