Ammonia (NH3) represents a perilous gas that poses a substantial hazard to both human well-being and the environment, particularly within agricultural regions. Agricultural activities constitute a primary source of ammonia emissions. Thus, effective monitoring and measurement of ammonia sources in agriculture are imperative for mitigating its adverse impact. However, not all existing ammonia detection methods are suitable for discerning the low concentrations typically encountered in agricultural ammonia volatilizing (ranging from 0.01 to 5 parts per million). Consequently, curtailing ammonia volatilization from farmland assumes paramount importance, with real-time monitoring serving as a crucial mechanism for assessing environmental contamination and minimizing agricultural ammonia losses. Deploying appropriate detection methodologies ensures that requisite measures are taken to safeguard human health and the environment from the deleterious repercussions of ammonia exposure.
1. Introduction
Ammonia (NH
3) is a gas with a potent odor, despite being colourless, and is classified by the United States Environmental Protection Agency (EPA) as one of the 366 extremely hazardous substances
[1]. NH
3 plays a role in the creation of fine particulate matter that is 2.5 micrometers or less in size: (PM2.5) particles
[2], which are known as pollution that significantly reduce the health index of residents and increase the incidence of chronic diseases and depression
[3]. The majority of ammonia utilized as a fertilizer is composed of key components such as ammonium nitrate and urea. These components are also used to produce ammonium and nitrate salts in agricultural areas and require accurate diagnosis and measurement of ammonia levels within fertilizer elements
[4]. On the other side It is important to note that these are general effects, and the actual symptoms and severity may vary depending on various factors such as the level and duration of exposure, individual susceptibility and preexisting health conditions.
Ammonia can contribute to air pollution, which can have negative impacts on both the environment and human health. It can also contribute to eutrophication, which can harm aquatic ecosystems
[5]. NH
3 is a hazardous gas that presents significant risks to human health and the environment, particularly in agricultural areas where it is a byproduct of production. Based on declared values, the outdoor air quality standard for ammonia is set at 50 parts per billion (ppb) over a one-hour period
[6]. However, the actual effects and symptoms caused by exposure to ammonia depend on a number of factors, including the level and duration of exposure, individual sensitivity, and pre-existing health conditions.
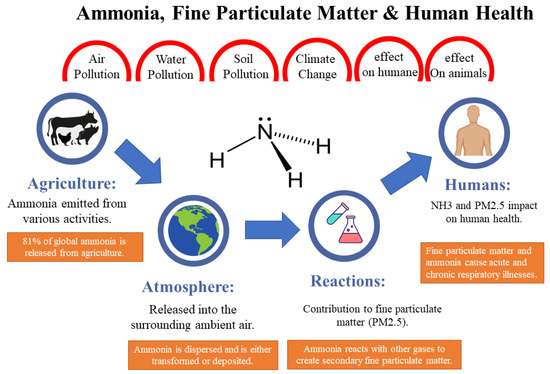
Figure 1 gives a thorough overview of the possible impacts of ammonia gas emissions, focusing on how they may affect humans, animals, the ecosystem, and the atmosphere through things like water contamination, air pollution, soil pollution, and changes in the climate.
Figure 1. Ammonia gas effect in agriculture, humans, and the atmosphere
[7].
The influence of ammonia gas emissions on several elements, including animals, people, the atmosphere, and the environment, is depicted in
Figure 1. It demonstrates how ammonia gas emissions can have a variety of negative consequences. The picture also depicts the consequences on animals and people, suggesting that ammonia exposure can be hazardous to their health and well-being. This may include breathing problems, eye and skin irritation, and long-term health implications. Furthermore, the image emphasises the impact on the environment, illustrating that ammonia gas emissions lead to air, water, and soil contamination. In general, exposure to ammonia can be harmful to the human body, as it readily dissolves in water and can interact with moist mucous membranes, potentially leading to eye, respiratory, lung, and skin diseases
[8][9][10]. Furthermore, some studies have reported that high levels of ammonia exposure can cause neurological symptoms, such as headaches, dizziness, and confusion. In this state, ammonia can cause liquefaction necrosis. Therefore, even there is no single policy regarding the control of ammonia gas, numerous countries have established regulations and policies to address the release of ammonia into the environment and minimize its impacts on human health and the environment
[11].
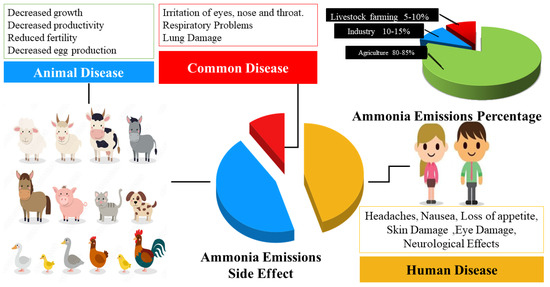
Ammonia is a ubiquitous chemical compound that is generated from both anthropogenic and natural sources. The Figure 2 shown the ammonia source emission and their effect on human and animals.
Figure 2. Ammonia Effect On Humans And Animals.
Figure 2 represents that agriculture stands out as the most significant contributor to ammonia emissions, accounting for approximately 80–85% of the total. This is due to the widespread use of nitrogen-rich fertilizers and animal manure in agriculture, which can release large amounts of ammonia into the atmosphere
[12]. The emissions stem from various agricultural practices, including the management of livestock waste and the use of fertilizers and crop residues
[13]. The research conducted by various researchers demonstrates the correlation of the diets of chickens, turkeys, ducks, meat pigs, cows, sheep, and goats with ammonia production. Four species (cattle, poultry, goats, and pigs) and three crops (wheat, maize, and rice) accounted for more than 70% of all ammonia emissions. This is because animal waste, including urine and feces, can release ammonia into the environment
[14]. Additionally, industrial activities, such as the production of fertilizers, plastics, and explosives, release ammonia into the environment, albeit to a lesser extent, contributing to around 10–15% of total emissions. Although ammonia is also emitted naturally from soil, water bodies, and vegetation, these natural sources generally account for less than 5–10% of the total
[15].
It is important to note that the exact proportion of ammonia emissions from different sources can vary depending on the country and the specific agricultural and industrial practices in use. As depicted in the visual representation presented in
Figure 2, even ammonia is an essential part of plant nutrition because it gives plants the nitrogen they need for vigorous, healthy growth. However, too much ammonia may be harmful to both plants and animals since it raises the amount of acidity in the environment, which can cause health problems including respiratory illnesses or even death if not controlled for an extended period
[16].
High levels of ammonia can cause respiratory problems in humans and animals, including irritation of the eyes, nose
[17], and throat. It can also cause respiratory problems, as well as damage to the lungs
[18][19]. Chronic exposure to high levels of ammonia can also lead to decreased growth and productivity
[20], reduced fertility and decreased egg production
[21]. Then monitoring ammonia levels can help farmers ensure compliance with environmental regulations and promote sustainable agricultural practices for example for good crop production because it enables growers to foresee possible issues with soil fertility before planting.
Finally, reducing ammonia emissions can help farmers reduce their environmental footprint and promote more sustainable agricultural practices. However, detecting low concentrations of agricultural ammonia volatilization (0.01–5 ppm) can be challenging, and not all ammonia detection methods are suitable for this task
[22][23][24]. The ammonia effect range is based on parts per million (ppm) as shown in
Figure 3.
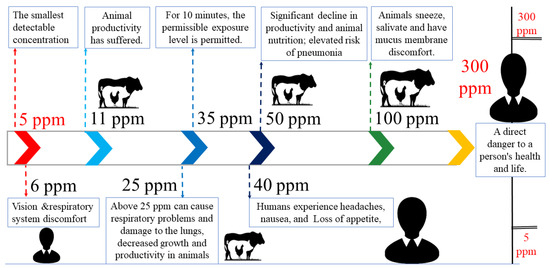
Figure 3. The ammonia level and its effect on humans and animals.
Figure 3 shows that the ammonia concentration suitable for agriculture is 0.01–100 ppm and the regional ammonia concentration after manure or synthetic fertilizer application was 0.01–5 ppm
[25]. Different concentrations of ammonia can have severe consequences for poultry and humans. The smallest detectable level of ammonia is 5 ppm, but even 6 ppm can cause discomfort in the respiratory and visual systems. Animal productivity can be affected at concentrations as low as 11 ppm. Although the United States Environmental Protection Agency (EPA) considered 25 ppm of ammonia safe for human exposure for one hour, exposure to 35 ppm is limited to just 10 min. Concentrations of 40–50 ppm of ammonia can result in headaches, nausea, loss of appetite, and decreased productivity in humans. Exposure to 100 ppm of ammonia can cause sneezing, salivation, and discomfort in the mucus membranes in animals. The most hazardous levels of ammonia concentration are 300 ppm or higher, which pose a direct threat to human health and life
[26]. Agricultural sources should be thoroughly examined from both the source and its side effects and appropriate measures should be implemented to decrease the concentration of tropospheric NH
3, as noted in reference
[7]. There are many methods for detecting ammonia concentration, but due to ammonia’s nature to react with water and its strong adsorption few methods are applicable for detecting volatile ammonia in agriculture. Furthermore, the inherent environmental complexity in agriculture with small changes make this task difficult.
2. Performance Evaluation of Traditional Methods vs. E-Nose
The traditional methods have been used in agriculture for many years and are still widely used due to their simplicity, affordability, and ease of use. However, they also have limitations, such as the need for manual sampling, the potential for measurement errors, and time-consuming laboratory analysis. These methods can provide accurate and reliable results for ammonia detection, but their performance may vary depending on factors such as sensitivity, specificity, real-time monitoring capability, ease of use, and maintenance requirements. This has led to the emergence of alternative methods, such as E-nose, which offer potential advantages in terms of real-time, non-invasive, and automated ammonia detection in agricultural settings. When selecting a method for ammonia detection, it is crucial to consider both the specific application and the desired level of accuracy. The cost, speed, and accuracy of ammonia detection methods in agriculture can vary greatly depending on the method used and should be taken into account.
According to
[27], the most used methods in agriculture, one of the most commonly used methods for ammonia detection is the use of Electrochemical sensors. Electrochemical sensors are widely employed for their high sensitivity, good selectivity, and relatively fast response time. These sensors are capable of accurately measuring ammonia gas concentrations in the agricultural environment, making them suitable for applications such as monitoring livestock facilities, assessing fertilizer efficiency, and managing air quality in crop production. Electrochemical sensors offer several advantages for ammonia detection in agriculture. They are cost-effective, easy to use, and provide real-time measurements.
Additionally, they can detect low concentrations of ammonia gas accurately, enabling farmers to monitor and adjust ammonia levels to optimize crop growth and minimize environmental impact. It is important to note that, while electrochemical sensors are commonly used, the choice of ammonia detection method can vary depending on specific agricultural practices, regulatory requirements, and the desired level of accuracy and monitoring capabilities. A deeper comparison shows that the cost of these methods can vary, with Calorimetric Methods and Spectroscopic Methods generally being more expensive, while Electrochemical and Gas Sensitive Tube methods tend to be more affordable. In terms of speed, Electrochemical sensors and Gas Sensitive Tubes offer fast response times, while Sampling and Laboratory Analysis and Spectroscopic Methods can be slower due to the need for sample collection and analysis. Regarding accuracy, Calorimetric Methods
[28], Electrochemical sensors
[29], Ion Selective Electrode (ISE)
[30], and Spectroscopic Methods
[31] are known for providing high accuracy in ammonia detection. The measurement range can vary depending on the specific method and equipment used. Generally, Electrochemical sensors have a moderate to wide measurement range, while Calorimetric Methods and Ion Selective Electrode (ISE) have a narrower to moderate range.
E-nose can also be used for spatial mapping of ammonia levels across large areas, providing valuable information for site-specific management strategies. Additionally, E-nose has the potential to detect other odor compounds or gases that may be indicative of specific agricultural practices or environmental conditions
[23]. However, E-nose also has limitations. It may require calibration and validation against reference methods to ensure accuracy and reliability. The cost of initial investment and maintenance may be higher compared to some traditional methods. The complexity of data analysis and interpretation may also pose challenges, as E-nose generates large amounts of data that require advanced analytical techniques
[26]. The comparative analysis of advantages and disadvantages pertaining to diverse domains concerning Electronic nose (E-nose) and conventional methodologies are elucidated in
Table 1 presented hereunder.
Table 1. Advantages of E-nose and Traditional Methods.
| Advantages |
E-Nose |
Traditional Methods |
| Sensitivity |
High detection limits in the low ppm or even ppb range |
Highly selective for ammonia |
| Precision and Accuracy |
Precise and accurate measurements |
Familiarity and wide adoption |
| Real-time Monitoring and Rapid Results |
Provides real time monitoring and quick results |
Easier to implement and validate |
| Portability and Ease of Use |
Very portable and easy to use |
High accuracy and precision in ammonia measurement |
| Potential Selectivity towards Specific Analytes |
Including ammonia |
Potentially reducing interference from other compounds in the sample |
| Disadvantages |
E-Nose |
Traditional Methods |
| Sensor Limitations |
It has sensor limitations |
Require complex sample preparation steps |
| Cost |
E-nose devices can have varying costs depending on the complexity of the sensor array |
Longer analysis times compared to E-nose |
| Standardization |
Lack standardized methods or regulatory guidelines for ammonia measurement |
Require specialized equipment, reagents, and skilled personnel for operation and maintenance |
| Application Range |
Limited |
Less portable compared to E-nose devices |
According to the findings presented in Table 1, it can be deduced that E-nose devices present a plethora of advantages in the realm of ammonia detection. These advantages encompass heightened sensitivity, instantaneous tracking capabilities, convenient portability, swift response times, and the ability to discern specific analytes. Consequently, E-nose devices have found widespread utility across diverse industries and sectors. Conversely, traditional methods possess well-established methodologies, exhibit selectivity towards ammonia, boast familiarity among operators, and have demonstrated commendable precision and accuracy. Nevertheless, E-nose devices do have certain limitations, such as limited sensing strength, susceptibility to cross-sensitivity, calibration prerequisites, and a lack of standardized procedures. Moreover, they necessitate significant upfront investment and ongoing maintenance. A comprehensive comparison was conducted between traditional methods such as calorimetric methods, electrochemical sensors, titration methods, ion selective electrodes (ISE), gas-sensitive tubes, spectroscopic methods, sampling with laboratory analysis, and the emerging technology of E-nose.
In this comparative analysis, a comprehensive analysis was conducted utilizing a radar graph framework, drawing upon meticulously documented data. In the analysis the sensitivity, accuracy, analysis time, real-time monitoring, portability, versatility, and cost were as the main parameters. To facilitate a systematic evaluation, each of these critical parameters assigned a numerical rating, adhering to a refined metric scale ranging from 0 to 5. In this scale, the quantitative correspondences are assigned as follows: low corresponds to 0, moderate corresponds to 2.5, high corresponds to 5, limited corresponds to 1.5, and affirmatives like “yes” and “no” are respectively associated with the numerical values of 5 and 0. The findings of this analysis are presented in Figure 4 as a radar chart.
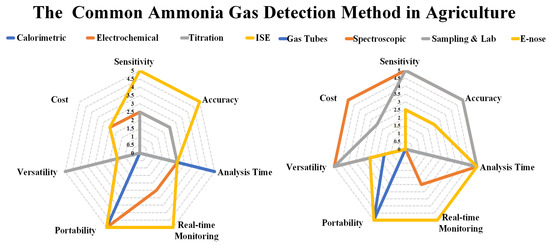
Figure 4. The trend of E-nose VS ammonia detection.
Figure 4 shows, the sensitivity of various analytical methods plays a crucial role in determining their effectiveness in detecting and measuring substances of interest. Calorimetric methods exhibit a moderate to high sensitivity, which depends on the specific method and equipment employed. The gas-sensitive tubes method displays moderate sensitivity, subject to variation based on the type of tube and gas being detected. The Ion Selective Electrodes (ISE) method shows high sensitivity for specific ions, although this can vary depending on the electrode and analyte involved. Electrochemical methods offer a sensitivity ranging from moderate to high, depending on the electrode and technique utilized. Titration methods generally possess moderate sensitivity, with variations based on the specific method and indicator employed. Spectroscopic methods, particularly UV-Vis, fluorescence, or atomic absorption spectroscopy, exhibit high sensitivity. Sensitivity in sampling and laboratory analysis relies on the specific analytical methods utilized. E-nose demonstrates moderate to high sensitivity, contingent upon the sensors and algorithms employed.
The accuracy of different methods used in scientific measurements varies depending on specific factors and conditions. Calorimetric methods generally exhibit a high level of accuracy, although this accuracy can fluctuate depending on the chosen method and experimental conditions. Electrochemical methods are generally accurate, but their precision relies heavily on proper calibration and the specific experimental conditions in place. Titration methods can achieve accurate results when executed and calibrated correctly. The Ion Selective Electrodes (ISE) method provides accurate measurements for targeted ions, provided proper calibration is carried out. The gas-sensitive tubes method accuracy is influenced by environmental factors and limited to specific ranges and conditions. Spectroscopic methods yield accurate results when appropriately calibrated and operated. The accuracy of sampling and laboratory analysis techniques varies depending on the utilized equipment and techniques. Lastly, the accuracy of the E-nose technique is subject to variation and can be affected by environmental factors and the limitations of the sensors employed.
The analysis time varies across different methods used in scientific research and analysis. Electronic noses (E-nose) and Ion Selective Electrodes (ISE) exhibit relatively fast analysis times, typically within minutes. The gas-sensitive tubes method also provides generally quick analysis times, often within minutes. However, in Spectroscopic Methods, the analysis times can vary greatly, ranging from seconds to minutes, depending on the complexity and technique employed. Sampling and laboratory analysis involves analysis times that can span from minutes to hours, depending on the intricacy of the sample and the required analyses. For Calorimetric Methods, particularly simple tests, the analysis time is relatively fast. On the other hand, analysis times for Titration Methods can vary from a few minutes to several hours, depending on the specific method employed. Understanding the analysis times associated with these different methods is crucial for researchers and scientists to plan their experiments and allocate resources accordingly.
Real-time monitoring techniques vary in their applicability across different analytical methods. Titration methods typically do not employ real-time monitoring, while Ion Selective Electrodes (ISE) method often utilizes it to monitor specific ion concentrations. Gas-sensitive tubes method commonly employs real-time monitoring to track gas concentrations. Spectroscopic methods can be used for real-time or near real-time monitoring, depending on the equipment and method employed. Real-time monitoring is not typically used in Calorimetric Methods
[28]. Electrochemical methods, however, generally offer quick results, especially when well-optimized, making real-time monitoring a common practice due to their fast response times. In the context of sampling and laboratory analysis
[32], real-time monitoring is not commonly employed as it typically involves sample collection and subsequent analysis. Finally, real-time monitoring finds extensive usage in E-nose for the real-time assessment of odor or volatile compound profiles. The choice of employing real-time monitoring depends on the specific method and the desired monitoring objectives.
The portability of various analytical methods depends on the specific techniques and equipment employed. Calorimetric methods may offer portability, contingent upon the equipment utilized. Electrochemical methods, especially those utilizing handheld devices, exhibit potential for portability. Conversely, titration methods are generally not portable due to the requirement of a controlled laboratory environment. Ion selective electrodes (ISE) methods
[33] can be made portable, particularly with handheld ISE devices. The gas-sensitive tubes method can also be conveniently portable, especially with the use of handheld devices. The portability of spectroscopic methods varies depending on the technique and equipment utilized. Sampling and laboratory analysis are not portable as they require specialized equipment and controlled laboratory conditions. On the other hand, in the realm of E-nose, portability can be achieved, particularly with handheld E-nose devices.
The parameter of versatility serves as a critical factor in evaluating the effectiveness of different methods in various scientific analyses. Calorimetric methods, while valuable in specific types of reactions, exhibit limited versatility. On the other hand, electrochemical methods offer a high degree of versatility, accommodating a wide range of analytes and applications. Titration methods also display significant versatility, making them suitable for diverse applications and a broad spectrum of analytes. However, the ion-selective electrodes (ISE) method falls short in terms of versatility, as it is restricted to specific ions and applications compared to other methods. Similarly, the gas-sensitive tubes method is limited to particular gases and applications. Spectroscopic methods, in contrast, provide extensive versatility, allowing for analysis across a wide range of analytes and applications. The field of sampling and laboratory analysis demonstrates remarkable versatility, covering a diverse array of samples and analytes. Lastly, the E-nose technique proves highly versatile in detecting and distinguishing various odors and volatile compounds. Overall, versatility plays a crucial role in determining the suitability and applicability of different methods in scientific analyses. The cost considerations for various analytical methods differ significantly. The Calorimetric Methods are generally considered cost-effective, offering an economical approach. The Electrochemical Methods are particularly cost-effective, especially for basic electrochemical techniques. Titration Methods are relatively cost-effective due to the affordability of equipment and reagents. However, the Ion Selective Electrodes (ISE) method is moderately expensive due to the specialized electrodes and equipment required. The Gas-sensitive tubes method is relatively cost-effective, as gas-sensitive tubes are often affordable. The cost of Spectroscopic Methods varies greatly depending on the specific technique and equipment used, with some advanced instruments being quite expensive. Sampling and laboratory analysis costs can vary based on complexity and the number of required analyses, with specialized tests tending to be more expensive. Lastly, the cost of E-nose devices can be relatively high, depending on complexity and quality. It is important for researchers and analysts to consider the cost implications when choosing the most suitable analytical method for their specific needs.
In contrast, traditional methods tend to be time-consuming, necessitate specialized equipment and personnel, and suffer from limited portability. In scenarios where adherence to regulations or standardized procedures is indispensable, traditional methods may be the preferred choice.