
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luís R. Silva | -- | 4073 | 2023-08-07 11:51:20 | | | |
| 2 | Peter Tang | + 1 word(s) | 4074 | 2023-08-08 02:45:30 | | |
Video Upload Options
The consumption of natural-based foods, including beans, fruits, legumes, nuts, oils, vegetables, spices, and whole grains, has been encouraged. This fact is essentially due to their content in bioactive phytochemicals, with the phenolic compounds standing out. Among them, anthocyanins have been a target of many studies due to the presence of catechol, pyrogallol, and methoxy groups in their chemical structure, which confer notable scavenging, anti-apoptotic, and anti-inflammatory activities, being already recommended as supplementation to mitigate or even attenuate certain disorders, such as diabetes, cancer, and cardiovascular and neurological pathologies. The most well-known anthocyanins are cyanidin 3-O-glucoside and cyanidin 3-O-rutinoside. They are widespread in nature, being present in considerable amounts in red fruits and red vegetables.
1. Introduction
2. Chemical Structure and Function of Anthocyanins
3. Major Sources of Anthocyanins
|
Source |
Maximum Anthocyanin Amount in FW (mg C3G/100 g) |
Maximum Anthocyanin Amount in DW (mg C3G/100 g) |
Dominant Anthocyanins |
Reference |
|---|---|---|---|---|
|
Fruits |
||||
|
Açaí |
282.5–303.7 |
Cy 3,5-hexose pentose, Cy 3-O-glucoside, Cy 3-O-rutinoside, Pg 3-O-glucoside, Pn 3-O-glucoside, Pn 3-O-rutinoside, Cy 3-(acetyl)hexose |
[51] |
|
|
Acerola |
6.5–8.4 |
Cy 3-rhamnoside, Pg 3-rhamnoside Cy, Pg |
[51] |
|
|
Apple |
30.07–71.49 * |
Cy 3-galactoside, Cy 3-O-glucoside, Cy 3-arabinoside, Pn 3-galactoside, Cy 7-arabinoside, Cy3-xyloside |
||
|
Blackberries |
70.3–201 |
909.3 |
Cy 3-O-glucoside, Cy 3-O-rutinoside, Cy 3-xyloside, Cy 3-malonylglucoside, Cy 3-dioxalylglucoside |
|
|
Black currants |
218.93 |
32,300 1 |
Dp 3-O-glucoside, Dp 3-O- rutinoside, Cy 3-O-glucoside, Cy 3-O- rutinoside |
|
|
Blueberries |
406.90 |
11601 |
Dp 3-O-galactoside, Dp 3-O-glucoside, Cy-3-O-galactoside, Dp 3-O-arabinoside, Cy-3-glucoside, Pt-3-galactoside, Cy 3-O-arabinoside, Pt 3-O-glucoside, Pn 3-O-arabinoside, Mv 3-O-galactoside, Mv 3-O-glucoside |
|
|
Chokeberries |
357.20 |
Cy 3-O-galactoside, Cy 3-arabinoside, Cy 3-O-glucoside, Cy xyloside |
||
|
Cranberries |
40.7–207.3 1 |
Cy 3-O-galactoside Cy 3-O-glucoside, Cy arabinoside, Pn galactoside, Pn 3-O-glucoside, Pn arabinoside |
[59] |
|
|
Elderberries |
317.51 |
408.6–1066.6 |
Cy 3-O-glucoside, Cy 3-O-sambubioside |
|
|
Fig |
0.3–10.9 |
4.6–83 |
Cy 3-O-glucoside, Cy 3-O-rutinoside, Cy 3-sambubioside-5-glucoside, Cy 3,5-diglucoside |
|
|
Grapes |
38.70–186.02 1 |
135,960–533,630 1 |
Cy, Dp, Mv, Pn, Pt 3-O-glycosides; Mv, Pn, Pt 3-O-coumarylglucosides |
|
|
Haskaps |
449–697 |
2273 |
Cy 3,5-di-glucoside; Cy 3-galactoside, Cy 3-O-glucoside, Cy 3-O-rutinoside, Pg 3-O-glucoside; Pn 3-O-glucoside |
|
|
Nectarine |
0.22 |
Cy 3-O-glucoside, Cy 3-O-rutinoside |
||
|
Plums |
7.4–36.6 1 |
Cy 3-xyloside, Cy 3-O-glucoside, Cy 3-O-rutinoside, Pn 3-O-rutinoside, Pn 3-O-glucoside, Cy 3-galactoside, Cy 3-(6’’-acetoyl)glucoside |
||
|
Pomegranate |
1500–2000 |
Dp 3,5-diglucoside, Cy 3-O-glucoside, Cy 3,5-diglucoside, Pg 3-O-glucoside, Pg 3,5-diglucoside |
||
|
Peaches |
0.27–2.50 |
0.28–15.34 1 |
Cy 3-O-rutinoside and glucoside |
|
|
Red cabbages |
109–185 |
1111–1780 |
Cy 3-diglucoside-5-glucoside, Cy 3-(sinapoyl)(sinapoyl)-diglucoside-5-glucoside, Cy 3-(ρ-coumaroyl)-diglucoside-5-glucoside |
|
|
Red currants |
19.78 |
149.91 1 |
Cyanidin-3-O-sambusoside, Cy 3-O-glucoside, Cy 3-O-rutinoside |
|
|
Red pears |
1.2–12.0 1 |
Cy 3-O-galactoside, Cy 3-O-glucoside, Cy pentoside, Cy 3-O-arabinoside, Cy 3-O-rutinoside |
[76] |
|
|
Red raspberries |
23.17–68.0 |
260.9–571.8 |
Cy 3-O-sophoroside, Cy 3-O-(2’’-O-glucosyl)rutinoside, Cy 3-O-glucoside, Cy 3-O-rutinoside, Cy 3-O-(2’’-O-xylosyl)rutinoside, Pg 3-O-sophoroside, Pg 3-O-glucoside, Cy 3,5-O-diglucoside |
|
|
Strawberries |
20–60 1 |
31.9–315.2 2 |
Pg 3-O-glucoside, Pg 3-O-glucoside, Cy 3-O-glucoside, Cy 3-O-rutinoside, Pg 3-O-glucoside, Pg 3-O-rutinoside, Pg 3-(malonoyl)glucoside, Pg 3-(6’’-acetoyl)glucoside |
|
|
Sweet cherries |
2.06–462.77 1 |
107.70–218.36 1 |
Cy, Dp, Pg, Pn 3-O-rutinosides and glucosides, Cy 3-coumaroyl-diglucoside, Cy 3-O-sambubioside, Cy 3-5-diglucoside, Cy 3-sophoroside Cy 3-O-arabinoside Mv 3-O-glucoside-acetaldehyde |
|
|
Tamarillo |
29.70–486.84 1 |
Dp 3-O-rutinoside, Cy 3-O-rutinoside, Cy 3-O-glucoside, Pg 3-O-rutinoside |
[82] |
|
|
Tart cherries |
65.06–82.40 |
114.59 |
Cy, Cy 3-O-sophoroside, Cy 3-glucosylrutinoside, Cy 3-O-glucoside, Cy 3-O-rutinoside, Pn 3-O-rutinoside |
|
|
Tomato |
7.1 1 |
5.48–29.86 3 |
Dp glycoside, Dp rutinoside, Dp ρ-coumaroyl-rutinoside Mv glycoside, Mv rutinoside, Mv ρ-coumaroyl-rutinoside-glycoside, Pt rutinoside, Pt ρ-coumaroyl-rutinoside, Pt ρ-coumaroyl-rutinoside-glycoside |
|
|
Vegetables |
||||
|
Black carrot |
22.45 * |
1.74–4.54 1 |
Cy 3-(ρ-coumaroyl)-diglucoside-5-glucoside |
|
|
Eggplant |
6.31 |
138 4 |
Dp 3-(ρ-coumaroylrutinoside)-5-glucoside, Dp 3-O-glucoside, Dp 3-glucosyl-rhamnoside, Pt -3-O-rutinoside, Cy -3-O-rutinoside |
|
|
Purple sweet potato |
42.37 * |
Pn 3-O-sophoroside-5-O-glucoside, Pn 3-O-glucoside, Cy 3-ρ-hydroxybenzoylsophoroside-5-glucoside, Pn 3-ρ-hydroxybenzoylsophoroside-5-glucoside, Cy 3-caffeoylsophoroside-5-glucoside, Pn 3-caffeoylsophoroside-5-glucoside, Cyanidin-3-caffeoyl- ρ-hydroxybenzoylsophoroside-5-glucoside, Pn 3-dicaffeoylsophoroside-5-glucoside, Pn 3-caffeoyl-ρ-hydroxybenzoylsophoroside-5-glucoside, Pn 3-caffeoy-feruloylsophoroside-5-glucosie |
[47] |
|
|
Red Chicory |
39.20 * |
Cy 3-O-glucoside, Cy 3-O-(6”-malonyl-glucoside) |
[47] |
|
|
Red onion |
29.99 |
Cy 3-O-glucoside, Cy 3-O-laminaribioside, Cy 3-(6’’-malonyl-glucoside), Cy 3-(6”-malonyl- laminaribioside), Cy 3-xylosylglucosylgalactoside, Dp 3,5-diglycosides |
||
|
Beverages |
||||
|
Blackberry juice |
12.3–107 |
Cy 3-O-glycoside, Cy 3-O-rutinoside, Cy 3-xyloside, Cy malonylglucoside, Cy dioxalylglucoside |
[54] |
|
|
Pomegranate juice |
7.2–20 1 |
Cy 3-O-glucoside, Cy 3,5-diglucoside, Dp 3,5-diglucoside, Cy 3,5-diglucoside, Pg 3,5-diglucoside, Dp 3-glucoside, Cy 3- O-glucoside; Pg 3-O-glucoside |
[88] |
|
|
Red wine |
32.71–87.17 1 |
Cy, Dp, Mv, Pn, Pt 3-O-glycosides, Pn 3-O-acetylglucoside, Mv 3-O-acetylglucoside, Mv 3-O-coumarylglucoside, Pn 3-O-ρ-coumarylglucoside; |
||
|
Tart cherry juice |
72.2 |
Cy 3-sophoroside, Cy 3-glucosylrutinoside, Cy 3-O-glucoside, Cy 3-O-rutinoside, Cy, Pg, Pn 3-O-glucoside |
[90] |
|
FW, fresh weight; DW, dry weight; Cy, cyanidin; Dp, delphinidin; Mv, malvidin; Pg, pelargonidin; Pn, peonidin; Pt, petunidin. * mg cyanidin 3-galactoside equivalent per 100 g. 1 Total amount (weight) of anthocyanins identified by HPLC method. 2 mg pelargonidin 3-glucoside equivalent per 100 g. 3 mg malvidin 3-rutinoside equivalent per 100 g. 4 Delphinidin 3-glucoside equivalent per 100 g.
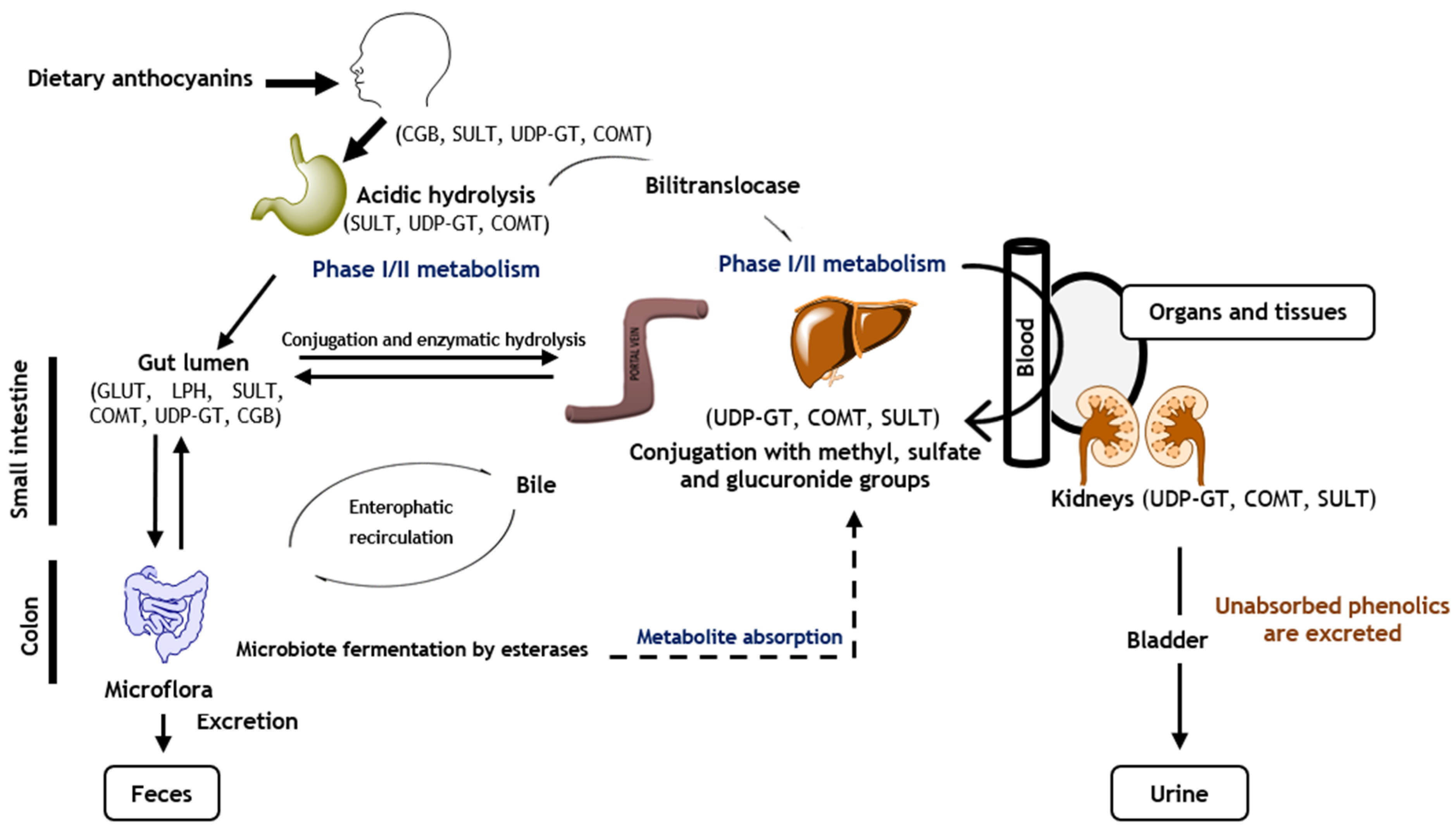
4. Anthocyanins’ Bioavailability and Metabolism
|
Intake |
n (a) |
Total Anthocyanins Intake |
Cmax (b) |
tmax (h) (c) |
AUC (d) |
Urinary Excretion (%) |
Reference |
|---|---|---|---|---|---|---|---|
|
Foods |
|||||||
|
Blueberries (100 g) |
5 |
1200 mg |
13.1 ng/mL |
4 |
[57] |
||
|
Elderberries (12 g) |
4 |
720 mg |
97.4 nmol/L |
1.2 h |
[94] |
||
|
Red raspberries (300 g) |
9 |
292 µmol |
0.1–180 nmol/L |
1–1.5 |
0.007% (1–1.5 h) |
[77] |
|
|
Beverages |
|||||||
|
Red wine (300 mL) |
6 |
218 mg |
6 |
1.5–5.1% (12 h) |
[89] |
||
|
Red grape juice (400 mL) |
9 |
Cy 3-O-glucoside |
0.42 ng/mL |
0.5 |
0.60 ng × h/mL (3 h) |
0.09% (7 h) |
[97] |
|
Dp 3-O-glucoside |
6.12 ng/mL |
0.5 |
11.9 ng × h/mL (3 h) |
0.20% (7 h) |
|||
|
Mv 3-O-glucoside |
48.8 ng/mL |
0.5 |
71.7 ng × h/mL (3 h) |
0.18% (7 h) |
|||
|
Pn 3-O-glucoside |
27.3 ng/mL |
0.5 |
49.7 ng × h/mL (3 h) |
0.29% (7 h) |
|||
|
Pt 3-O-glucoside |
16.1 ng/mL |
0.5 |
31.5 ng × h/mL (3 h) |
0.32% (7 h) |
|||
|
Σ = 283.5 mg |
100.1 ng/mL |
0.5 |
168.4 ng × h/mL |
||||
|
Black currant juice (150 mL) |
8 |
Cy 3-O-glucoside: 0.165 mg |
5.0 nmol/L |
1.34 |
11.0–19.6 ng × h/mL (4 h) |
0.060% (8 h) |
[96] |
|
Cy 3-O-rutinoside: 1.24 mg |
46.3 nmol/L |
3.45 |
19.6–24.9 ng × h/mL (4 h) |
0.098% (8 h) |
|||
|
Dp 3-O-glucoside: 0.488 mg |
22.7 nmol/L |
4.19 |
11.0–16.3 ng × h/mL (4 h) |
0.066 (8 h) |
|||
|
Dp 3-O-rutinoside: 1.68 mg |
73.4 nmol/L |
3.18 |
16.3–24.9 ng × h/mL (4 h) |
0.11% (8 h) |
|||
|
Açaí Juice (7 mL/kg of body weight) |
12 |
165.9 mg/L |
1138.51 ng/L |
2 |
3314.04 ng × h/L (12 h) |
[11] |
|
|
Cranberry Juice (480 mL) |
15 |
Cy 3-O-galactoside (18.7 mg) |
1.38 nmol/L |
1.27 |
3.91 nmol × h/L (4 h) |
0.007% (4 h) |
[98] |
|
Cy 3-O-glucoside (1.58 mg) |
0.93 nmol/L |
1.13 |
1.99 nmol × h/L (4 h) |
0.007% (4 h) |
|||
|
Cy 3-O-arabinoside (16.47 mg) |
3.61 nmol/L |
1.47 |
9.16 nmol × h/L (4 h) |
0.010% (4 h) |
|||
|
Mv 3-O-glucoside |
0.56 nmol/L |
0.93 |
1.25 nmol × h/L (4 h) |
||||
|
Pn 3-O-galactoside (30.83 mg) |
4.64 nmol/L |
1.47 |
12.00 nmol × h/L (4 h) |
0.015% (4 h) |
|||
|
Pn 3-O-glucoside (5.85 mg) |
0.71 nmol/L |
1.40 |
1.85 nmol × h/L (4 h) |
0.029% (4 h) |
|||
|
Pn 3-O-arabinose (21.03 mg) |
1.78 nmol/L |
1.27 |
4.13 nmol × h/L (4 h) |
0.010% (4 h) |
|||
|
Σ = 94.47 mg |
|||||||
|
Tart cherry juice (60 mL) |
12 |
62.47 mg/L |
2.75 µg × h/mL |
1 |
106.4 µg × h/mL |
[99] |
|
|
Grape/blueberry juice (330 mL) |
10 |
3,4-dihydroxybenzoic acid |
7.6 nmol/L |
1 |
568 nmol × min/L |
[100] |
|
|
Cy 3-O-glucoside |
0.10 nmol/L |
1 |
6 nmol × min/L |
||||
|
Dp 3-O-glucoside |
0.18 nmol/L |
1.1 |
10 nmol × min/L |
||||
|
Mv 3-O-glucoside |
1.5 nmol/L |
1.1 |
103 nmol × min/L |
||||
|
Mv 3-O-glucuronide |
1.1 nmol/L |
2 |
114 nmol × min/L |
||||
|
Pn 3-O-glucuronide |
1.1 nmol/L |
1.8 |
114 nmol × min/L |
||||
|
Pn 3-O-glucoside |
1.7 nmol/L |
1 |
52 nmol × min/L |
||||
|
Pt 3-O-glucoside |
0.8 nmol/L |
1 |
12 nmol × min/L |
||||
|
Σ = 841 mg/L |
1.21 nmol/L |
||||||
|
Orange juice |
18 |
53.09 mg/L |
0.63 nmol/L |
0.96 |
8.99 nmol × h/L (8 h) |
43–53% (2 h) |
[101] |
|
Chokeberry juice (0.8 mg/kg of body weight) |
13 |
32.7 nmol/L |
1.3 |
109.4 nmol × h/L (1 h) |
0.25 (24 h) |
[102] |
|
|
Strawberry juice (34.7 mg) |
14 |
Cy 3-O-glucoside: 7.8 µmol |
0.6 nmol/L |
2.1 |
1.7 nmol × h/L (10 h) |
[95] |
|
|
Pg glucuronide |
38.0 nmol/L |
1.7 |
123.8 nmol × h/L (10 h) |
||||
|
Pg-3-O-glucoside: 58.8 µmol |
5.2 nmol/L |
1.3 |
15.0 nmol × h/L (10 h) |
||||
|
Pg 3-O-rutinoside: 9.7 µmol |
0.4 nmol/L |
1.9 |
1.4 nmol × h/L (10 h) |
||||
|
Σ = 76.6 µmol |
|||||||
5. Anthocyanin Encapsulation
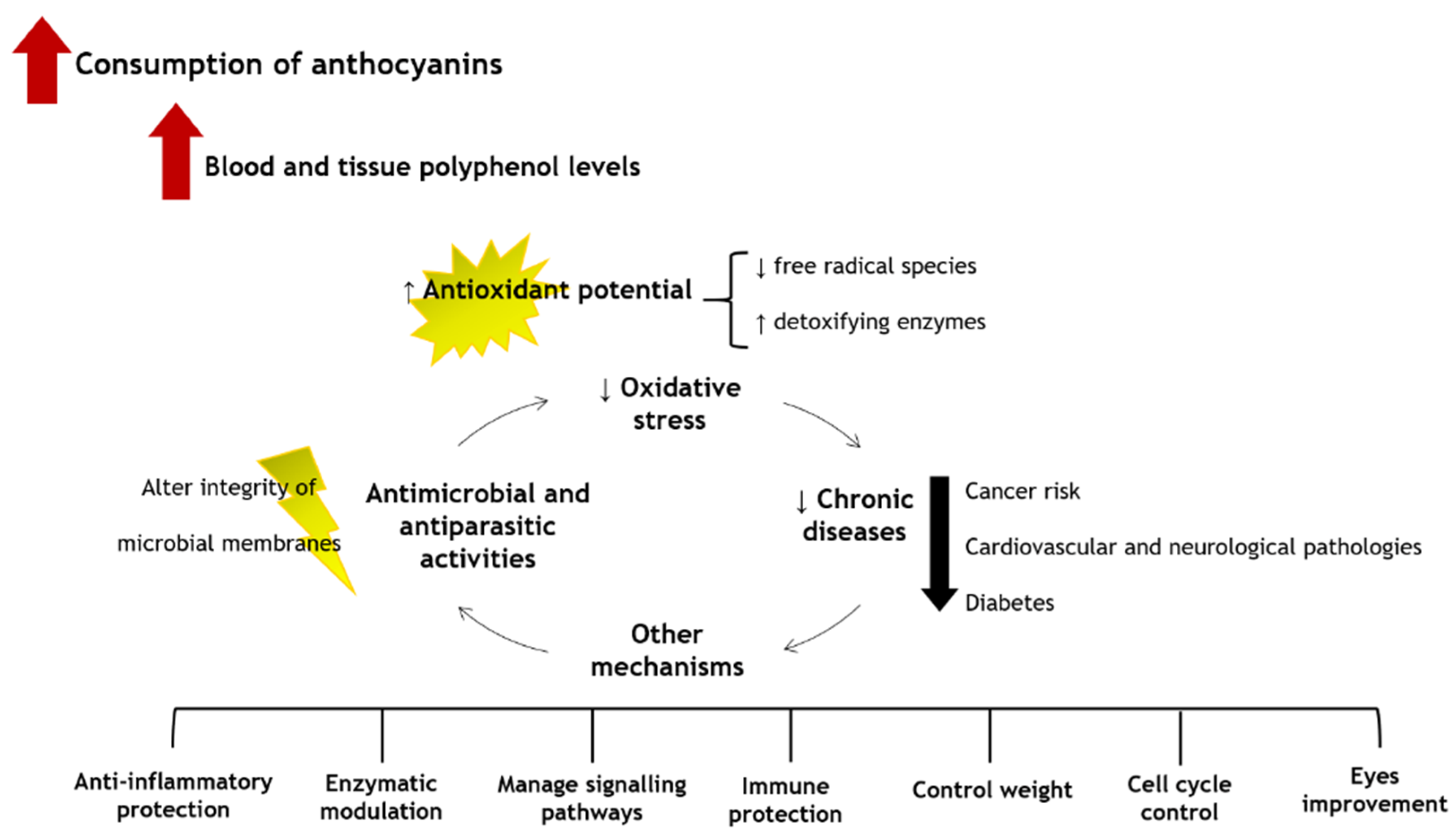
6. Putative Health Benefits
References
- Mannino, G.; Perrone, A.; Campobenedetto, C.; Schittone, A. Phytochemical profile and antioxidative properties of Plinia trunciflora fruits: A new source of nutraceuticals. Food Chem. 2020, 307, 125515.
- Ożarowski, M.; Karpiński, T.M.; Szulc, M.; Wielgus, K.; Kujawski, R.; Wolski, H.; Seremak-Mrozikiewicz, A. Plant phenolics and extracts in animal models of preeclampsia and clinical trials—Review of perspectives for novel therapies. Pharmaceuticals 2021, 14, 269.
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803.
- Martín, J.; Kuskoski, E.M.; Navas, M.J.; Asuero, A.G. Antioxidant capacity of anthocyanin pigments. In Flavonoids-From Biosynthesis to Human Health; Justino, G.C., Ed.; IntechOpen: London, UK, 2017; pp. 205–255.
- Aziz, M.A.; Sarwar, M.S.; Akter, T.; Uddin, M.S.; Xun, S.; Zhu, Y.; Islam, M.S.; Hongjie, Z. Polyphenolic molecules targeting STAT3 pathway for the treatment of cancer. Life Sci. 2021, 268, 118999.
- Grosso, G.; Stepaniak, U.; Topor-Madry, R.; Szafraniec, K.; Pajak, A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition 2014, 30, 1398–1403.
- Alappat, B.; Alappat, J. Anthocyanin pigments: Beyond aesthetics. Molecules 2020, 25, 5500.
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779.
- Bendokas, V.; Skemiene, K.; Trumbeckaite, S.; Passamonti, S.; Borutaite, V.; Liobikas, J. Anthocyanins: From plant pigments to health benefits at mitochondrial level. Crit. Rev. Food Sci. Nutr. 2020, 60, 3352–3365.
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The influence of supplementation of anthocyanins on obesity-associated comorbidities: A concise review. Foods 2020, 9, 687.
- Mertens-Talcott, S.U.; Rios, J.; Jilma-Stohlawetz, P.; Pacheco-Palencia, L.A.; Meibohm, B.; Talcott, S.T.; Derendorf, H. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich açai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J. Agric. Food Chem. 2008, 56, 7796–7802.
- Lynn, A.; Mathew, S.; Moore, C.T.; Russell, J.; Robinson, E.; Soumpasi, V.; Barker, M.E. Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: A randomised controlled trial. Plant Foods Hum. Nutr. 2014, 69, 122–127.
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849.
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2017, 56, 333–341.
- Bowtell, J.L.; Aboo-Bakkar, Z.; Conway, M.; Adlam, A.-L.R.; Fulford, J. Enhanced task related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl. Physiol. Nutr. Metab. 2017, 42, 773–779.
- Kwon, S.H.; Ahn, I.S.; Kim, S.-O.; Kong, C.S.; Chung, H.Y.; Do, M.S.; Park, K.Y. Anti-obesity and hypolipidemic effects of black soybean anthocyanins. J. Med. Food 2007, 10, 552–556.
- Thompson, K.; Hosking, H.; Pederick, W.; Singh, I.; Santhakumar, A.B. The effect of anthocyanin supplementation in modulating platelet function in sedentary population: A randomised, double-blind, placebo-controlled, cross-over trial. Br. J. Nutr. 2017, 118, 368–374.
- Shim, S.H.; Kim, J.M.; Choi, C.Y.; Kim, C.Y.; Park, K.H. Ginkgo biloba extract and bilberry anthocyanins improve visual function in patients with normal tension glaucoma. J. Med. Food 2012, 15, 818–823.
- Li, L.; Lyall, G.K.; Martinez-blazquez, J.A.; Vallejo, F.; Tomas-barberan, F.A.; Birch, K.M.; Boesch, C. Blood orange juice consumption increases flow-mediated dilation in adults with overweight and obesity: A randomized controlled trial. J. Nutr. 2020, 150, 2287–2294.
- Tang, P.; Monica Giusti, M. Metal chelates of petunidin derivatives exhibit enhanced color and stability. Foods 2020, 9, 11–15.
- Mladěnka, P.; Říha, M.; Martin, J.; Gorová, B.; Matějíček, A.; Spilková, J. Fruit extracts of 10 varieties of elderberry (Sambucus nigra L.) interact differently with iron and copper. Phytochem. Lett. 2016, 18, 232–238.
- Sinopoli, A.; Calogero, G.; Bartolotta, A. Computational aspects of anthocyanidins and anthocyanins: A review. Food Chem. 2019, 297, 124898.
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075.
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Slimani, N.; Romieu, I.; Fedirko, V.; Magistris, M.S.; Ericson, U.; Amiano, P.; Trichopoulou, A.; et al. Estimated dietary intakes of flavonols, flavanones and flavones in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24 hour dietary recall cohort. Br. J. Nutr. 2011, 106, 1915–1925.
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape berry secondary metabolites and their modulation by abiotic factors in a climate change scenario—A review. Front. Plant Sci. 2021, 12, 1–26.
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118.
- Legua, P.; Domenech, A.; Martinez, J.J.; Sánchez-Rodríguez, L.; Hernández, F.; Carbonell-Barrachina, A.A.; Melgarejo, P. Bioactive and volatile compounds in sweet cherry cultivars. J. Food Nutr. Res. 2017, 5, 844–851.
- Bresciani, L.; Martini, D.; Mena, P.; Tassotti, M.; Calani, L.; Brigati, G.; Brighenti, F.; Holasek, S.; Malliga, D.E.; Lamprecht, M.; et al. Absorption profile of (poly)phenolic compounds after consumption of three food supplements containing 36 different fruits, vegetables, and berries. Nutrients 2017, 9, 194.
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23.
- Landete, J.M. Dietary intake of natural antioxidants: Vitamins and polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721.
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263.
- Diaconeasa, Z.; Ioana, S.; Xiao, J.; Leopold, N.; Ayvaz, Z.; Danciu, C.; Ayvaz, H.; Sttǎnilǎ, A.; Nistor, M.; Socaciu, C. Anthocyanins, vibrant color pigments, and their role in skin cancer prevention. Biomedicines 2020, 8, 336.
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809.
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural antioxidant anthocyanins—A hidden therapeutic candidate in metabolic disorders with major focus in neurodegeneration. Nutrients 2019, 11, 1195.
- Ribnickya, D.M.; Roopchand, D.E.; Oren, A.; Grace, M.; Poulev, A.; Lila, M.A.; Havenaar, R.; Raskin, I. Effects of a high fat meal matrix and protein complexation on the bioaccessibility of blueberry anthocyanins using the TNO gastrointestinal model (TIM-1). Food Chem. 2014, 142, 349–357.
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622.
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radic. Res. 2006, 40, 1014–1028.
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451.
- White, B.L.; Howard, L.R.; Prior, R.L. Proximate and polyphenolic characterization of cranberry pomace. J. Agric. Food Chem. 2010, 58, 4030–4036.
- Gonçalves, A.C.; Campos, G.; Alves, G.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Physical and phytochemical composition of 23 Portuguese sweet cherries as conditioned by variety (or genotype). Food Chem. 2021, 335, 127637.
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508.
- Escobar-Cévoli, R.; Castro-Espín, C.; Béraud, V.; Buckland, G.; Zamora-Ros, R. An overview of global flavonoid intake and its food sources. In Flavonoids—From Biosynthesis to Human Health; Justino, G.C., Ed.; InTech: London, UK, 2017; pp. 371–391.
- Igwe, E.O.; Charlton, K.E.; Probst, Y.C. Usual dietary anthocyanin intake, sources and their association with blood pressure in a representative sample of Australian adults. J. Hum. Nutr. Diet. 2019, 32, 578–590.
- Chinese Nutrition Society. Chinese DRIs Handbook; Standards Press of China: Beijing, China, 2013.
- Kim, K.; Vance, T.M.; Chun, O.K. Estimated intake and major food sources of flavonoids among US adults: Changes between 1999–2002 and 2007–2010 in NHANES. Eur. J. Nutr. 2016, 55, 833–843.
- Wu, X.; Prior, R.L. Identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: Fruits and berries. J. Agric. Food Chem. 2005, 53, 2589–2599.
- Frond, A.D.; Iuhas, C.I.; Stirbu, I.; Leopold, L.; Socaci, S.; Andreea, S.; Ayvaz, H.; Andreea, S.; Mihai, S.; Zorita, D.; et al. Phytochemical characterization of five edible purple-reddish vegetables: Anthocyanins, flavonoids, and phenolic acid derivatives. Molecules 2019, 24, 1536.
- Kharadze, M.; Japaridze, I.; Kalandia, A.; Vanidze, M. Anthocyanins and antioxidant activity of red wines made from endemic grape varieties. Ann. Agrar. Sci. 2018, 16, 181–184.
- Horincar, G.; Enachi, E.; Bolea, C.; Râpeanu, G.; Aprodu, I. Value-added lager beer enriched with eggplant (Solanum melongena L.) peel extract. Molecules 2020, 25, 731.
- Zambrano-Moreno, E.L.; Chávez-Jáuregui, R.N.; Plaza, M.d.L.; Wessel-Beaver, L. Phenolic content and antioxidant capacity in organically and conventionally grown eggplant (Solanum melongena) fruits following thermal processing. Food Sci. Technol. 2015, 35, 414–420.
- de Rosso, V.V.; Hillebrand, S.; Montilla, E.C.; Bobbio, F.O.; Winterhalter, P.; Mercadante, A.Z. Determination of anthocyanins from acerola (Malpighia emarginata DC.) and açai (Euterpe oleracea Mart.) by HPLC–PDA–MS/MS. J. Food Compos. Anal. 2008, 21, 291–299.
- Polat, M.; Okatan, V.; Güclü, S.F.; Çolak, A.M. Determination of some chemical characteristics and total antioxidant capacity in apple varieties grown in Posof/Ardahan region. Int. J. Agric. Environ. Food Sci. 2018, 2, 131–134.
- Wang, S.Y.; HsinShan, L. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146.
- Fan-Chiang, H.-J.; Wrolstad, R.E. Anthocyanin pigment composition of blackberries. JFS C Food Chem. Toxicol. 2005, 70, 198–202.
- Jakobek, L.; Seruga, M.; Novak, I.; Medvidovic-Kosanovic, M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch. Leb. 2007, 103, 369–378.
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black currant anthocyanins attenuate weight gain and improve glucose metabolism in diet-induced obese mice with intact, but not disrupted, gut microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180.
- Mazza, G.; Kay, C.D.; Cottrell, T.; Holub, B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 2002, 50, 45–48.
- Zielińska, A.; Siudem, P.; Paradowska, K.; Gralec, M.; Kaźmierski, S.; Wawer, I. Aronia melanocarpa fruits as a rich dietary source of chlorogenic acids and anthocyanins: 1H-NMR, HPLC-DAD, and chemometric studies. Molecules 2020, 25, 3234.
- Jasutiene, L.; Cesonienė, I.; Sarkinas, A. Phenolics and anthocyanins in berries of European cranberry and their antimicrobial activity. Medicina 2009, 45, 992–999.
- Duymuş, H.G.; Göger, F.; Başer, K.H.C. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119.
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gottlieb, H.E.; Altman, A.; Kerem, Z.; Flaishman, M.A. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J. Agric. Food Chem. 2006, 54, 7717–7723.
- Kamiloglu, S.; Capanoglu, E. Polyphenol content in figs (Ficus carica L.): Effect of sun-drying. Int. J. Food Prop. 2015, 18, 521–535.
- Silva, L.R.; Queiroz, M. Bioactive compounds of red grapes from Dão region (Portugal): Evaluation of phenolic and organic profile. Asian Pac. J. Trop. Biomed. 2016, 6, 315–321.
- Kallithraka, S.; Aliaj, L.; Makris, D.P.; Kefalas, P. Anthocyanin profiles of major red grape (Vitis vinifera L.) varieties cultivated in Greece and their relationship with in vitro antioxidant characteristics. Int. J. Food Sci. Technol. 2009, 44, 2385–2393.
- Khattab, R.; Brooks, M.S.-L.; Ghanem, A. Phenolic analyses of haskap berries (Lonicera caerulea L.): Spectrophotometry versus high performance liquid chromatography. Int. J. Food Prop. 2016, 19, 1708–1725.
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Optimization of ultrasound-assisted extraction of anthocyanins from haskap berries (Lonicera caerulea L.) using Response Surface Methodology. Ultrason. Sonochem. 2015, 27, 449–455.
- Cantín, C.M.; Moreno, M.A.; Gogorcena, Y. Evaluation of the antioxidant capacity, phenolic compounds, and vitamin C content of different peach and nectarine breeding progenies. J. Agric. Food Chem. 2009, 57, 4586–4592.
- Usenik, V.; Štampar, F.; Veberič, R. Anthocyanins and fruit colour in plums (Prunus domestica L.) during ripening. Food Chem. 2009, 114, 529–534.
- Passafiume, R.; Perrone, A.; Sortino, G.; Gianguzzi, G.; Saletta, F.; Gentile, C.; Farina, V. Chemical-physical characteristics, polyphenolic content and total antioxidant activity of three Italian-grown pomegranate cultivars. NFS J. 2019, 16, 9–14.
- Zhu, F.; Yuan, Z.; Zhao, X.; Yin, Y.; Feng, L. Composition and contents of anthocyanins in different pomegranate cultivars. Acta Hortic. 2015, 1089, 35–41.
- Mihaylova, D.; Popova, A.; Desseva, I.; Petkova, N.; Stoyanova, M.; Vrancheva, R.; Slavov, A.; Slavchev, A.; Lante, A. Comparative study of early- and mid-ripening peach (Prunus persica L.) varieties: Biological activity, macro-, and micro- nutrient profile. Foods 2021, 10, 164.
- Bento, C.; Gonçalves, A.C.; Silva, B.; Silva, L.R. Assessing the phenolic profile, antioxidant, antidiabetic and protective effects against oxidative damage in human erythrocytes of peaches from Fundão. J. Funct. Foods 2018, 43, 224–233.
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309.
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Anthocyanins contents, profiles, and color characteristics of red cabbage extracts from different cultivars and maturity stages. J. Agric. Food Chem. 2014, 62, 7524–7531.
- Jara-Palacios, M.J.; Santisteban, A.; Gordillo, B.; Hernanz, D.; Heredia, F.J.; Escudero-Gilete, M.L. Comparative study of red berry pomaces (blueberry, red raspberry, red currant and blackberry) as source of antioxidants and pigments. Eur. Food Res. Technol. 2019, 245, 1–9.
- Galvis Sánchez, A.C.; Gil-Izquierdo, A.; Gil, M.I. Comparative study of six pear cultivars in terms of their phenolic and vitamin C contents and antioxidant capacity. J. Sci. Food Agric. 2003, 83, 995–1003.
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.J.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015, 89, 758–769.
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Booren, M.; Gray, I.; Dewitt, D.L. Cyclooxygenase active bioflavonoids from Balaton tart cherry and their structure activity relationships. Phytomedicine 2000, 7, 15–19.
- Silva, F.L.; Escribano-Bailón, M.T.; Pérez Alonso, J.J.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT-Food Sci. Technol. 2007, 40, 374–382.
- Gonçalves, A.C.; Bento, C.; Silva, B.M.; Silva, L.R. Sweet cherries from Fundão possess antidiabetic potential and protect human erythrocytes against oxidative damage. Food Res. Int. 2017, 95, 91–100.
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26.
- Diep, T.; Pook, C.; Yoo, M. Phenolic and anthocyanin compounds and antioxidant activity of Tamarillo (Solanum betaceum Cav.). Antioxidants 2020, 9, 169.
- Cao, J.; Jiang, Q.; Lin, J.; Li, X.; Sun, C.; Chen, K. Physicochemical characterisation of four cherry species (Prunus spp.) grown in China. Food Chem. 2015, 173, 855–863.
- Borghesi, E.; González-Miret, M.L.; Escudero-Gilete, M.L.; Malorgio, F.; Heredia, F.J.; Meléndez-Martínez, A.J. Effects of salinity stress on carotenoids, anthocyanins, and color of diverse tomato genotypes. J. Agric. Food Chem. 2011, 59, 11676–11682.
- Blando, F.; Berland, H.; Maiorano, G.; Durante, M.; Mazzucato, A.; Picarella, M.E.; Nicoletti, I.; Gerardi, C.; Mita, G.; Andersen, Ø.M. Nutraceutical characterization of anthocyanin-rich fruits produced by “Sun Black” tomato line. Front. Nutr. 2019, 6, 133.
- Kammerer, D.; Carle, R.; Schieber, A. Quantification of anthocyanins in black carrot extracts (Daucus carota ssp. sativus var. atrorubens Alef.) and evaluation of their color properties. Eur. Food Res. Technol. 2004, 219, 479–486.
- Shi-Lin, Z.; Peng, D.; Yu-Chao, X.; Jian-Jun, W. Quantification and analysis of anthocyanin and flavonoids compositions, and antioxidant activities in onions with three different colors. J. Integr. Agric. 2016, 15, 2175–2181.
- Legua, P.; Melgarejo, P.; Martínez, J.J.; Martínez, R.; Hernández, F. Evaluation of Spanish pomegranate juices: Organic acids, sugars, and anthocyanins. Int. J. Food Prop. 2012, 15, 481–494.
- Lapidot, T.; Harel, S.; Granit, R.; Kanner, J. Bioavailability of red wine anthocyanins as detected in human urine. J. Agric. Food Chem. 1998, 46, 4297–4302.
- Kirakosyan, A.; Seymour, E.M.; Llanes, D.E.U.; Kaufman, P.B.; Bolling, S.F. Chemical profile and antioxidant capacities of tart cherry products. Food Chem. 2009, 115, 20–25.
- Oliveira, H.; Roma-Rodrigues, C.; Santos, A.; Veigas, B.; Brás, N.; Faria, A.; Calhau, C.; De Freitas, V.; Baptista, P.V.; Mateus, N.; et al. GLUT1 and GLUT3 involvement in anthocyanin gastric transport- Nanobased targeted approach. Sci. Rep. 2019, 789, 1–14.
- European Food Safety Authority. Scientific opinion on the re-evaluation of anthocyanins (E 163) as a food additive. EFSA J. 2013, 11, 3145.
- Matsumoto, H.; Inaba, H.; Kishi, M.; Tominaga, S.; Hirayama, M.; Tsuda, T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J. Agric. Food Chem. 2001, 49, 1546–1551.
- Cao, G.; Muccitelli, H.U.; Sánchez-Moreno, C.; Prior, R.L. Anthocyanins are absorbed in glycated forms in elderly women: A pharmacokinetic study. Am. J. Clin. Nutr. 2001, 73, 920–926.
- Sandhu, A.K.; Huang, Y.; Xiao, D.; Park, E.; Edirisinghe, I.; Burton-Freeman, B. Pharmacokinetic characterization and bioavailability of strawberry anthocyanins relative to meal intake. J. Agric. Food Chem. 2016, 64, 4891–4899.
- Nielsen, I.L.F.; Dragsted, L.O.; Ravn-haren, G.; Freese, R.; Rasmussen, S.E. Absorption and excretion of black currant anthocyanins in humans and watanabe heritable hyperlipidemic rabbits. J. Agric. Food Chem. 2003, 51, 2813–2820.
- Bitsch, R.; Netzel, M.; Frank, T.; Strass, G.; Bitsch, I. Bioavailability and biokinetics of anthocyanins from red grape juice and red wine. J. Biomed. Biotechnol. 2004, 2004, 293–298.
- Milbury, P.E.; Vita, J.A.; Blumberg, J.B. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J. Nutr. 2010, 140, 1099–1104.
- Keane, K.M.; Bell, P.G.; Lodge, J.K.; Constantinou, C.L.; Jenkinson, S.E.; Bass, R.; Howatson, G. Phytochemical uptake following human consumption of Montmorency tart cherry (L. Prunus cerasus) and influence of phenolic acids on vascular smooth muscle cells in vitro. Eur. J. Nutr. 2016, 55, 1695–1705.
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Römpp, A.; Kunz, C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br. J. Nutr. 2015, 113, 1044–1055.
- Giordano, L.; Coletta, W.; Tamburrelli, C.; D’Imperio, M.; Crescente, M.; Silvestri, C.; Rapisarda, P.; Reforgiato Recupero, G.; De Curtis, A.; Iacoviello, L.; et al. Four-week ingestion of blood orange juice results in measurable anthocyanin urinary levels but does not affect cellular markers related to cardiovascular risk: A randomized cross-over study in healthy volunteers. Eur. J. Nutr. 2012, 51, 541–548.
- Wiczkowski, W.; Romaszko, E.; Piskula, M.K. Bioavailability of cyanidin glycosides from natural chokeberry (Aronia melanocarpa) juice with dietary-relevant dose of anthocyanins in humans. J. Agric. Food Chem. 2010, 58, 12130–12136.
- McGhie, T.K.; Ainge, G.D.; Barnett, L.E.; Cooney, J.M.; Jensen, D.J. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. J. Agric. Food Chem. 2003, 51, 4539–4548.
- Morazzoni, P.; Bombardelli, E. Vaccinium myrtillus L. Fitoterapia 1996, 67, 3–29.
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187.
- Oliveira, H.; Perez-Gregório, R.; Freitas, V.; Mateus, N.; Fernandes, I. Comparison of the in vitro gastrointestinal bioavailability of acylated and non-acylated anthocyanins: Purple-fleshed sweet potato vs red wine. Food Chem. 2018, 276, 410–418.
- Passamonti, S.; Vrhovsek, U.; Mattivi, F. The interaction of anthocyanins with bilitranslocase. Biochem. Biophys. Res. Commun. 2002, 296, 631–636.
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.Y.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197.
- Han, F.; Oliveira, H.; Brás, N.F.; Fernandes, I.; Cruz, L.; de Freitas, V.; Mateus, N. In vitro gastrointestinal absorption of red wine anthocyanins–Impact of structural complexity and phase II metabolization. Food Chem. 2020, 317, 126398.
- Ramos, P.; Herrera, R.; Moya-león, M.A. Anthocyanins: Food sources and benefits to consumer’s health. In Handbook of Anthocyanins; Warner, L.M., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 363–384.
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Brncic, S.R.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; De Pascual-Teresa, S. A review of factors affecting anthocyanin bioavailability: Possible implications for the inter-individual variability. Foods 2020, 9, 2.
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Kulozik, U.; Schwarz, K.; Richling, E. Encapsulation of anthocyanins from bilberries–Effects on bioavailability and intestinal accessibility in humans. Food Chem. 2018, 248, 217–224.
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853.
- Norberto, S.; Silva, S.; Meireles, M.; Faria, A.; Pintado, M.; Calhau, C. Blueberry anthocyanins in health promotion: A metabolic overview. J. Funct. Foods 2013, 5, 1518–1528.
- Azzini, E.; Bugianesi, R.; Romano, F.; Di Venere, D.; Miccadei, S.; Durazzo, A.; Foddai, M.S.; Catasta, G.; Linsalata, V.; Maiani, G. Absorption and metabolism of bioactive molecules after oral consumption of cooked edible heads of Cynara scolymus L. (cultivar Violetto di Provenza) in human subjects: A pilot study. Br. J. Nutr. 2007, 97, 963–969.
- Martini, S.; Conte, A.; Tagliazucchi, D. Bioactivity and cell metabolism of in vitro digested sweet cherry (Prunus avium) phenolic compounds. Int. J. Food Sci. Nutr. 2018, 70, 335–348.
- Dharmawansa, K.V.S.; Hoskin, D.W.; Rupasinghe, H.P. Chemopreventive effect of dietary anthocyanins against gastrointestinal cancers: A review of recent advances and perspectives. Int. J. Mol. Sci. 2020, 21, 6555.
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286.
- Lin, S.; Wang, Z.; Lam, K.L.; Zeng, S.; Tan, B.K.; Hu, J. Role of intestinal microecology in the regulation of energy metabolism by dietary polyphenols and their metabolites. Food Nutr. Res. 2019, 63, 1–12.
- Yang, L.; Ling, W.; Yang, Y.; Chen, Y.; Tian, Z.; Du, Z.; Chen, J.; Xie, Y.; Liu, Z.; Yang, L. Role of purified anthocyanins in improving cardiometabolic risk factors in chinese men and women with prediabetes or early untreated diabetes—A randomized controlled trial. Nutrients 2017, 9, 1104.
- Kim, M.J.; Rehman, S.U.; Amin, F.U.; Kim, M.O. Enhanced neuroprotection of anthocyanin-loaded PEG-gold nanoparticles against Aβ1-42-induced neuroinflammation and neurodegeneration via the NF-KB /JNK/GSK3β signaling pathway. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2533–2544.
- Amin, F.U.; Shah, S.A.; Badshah, H.; Khan, M.; Kim, M.O. Anthocyanins encapsulated by nanoparticles potentially improved its free radical scavenging capabilities via p38/JNK pathway against Aβ1-42-induced oxidative stress. J. Nanobiotechnol. 2017, 15, 12.
- Thibado, S.; Thornthwaite, J.; Ballard, T.; Goodman, B. Anticancer effects of bilberry anthocyanins compared with NutraNanoSphere encapsulated bilberry anthocyanins. Mol. Clin. Oncol. 2017, 8, 330–335.




