Among the phenolic compounds, anthocyanidins and their conjugated acyl-glycosylated or glycosylated forms, called anthocyanins, are both members of the flavonoids and an interesting class of water-soluble vacuolar pigments [
7]. They are synthetized via the flavonoid path and considered the major contributors to the vivid red, orange, violet, and blue colours exhibited by various edible flowers, vegetables, fruits, some cereals, seeds and plant leaves, and their derivatives, such as juices, tea, and red wines [
8]. They also have received much attention owing to their nutritional value, pharmacokinetic profile, pharmacological mechanisms, and health-promoting properties [
9,
10]. Indeed, recent human and animals surveys revealed that they are functional compounds able to increase antioxidant defences, diminish free radical damage, chronic inflammation and the risk of mutations, and attenuate, or even mitigate, the development and progression of many non-communicable and degenerative chronic disorders, namely, atherosclerosis, metabolic syndrome, eye and kidney complications, many cancer types, and also to control weight [
6,
11,
12,
13,
14,
15,
16,
17,
18,
19]. These biological activities are associated with their chemical structure, the presence of the catechol and pyrogallol groups standing out, allowing them to have the ability to chelate metal ions and neutralize free radicals and reactive species [
4,
20,
21,
22]. The predominant ones found in foodstuffs are cyanidin, delphinidin, pelargonidin, peonidin, petunidin, and malvidin glycosides [
6,
23,
24].
2. Chemical Structure and Function of Anthocyanins
Phenolic compounds are secondary metabolites produced by plants to protect them against pathogens and predators, ultraviolet radiation, climate conditions, and acidified soils, acting also as attractants for pollinators, antifeedants, and phytoalexins [
25,
26]. They are also considered the main contributors to plants’ colour, nutritional, and sensory characteristics [
27]. Their structure presents at least one benzene ring coupled to one or more hydroxyl groups and can range from simple phenolic, low molecular weight and single-aromatic molecules to highly polymerized compounds [
28]. In order to facilitate their distinction, phenolics are classified into two major groups: (i) non-flavonoid compounds (phenolic acids, tannins, lignans, coumarins, stilbenes, and curcuminoids) and (ii) flavonoid compounds (anthocyanidins, flavan-3-ols, and their oligomeric structures, recognized as proanthocyanidins, flavanones, flavanonols, flavones, flavonols, and isoflavones) [
7,
25,
26]. Their biosynthesis, comprises the shikimate, phenylpropanoid, and flavonoid pathways, and involves deamination, hydroxylation, and methylation reactions [
25].
Concerning the flavonoids, they represent about two-thirds of the total dietary phenolics consumed, and currently, more than 9000 different flavonoids have been identified so far [
29,
30]. They all possess a common flavan nucleus, i.e., a 15 carbon-structure (C6 (A ring)-C3 (C ring)-C6 (B ring)), composed of 2 aromatic rings (A and B rings derived from the acetate/malonate and shikimate pathways, respectively), linked by a heterocyclic benzopyran 3-carbon ring that contains an oxygen atom (C ring) [
26,
31]. However, they differ in (i) the C ring unsaturation and oxidation state; (ii) A, B, and C ring substituents, such as the presence or absence of double bonds and carbonyl groups, and the possible occurrence of acylation, alkylation, glycosylation, oxygenation, and sulphonation processes; (iii) the position where the B ring is linked to the C ring; and (iv) location and number of hydroxy and methoxy groups on the B ring [
26].
Focusing on anthocyanidins, their B ring is joined to the C ring through carbon 2 and the sugar residue of anthocyanins is typically found conjugated at carbon 3 [
32,
33]. Furthermore, their capacity to create flavylium cations makes them distinguishable from other flavonoid subclasses [
22]. To date, about 27 different structural anthocyanin aglycones and 1000 anthocyanins are known [
34,
35]. In foods, the most commonly found are sugar moieties of cyanidin (50%), which is the most studied given their large distribution and number of hydroxyl groups, followed by pelargonidin, peonidin, and delphinidin (12%), and finally by petunidin and malvidin glycosides (7%) [
8]. Together, they represent 90% of the total anthocyanins identified until now [
36]. Their structure directly influences their biological potential, namely, the number of hydroxyl groups, the degree of glycosylation and acylation, the catechol residue on the B ring, and the oxonium ion on the C ring [
37].
Therefore, the classification of anthocyanins is made according to (i) the number, position, and degree of methylation of the hydroxyl groups; (ii) the number and nature of the sugar moieties bonded to the aglycone; and (iii) the position of the aliphatic and/or aromatic carboxylate acids on the sugar molecule [
38]. The stability of the anthocyanins is usually affected by the storage and processing conditions, temperature, and cooking, as well as by exposure to light and oxygen, the presence of enzymes, other phenolic compounds, metal ions, ascorbic acid, hydrogen peroxide (H
2O
2), water, and/or sulphites [
37].
3. Major Sources of Anthocyanins
Anthocyanins are widely spread in nature and are considered mainly responsible for the vibrant red, blue, and purple colours exhibited by vegetables, fruits, and their derivatives [
37,
39]. Their levels differ markedly among different species, being largely influenced by plant genotypes, and less by agricultural practices, growing area, climatic conditions, seasonal variability, temperature and light exposure, ripening stage, harvest time, and the adopted methods for processing and storage [
9,
40].
The daily average intake of anthocyanins is estimated to vary from several milligrams to hundreds of milligrams, although its evaluation is inaccurate and depends on the diet, gender, existence (or not) of food intolerances in individuals, and their quantities in foods [
41]. Their intake by humans is higher in countries with a Mediterranean diet, plentiful in reddish berries and other red and blue-coloured fruits and vegetables, and red wine [
23,
42]. In Europe, the ingestion ranges from 19.8 mg per day (the Netherlands) to 64.9 mg per day (Italy) in men and 18.4 mg per day (Spain) to 44.1 mg per day (Italy) in women [
24]. In the United States of America, Australia, and Asian countries, the intake is about 12.5, 24.2, and 37 mg per day per person, respectively [
23,
42,
43]. Furthermore, and although it is not well established since they are non-essential nutrients, the recommended daily consumption of these coloured compounds has been evaluated, with China already recommending a daily intake of 50 mg per day/person in order to reduce oxidative stress levels and consequently the risk of cancer, metabolic syndrome, diabetes, degenerative diseases, and other pathologies [
44].
In a general way, the primary sources of anthocyanins are berries (43% in Europe and 39% in the USA), red wine (22% in Europe and 18% in the USA), vegetables, and other fruits (19% in Europe and 9% in the USA) [
45].
Table 1 presents the main sources of anthocyanins. A major amount of anthocyanins are found in berries, especially elderberries, chokeberries, blueberries, pomegranate, and açaí, presenting values superior to 282.5 mg cyanidin 3-glucoside equivalent per 100 g of fresh product [
46,
47]. Among the anthocyanins, cyanidin, malvidin, and delphinidin glycosides are the most found [
47,
48,
49,
50].
Table 1. Concentration of anthocyanins in fresh weight (FW) and dried weight (DW) in fruits, beverages, and vegetables.
FW, fresh weight; DW, dry weight; Cy, cyanidin; Dp, delphinidin; Mv, malvidin; Pg, pelargonidin; Pn, peonidin; Pt, petunidin. * mg cyanidin 3-galactoside equivalent per 100 g. 1 Total amount (weight) of anthocyanins identified by HPLC method. 2 mg pelargonidin 3-glucoside equivalent per 100 g. 3 mg malvidin 3-rutinoside equivalent per 100 g. 4 Delphinidin 3-glucoside equivalent per 100 g.
4. Anthocyanins’ Bioavailability and Metabolism
Understanding the bioavailability of phenolics is crucial since, after consumption, their constituents undergo many modifications throughout the digestive tract (digestion, absorption, metabolization, and elimination), which have a great impact on their nutritional value and health-promoting properties [
28,
31].
Nowadays, it is established that the bioavailability of phenolics differs significantly between them, and therefore, the most abundant polyphenols in our dietary habits are not necessarily those that show the highest concentrations of active metabolites in organs and tissues [
35]. Indeed, their bioavailability is highly dependent on the chemical structure of the phenolics, i.e., their molecular size, pattern of glycosylation and/or acylation (where acylation increases anthocyanin stability but declines their absorption), degree of polymerization, and conjugations and/or combinations with other compounds [
36,
91]. Furthermore, it is also influenced by the pH values observed along the gastrointestinal tract, facilitation of the compounds to cross membranes, digestibility, solubility, and absorption actions carried out by digestive enzymes, biliary acids, gut microbiota, and enterocytes. The food matrix maturity degree and cooking also influence the availability rates [
35]. Although the thermal processing reduces anthocyanins’ stability, at the same time, it damages the anthocyanin cell walls, increasing their body absorption [
28].
It is also important to take into account that the bioavailability also varies among individuals—inter-individual variability—due to intrinsic aspects (e.g., age, sex, physiological and/or pathological states, and genetic factors), which induce marked differences regarding enzymes and microbiota activity [
43].
Unlike other phenolics, anthocyanins are quickly metabolized and eliminated, and even in high amounts, they rarely reach concentration values considered active. In fact, the intake of 10 and 720 mg of anthocyanins only results in maximal plasma concentrations of 1.4 and 200 nanomolar, respectively, achieved between 30 min and 2 h [
92,
93,
94].
Table 2 summarizes the principal human trials focused on the pharmacokinetic profile of anthocyanins observed after their ingestion in common foods and beverages. In a general way, less than 1.8% of consumed anthocyanins is normally absorbed. However, this percentage can decrease if they are ingested alone, with or after other compounds; i.e., if anthocyanins are consumed alone or after overnight fasting, their digestion happens in 1 hour [
93,
95], whereas if they are ingested accompanied by other foods and beverages, ingested with high-fat meals, or after a meal, it occurs only after 1.5 or 4 h, respectively [
96]. They disappear from the blood circulatory system in less than 6 h [
57].
Table 2. Human studies considering the pharmacokinetic parameters of anthocyanins, after ingestion of common foods and beverages rich in this type of compound.
(a) Number of participants. (b) Maximum concentration in plasma. (c) Time to reach Cmax in plasma. (d) Area under the curve, which describes the exposure of a compound over a set period of time.
Additionally, and considering that substitutions influence anthocyanin absorption, nowadays, it is well-described that pelargonidin derivatives are more readily absorbed than anthocyanins with more substituents on their B ring, such as peonidin-, delphinidin-, and cyanidin-based anthocyanins [
46]. Furthermore, and comparing the sugar moieties, it was already verified that malvidin 3-
O-arabinoside presents higher absorption rates than malvidin 3-
O-glucoside [
103].
Despite their low absorption and rapid metabolism, the regular consumption of anthocyanins is considered safe, and together with physical activity, it is encouraged as both can reduce the occurrence of several pathologies related to oxidative stress [
36]. As far as we know, until now, no adverse effects regarding anthocyanin consumption have been reported. In fact, and focusing on human studies, most people who consumed 160 mg of anthocyanins twice a day for 2 months tolerated the extract; only 4% of the participants revealed side effects, namely at the gastrointestinal level and eczema [
104,
105].
Therefore, after being consumed, anthocyanins are metabolized in the mouth, where the action of oral microbiota removes the glycosidic groups and transforms them into their corresponding chalcones [
106].
After that, they pass along the gastrointestinal tract, starting in the stomach, where they do not suffer considerable changes, despite the acidic pH, and can be absorbed by bilitranslocase, becoming available for absorption (bioaccessibility) [
106,
107], or reach intestinal epithelial cells [
108]. In fact, the literature suggests that it is possible that the gastric and intestinal bioavailability of anthocyanins are mainly done with the 3-monoglucosides, 3-monoglucoside acylated, and 3,5-diglucosides forms [
106,
107,
109].
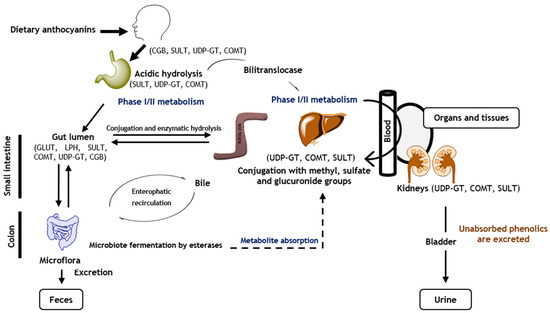
Therefore, anthocyanins can go through the portal vein to the liver and can be directed to the systemic circulation to be taken up by the target organs and tissues, or, if they are not absorbed, be discarded through urine and faeces (
Figure 1) [
41,
110]. It is important to note that in intestines, liver, and kidneys, anthocyanins are metabolized by enzymes of phase I and phase II, being conjugated with additional hydroxyl, methyl, sulfuric, or glycoside groups in order to increase their availability [
33,
36,
111].
Figure 1. Anthocyanin absorption, distribution, metabolism, and excretion. CGB—cytosolic β-glucosidase; SULT—sulfotransferase; UDP-GT—glucuronosyltransferase; COMT—catechol-O-methyl transferase; GLUT—glucose transporters; LPH—lactase-phlorizin hydrolase.
In general, both native anthocyanidins and their conjugated forms are found in plasma and urine; nevertheless, the intact ones are more rapidly absorbed in the stomach (20–25%) and detected in plasma a few minutes after their ingestion [
112]. This evidence is supported by previous studies based on the oral ingestion of red fruits, which revealed that anthocyanins are not metabolized into their aglycones, being directly absorbed and appearing in plasma 30 min after their consumption [
92]. Their glycosylated forms are excreted in urine [
8,
93].
Notwithstanding, the major absorption rate is observed in the gut [
33]. Therefore, in the gut, the lactase-phlorizin hydrolase (LPH) and
β-glucosidase enzymes release the aglycone of the coloured compounds, increasing their hydrophobic character, thus facilitating their entrance by passive diffusion in epithelial cells [
113]. Glycosides and acylated anthocyanins can also be absorbed by the small intestine due to the action of glucose transporters 1 and 3 (GLUT 1 and 3) [
91,
109,
111,
114]; however, the absorption of the acylated ones is four times lower than that of non-acylated anthocyanins [
33]. Particularly, molecular docking studies revealed that smaller molecules interact with GLUT 1 and 3 by their glucose residue and AC rings, while larger compounds penetrate in both transporters by their C5 glucose, as well as B and coumaroyl rings [
91,
109].
Unabsorbed anthocyanins reach the colon and are hydrolysated within 20 min−2 h by colonic bacteria (e.g.,
α-galactosidase,
β-D-glucuronidase,
β-D-glucosidase, and
α-rhamnosidase), which break down the glycosidic bonds and catalyse them into smaller phenolic compounds (e.g., benzaldehydes or hydroxytyrosol) or simple phenolic acids, such as
ρ-hydroxybenzoic, homovanillic, phenylpropionic, protocatechuic, syringic, and vanillic acids, to simplify their absorption by colonic mucosa and consequently increase their availability [
115]. This extensive metabolization is tremendously interesting and shows that the availability of anthocyanins is probably higher than we thought, and the reason why anthocyanins can be found in urine in amounts below 0.1% [
91,
116]. Indeed, a recent study indicates the intake of 300 g of red raspberries results in the identification of 18 different anthocyanin-derived metabolites [
77]. Basically, delphinidin-based anthocyanins are transformed into 2,4,6-trihydroxybenzaldehyde, gallic, and syringic acids, while syringic, 4-hydroxybenzoic, and vanillic acids are the primary metabolites of malvidin, pelargonidin, and peonidin glucosides, respectively [
111,
117]. Cyanidin glycosides can produce around 35 metabolites, 31 being found in urine samples, 28 in faeces, and 17 in the circulatory system, where the main ones are 2,4,6-trihydroxybenzaldehyde,
ρ-coumaric, protocatechuic and vanillic acids, and phenolic conjugates (e.g., hippuric, phenylacetic, and phenylpropenoic acids) [
77,
118].
Additionally, the cleavage of glycosidic bonds also enhances the beneficial effects exhibited by anthocyanins [
33]. In fact, this modulation on colon microflora leads to the formation of short-chain fatty acids that together with phenolic acids induce a decrease in pH values, creating favourable conditions for the proliferation of probiotic bacteria, such as
Actinobacteria, Bifidobacteria, and
Lactobacilli [
33,
111]. These bacteria exert positive effects in the control of gastrointestinal and digestive disorders, allergies, eczema, and improvements in delicate cases of cardiac and mental illness [
10,
119].
5. Anthocyanin Encapsulation
Knowing that the incorporation of phenolic compounds into foods and pharmaceutical products is a challenge, due to their instability and susceptibility to degradation, during processing and storage, various delivery systems have been developed [
4]. Among them, encapsulation is a good strategy. This technology allows entrapping an active agent, liquid, gas, or solid within a matrix or a polymeric wall in micro or nanoparticles, to protect the active compound from environmental conditions, undesirable interactions, and to control their transportation, release, and handling. The most common polymers used are carbohydrates (e.g., cellulose derivatives and maltodextrins), natural gums (e.g., alginates and gum arabic), lipids (e.g., emulsifiers and waxes), and/or proteins (e.g., dairy proteins, gelatine, and soy proteins) [
33]. Additionally, their combination with other wall materials and some modifiers, such as antioxidants, chelate agents, and surfactants, can also increase the encapsulation benefits [
4]. In a general way, the process of encapsulation is based on the formation of the wall around the compound of interest, ensuring that unwanted materials are kept outside, and preventing undesired leaks to happen. It is important to take into account, along with its cost, the particle size and physicochemical properties of the core and the origin of wall constituents, to favour capsule stability. To that end, several methods have been developed, and the best-known ones for anthocyanin encapsulation are emulsification, ionotropic gelation, thermal gelation, and spray-drying. This last one is the most applied technique (80–90% of encapsulated formulations are spray-dried) due to their cost and easy procedure [
4]. Maltodextrins are the most used coating material given their ability to maintain anthocyanin stability [
120].
Therefore, and in order to enhance their bioefficacy and stability, and thus prevent their rapid degradation, anthocyanins are mainly encapsulated with liposomes, nanocomplexes of alginate and chitosan, and gel emersions [
34]. In fact, several studies already showed that anthocyanin nano-formulations, along with chemical modifications, favour their absorption and metabolization, and consequently increase their biological action [
33]. Mueller et al. [
112] conducted a human study and reported that the encapsulation of 2.4 g of blueberry anthocyanins with whey protein does not contribute to anthocyanin stabilization during intestinal passage given their ability to prolong their passage through the stomach, whereas the encapsulation with citrus pectin improves anthocyanin bioavailability and intestinal accessibility, thus increasing their concentrations in the bloodstream.
Furthermore, bioengineering-based, targeted drug delivery approaches using biodegradable PLGA@PEG nanoparticles revealed more notable results in both in vivo and in vitro Alzheimer’s disease models than anthocyanins alone, namely, lower levels of oxidative stress and neuroinflammatory hallmarks [
121,
122]. Furthermore, 50 mg/mL of blueberry anthocyanins encapsulated in liposomal micelles also revealed higher anticancer activity than non-encapsulated anthocyanins on K562 human erythroleukemic cancer cells [
123].
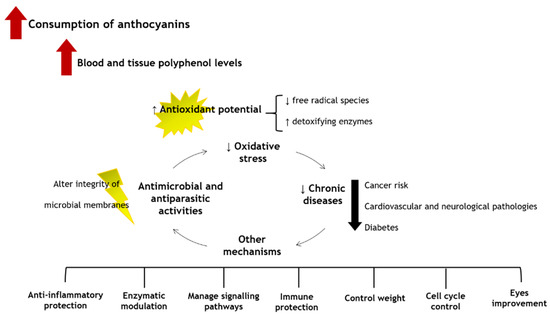
6. Putative Health Benefits
The low ingestion of fruits and vegetables causes around 1.7 million deaths worldwide, being related to 14% of gastrointestinal malignancies, 9% of stroke, and 11% of coronary artery disorders [
36]. Therefore, it is undeniable the role of anthocyanins in promoting human health and welfare [
105]. Several in vitro scavenging assays, animal and human cell lines studies, animal models, and human clinical trials already indicated that the consumption of foods, beverages, and supplements rich in anthocyanins brings numerous health benefits. In fact, this is due to the easy capacity of the anthocyanins to eliminate and/or neutralize free radicals and reactive species, chelate metals, control signalling pathways, diminish pro-inflammatory markers, and, thus, reduce the risk of cardiovascular pathologies, cancer, and neurodegeneration. Additionally, they also contribute to control weight and improve vision. The general health benefits resulting from the consumption of anthocyanins are shown in
Figure 2.
Figure 2. Health benefits of anthocyanins.