1. Introduction: GI MRI Strength and Weakness
Magnetic resonance imaging (MRI) is the most advanced cross-sectional imaging technique which currently allows the evaluation of several, if not all, intestinal segments from the oesophagus to the anal canal. Compared to other imaging methods, it has several important advantages. First of all, it does not require ionizing radiation, and thus can be used in young patients, and it is repeatable and useful in the follow-up and monitoring of many benign and malignant diseases. Second, MRI has a good-to-excellent spatial resolution and an excellent tissue contrast, higher than any other imaging tool; furthermore, it is able to analyze the normal and pathological human tissues with multiple imaging parameters, differently from any other imaging method.
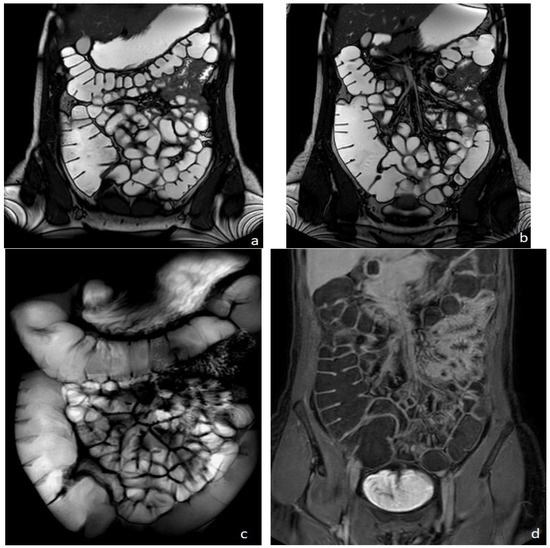
MRI was firstly introduced for bowel evaluation in the late 90s , when technology and software advanced to the point where it was possible to acquire images of the entire abdomen and bowel loops in breath-hold mode, using both T1- and T2-weighted sequences, with a sufficiently high spatial resolution. One of the main challenges in the MRI evaluation of the small intestine is still related to the intrinsic motility of the bowel, i.e., intestinal peristalsis, which causes motion artifacts, together with respiratory diaphragmatic movements. This problem can be partially resolved with antispasmodic drugs but, above all, with fast imaging acquisition. Breath-holding and fast imaging are both crucial for bowel evaluation, together with a good-to-high spatial resolution, high enough to assess the thin (2 mm) normal bowel wall, and together with a wide field of view, in order to assess the entire small and large bowel. Therefore, the combination of a rapid imaging acquisition (high temporal resolution), a high anatomical resolution (high spatial resolution), and a wide field of view are the three crucial requirements for a satisfactory evaluation of the bowel, but not easy to achieve simultaneously in a single examination, even using the most advanced MRI equipment (Figure 1).
Figure 1. Healthy woman. MR Enterography. (a,b) T2-weighted balanced coronal images showing normal small and large bowel loops. (c) T2-weighted fat-suppressed single shot coronal image (follow-through effect) and (d) coronal T1-weighted image after intravenous gadolinium-chelate injection.
Over the past two decades, MRI techniques for bowel evaluation (here including MR Enterography, MR Enteroclysis, MR Colonography, High-Resolution MRI of the rectum, and MR Defecography) have progressively improved and today, nearly 30 years after its introduction, the clinical value of MRI in the assessment of several intestinal diseases of the small and large bowels has grown significantly. To date, relevant information can be obtained in both benign and malignant intestinal diseases in a relatively short examination time, using the multiple imaging parameters currently available, such as diffusion-weighted imaging, motility imaging, and dynamic contrast enhancement (the so-called “multiparametric MRI”) . Recently, the diagnostic power of MRI has been added to the one offered by nuclear medicine; this advanced hybrid equipment combining MRI with PET, the so-called PET/MRI, could provide additional information on both oncological and inflammatory intestinal diseases .
GI MRI is nowadays the most accurate imaging tool for the diagnosis and monitoring of Crohn’s Disease (CD), for the preoperative evaluation of rectal cancer, and for its follow-up during treatment, as well as for the functional evaluation of pelvic floor disorders.
MRI is also able to study the motility of the esophagous, stomach, and small and large bowels, as well as of the ano-rectum, thanks to real-time dynamic fast sequences (cine-MRI).
However, there are still some inherent limitations. For example, to evaluate the gastrointestinal tract, at any site, oral contrast agents are crucial to distend the intestinal lumen and highlight the intestinal wall . Disappointingly, the intestinal contrast agents currently available for GI MRI are mostly osmotic laxatives only, such as polyethylene glycol (macrogol solution) or mannitol solution. Iso-osmotic laxatives, in fact, act as “biphasic contrast agents” in magnetic resonance, since their signal is hyperintense (positive) on T2-weighted images and hypointense (negative) on T1-weighted images. To distend the rectum, ultrasound gel (biphasic contrast agent) is also widely used. Unfortunately, no other specific intestinal or rectal contrast agent is currently available. Purely negative or purely positive contrast agents for magnetic resonance are no longer available, although highly “desirable” . Moreover, a common cause of artifact is the presence of air, which limits the diagnostic quality of the study of the intestine, especially on 3T magnets. The sequences most frequently affected by artifacts due to residual gas include the fast spin echo and the Steady-State Free Precession-Balanced sequences (magnetic susceptibility artifacts).
Limitations and contraindications to MRI also include the presence of metallic medical implants or pacemakers, although this problem no longer occurs frequently, thanks to the recent technical evolution that has produced numerous MRI-compatible devices. Claustrophobia is increasingly rare due to the larger space in which the patient is positioned during the examination (gantry) and can also be overcome with the use of anxiolytic drugs, but it remains a limitation. Furthermore, GI MRI requires a longer examination time than other imaging tools, therefore increased patient compliance in following instructions, standing still and holding breaths, which is often problematic for children or elderly patients, or for patients with severe and painful illnesses. In addition, because MRI requires a longer examination time, it is less accessible than CT and in general more expensive than other imaging techniques. Finally, longer examination time and higher costs determine a low accessibility to MRI, particularly for abdominal and gastrointestinal disorders.
2 Diagnostic role of GI MRI in benign and malignant diseases
MR Enterography (MRE) is nowadays considered a primary examination in the evaluation of Crohn’s disease (CD)
[1], being valuable to confirm the initial diagnosis, to stage disease severity and activity, to monitore the effects of treatment,s and for follow-up purposes, both in adult and pediatric patients. The MRE technique is nowadys well standardized. To fully display the small bowel, the patient is invited to drink approximately 1500–2000 mL of an iso-osmotic solution (macrogol or mannitol solutions), approximately 45 min before the examination. To reduce motion artifacts due to intestinal peristalsis, the use of an antispasmodic drug intravenously administered is suggested, before intravenous contrast injection. Distended bowel loops may be best evaluated in the prone position when possible. Basic MRE protocol for IBD includes the use of fast T2-weighted spin-echo and balanced steady-state sequences on axial and coronal planes in breath-hold or breath-hold free acquisition. Additional T2-weighted sequences may include the T2 weighted breath-hold HASTE fat-saturated thick slab in coronal acquisition (
Figure 1c), or the higher resolution T2-weighted BLADE or PROPELLER sequences; furthermore, motility sequences can be useful, as well as diffusion-weighted imaging (DWI). T1-weighted dynamic contrast-enhanced MRE provides considerable contrast between the parietal lesion and the healthy wall at early (20 s), late (60 s), and delayed (5 and 7 min) phases, acquired both on axial and coronal planes. For controls during treatment, the examination can be shortened to the non-contrast sequences only.
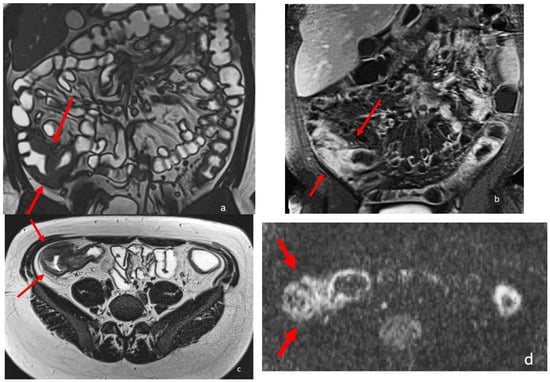
MRE is complemetary to endoscopy for the diagnosis and longitudinal monitoring of CD disease phenotype, complications, and disease activity. Crohn’s disease activity is associated with increased mural thickness and T2 signal intensity, but also with increased contrast-enhanced mural signal intensity. MRE in fact can detect intestinal Crohn’s disease inflammation with multiple inflammatory biomarkers, such as mural thickening, wall signal hyperintensity on T2 weighted images (wall edema), perivisceral signal hyperintensity on T2 weighted images (perivisceral edema), and post-contrast wall enhancement on T1-weighted images
[2][3][4][5][6][7] (
Figure 2). Recently, additional MRI biomarkers have also been evaluated, such as restricted signal of the affected wall at DWI, increased number, size, and signal of local lymphnodes, stratified and delayed wall contrast-enhancement, mesenteric vascularity, fibrofatty proliferation
[4][5]; overall, up to 13 different MRI biomarkers have been studied in the evaluation of chronic CD inflammation, with satisfactory results
[3][8]. Several CD activity scores based on MR Enterography have been proposed to stage the severity of the disease
[9], such as the Magnetic Resonance Index of Activity (MaRIA), which is one of the most widely used
[10], and the Magnetic Resonance Enterography Global Score (MEGS)
[11]. Additional scores also include DWI, such as the Nancy and Clermont scores
[6][7]. All of them are based on wall thickness and wall edema with some additional specific parameters. The transmural healing, assessed by MRI, is nowadays considered the most reliable endpoint of the medical treatment.
MRI is also useful in assessing most of the inflammatory disorders involving the gastrointestinal tract, not only Crohn’s Disease, such as ulcerative colitis, diverticulitis, appendicitis,, infectious colitis and the intestinal graft-versus-host-disease (GVHD), a severe acute or chronic complication of hematopoietic stem cell transplantation. Multiparametric MRI has recently showed good accuaacy also for the diagnosis and staging of acute and chronic GVHD, being able to identify several specific intestinal and extraintestinal signs (biomarkers), such as predominant and continuous small bowel involvement, edema of the intestinal wall, stratified “target” wall appearance on both T2-weighted and post contrast T1-weighted images, subcutaneous fat tissue edema, and mesenteric and retroperitoneal edema
[12][13].
Furthermore, MRI is currently considered the gold standard to diagnose and stage perianal fistulas
[5][14], being able to identify perianal fistulous tracts and abscesses, also assessing the involvement of both the internal and the external sphincters
[15][16]. Its role is crucial for therapeutic planning and monitoring perianal fistulas during medical therapy or after surgery. he MAGNIFI-CD score, recently developed, is an index of perianal fistula activity and can be used as a predictive tool
[17].
Figure 2. eighteen-year-old patient with Crohn’s disease; the arrows point to the inflammatory wall involvement of the last ileal loop and caecum showing wall thickening, post-contrast enhancement, and restricted diffusion. (a) Coronal TrueFISP image; (b) Coronal contrast-enhanced T1 weighted image; and (c) AxialT2 W high-resolution BLADE image. (d) Axial DWI b800 image.
In recent years, MRI has been also used in the diagnosis of all GI neoplasms, due to the development of fast, high-resolution imaging sequences that allow correct localization of parietal lesions and local staging through out the entire GI tract.
MRI can play a crucial role in preoperative staging, with accuracy ranging from 71.4% to 88% for T staging, and 52% to 55% for lymph node involvement; the overall accuracy of T2-weighted + DCE + DWI in T staging was significantly higher than T2-weighted + CE and T2-weighted + DWI
[18][19][20][21]. To assess gastric cancers, the gastric cavity is distended with 500–800 cc of water with the use of an antispasmodic drug intravenously administered, similarly to CT.
MRE has also shown 96.6% accuracy in the diagnosis of small bowel neoplasms, thanks to its excellent soft tissue contrast and multiplanar imaging, Small bowel mobility imagig can be useful for diagnosing low-grade stenosis and determining the level of neoplastic obstruction.
[22]. Finally, DWI MRI can be extremely effective and sensitive in the diagnosis and characterization of intestinal lymphomas
[23]. Many studies have highlighted the usefulness of MRI in the study of NETs, most of them focusing on pancreatic NETs, in which the routine morphological T2-weighted and T1-weighted sequences are implemented with contrast injection and multiple post-Gadolionium scans, including arterial, venous, and delayed (>5 min), and the use of diffusion-weighted sequences.
In rectal cancer the degree of invasion beyond the musculature is the most sensitive way to assess tumor stage compared with lymph node status, with a significant correlation to survival
[24]. AJCC staging manuals today consider T3 tumors as a single group characterized by tumor invasion through the muscularis propria. High-resolution MRI has proven to be highly accurate in T3 staging, its results over-lapping histopathological measurements: MRI has shown to be equivalent to histopathology on measures of tumor spread depth
[25]. Nowadays, the MRI role is crucial in the preoperative staging of rectal cancers, and in the therapeutic planning of locally advanced rectal cancer (LARC). Similarly, MRI plays a primary role in the evaluation of the therapeutic response after radio-chemotherapy of LARC, thanks to the high accuracy of morphological criteria associated with DWI
[24].
3. Advanced GI imaging techniques: Motility Imaging
The gastrointestinal tract is a moving organ whose peristaltic movement is critical to facilitate digestion and progression of food along the gastrointestinal tract, to expel waste and to maximize the action of digestive acids and enzymes on nutrients. Abnormalities of intestinal transit are common causes of functional disorders and abdominal pain, not associated with organic lesions. Imaging techniques that rely on static images and diagnostic tools may miss these functional alterations.
MRI is able to study the movement of the bowel and internal organs using fast and cine sequences. Cine MRI is currently widely used to study the heart; however, dynamic study of the gastrointestinal tract presents more difficulties than the heart because it is wider and bowel movements, although slower, are not periodic
[26][27].The radiological study of oesophageal motility has long been the prerogative of conventional radiography with fluoroscopy and barium swallow, but recently a few studies have been published on dynamic MRI assessment of oesophageal dysfunctions such as achalasia. The main limitation is the position of the patient, as most MRI machines only allow the patient to be in a supine position, whereas fluoroscopy allows the patient to swallow barium in a standing physiological position. Several MRI markers were found to be useful in diagnosing oesophageal motility disorders, such as the sphincter length, the oesophageal diameter (both numerically larger in patients with motility disorders), and the incomplete oesophageal clearance (markers of dysmotility)
[28].Manometry is the gold standard for assessing gastric motility, but it is a lengthy and invasive study that causes discomfort to the patient, and it can only quantify the motility of the gastric fundus. Another important parameter in the study of gastric function is gastric emptying, which is often studied using nuclear medicine (gastric scintigraphy). Fluoroscopy with barium swallow is still used to study gastric motility, as it is easy and accessible for the oesophagus.
To date, gastric motility has not been extensively studied using MRI, with only a few articles focusing on it. The study by Heissam et al
[29], based on fifteen healthy adults, compared motility cine-MRI performed with semi-automated techniques with simultaneous water-perfused manometry using water as a contrast agent and showed a strong correlation between the two methods. Another study by Hosseini et al [60] performed cine-MRI on four healthy volunteers using pineapple juice as a contrast agent and demonstrated the possibility of using MRI to measure and quantify gastric motility in human participants, even quantifying different motility patterns in different parts of the stomach. Interestingly, both studies used natural contrast agents.
Small bowel motility is often studied in patients with Crohn's disease, as reduced motility (fixity) of inflamed loops has long been considered a hallmark of the disease
[61]. The use of an oral contrast agent to distend the bowel loops is mandatory to correctly assess motility. A recent study by Dreja et al
[30] focused on the alteration of small bowel motility in patients with active Crohn's disease compared to healthy controls. A novelty was the measurement of motility not only at the level of the terminal ileum, where most studies focus, but also in the jejunum and the remaining ileum.
The analysis of small bowel motility may also be crucial in the study of functional disorders such as chronic pseudo-obstruction of the bowel; to date, only a few studies have focused on the potential diagnostic role of cine-MRI in this pathology
[31][32]. One of the most recent studies, by Sato et al
[32], compared seven patients with chronic bowel pseudo-obstruction and 11 healthy controls and found that all patients had altered small bowel motility and that the severity of the disease was reflected in the severity of the cine-MRI findings.
The assessment of bowel motility can be crucial in patients with functional constipation, a very common problem that should be carefully investigated when standard therapies fail. In clinical practice, radiological assessment of colonic transit time using radiopaque markers is widely used, although it is associated with moderate radiation exposure. Other radiological studies focus on the colon, such as virtual colonoscopy and double contrast colon enema, but provide only static information.
The current gold standard tests to investigate functional neuromuscular disorders of the colon are colon manometry and barostat, both of which are invasive, uncomfortable for the patient and costly, requiring anaesthesia or sedation and long examination times. On the other hand, MRI is non-invasive, more comfortable, requires less examination time and no anaesthesia or sedation except in cases of claustrophobia, and is also less expensive.
4. Advanced GI imaging techniques: DWI and IVIM GI Imaging
Diffusion-weighted magnetic resonance imaging (DWI) provides qualitative and quantitative data on tissue cellularity based on the random diffusion of water molecules. MR-DWI improves tumour detection by providing a quantitative assessment through measurement of the apparent diffusion coefficient (ADC).
DWI has an established diagnostic value in the diagnosis, staging, and follow-up of rectal cancer .
The most commonly used DWI b-values for rectal cancer detection are 800 and 1000 s/mm2. Lower b-values are always associated with T2 shine-through effects. DWI with ultra-high b-values (above 1000 s/mm2) may allow better visualization of rectal tumours due to highly effective suppression of the background signal.
Advancements in DWI technology now allow more information to be obtained for lesion detection and characterization by performing DWI with ultra-high b-values or multiple b-values. The use of DWI with a b-value of 2000 s/mm2 in rectal cancer helps to assess the primary rectal tumour and its response to chemoradiation therapy (CRT). . However, the resolution of ultra-high b-value images is limited for clinical diagnosis. One solution is to combine low, high and ultra-high b-values to balance spatial resolution and functional information. Another study conducted on patients with gastric cancer showed a high accuracy of 96.9% in tumour detection with WB-DWI/MRI, which was highly accurate in predicting inoperability (PPV 100%, NPV 100%) compared to CT (PPV 100%, NPV 53.3%) . In addition, WB-DWI/MRI revealed small peritoneal implants on the surface of the pancreas that were not detected by laparoscopy, suggesting a higher sensitivity of WB-DWI/MRI compared to laparoscopy. According to the recent literature, the role of MRI in gastric cancer imaging has become more important with the use of DWI and ADC quantification, as a possible biomarker in diagnosis, T-staging, and treatment response assessment. Several studies suggest that the diagnostic performance of (DWI)/MRI is not significantly different from 18F-FDG PET/CT or CT .
Furthermore, DWI is useful in assessing inflammation of the bowel wall and widely used in the evaluation of IBD, particularly in Crohn’s disease, to diagnose the presence of intestinal inflammation associated with fibrosis, and to assess disease severity and to identify inflammatory lymphnodes or abscesses. Some of the most commonly used Crohn's disease activity scores are based on DWI, such as the Nancy Score and the Clermont Score .
Intravoxel incoherent motion imaging (IVIM) was used to estimate tissue perfusion, as blood flow in randomly oriented capillaries mimics a pseudo-diffusion process. Furthermore, IVIM allows the contributions of perfusion and true molecular diffusion to be separated and evaluated as true diffusion coefficient (D slow), pseudo-diffusion coefficient (D fast) and perfusion fraction (f) using multiple values of b according to a bi-exponential model. Perfusion MRI based on IVIM, which does not require contrast agents, is increasingly used especially in oncology. The multiple b-value diffusion-driven IVIM method has recently shown to be a reliable tool for the differential diagnosis of malignant and benign tumours, as well as a promising imaging biomarker for the prognosis and treatment monitoring of various malignant tumours . A recent study by Yoo et al. also found a significant potential value of ADC in differentiating gastric stromal tumors from non-stromal tumors and in differentiating high-risk from low-risk gastric stromal tumors.
5. Advanced GI imaging techniques: Hybrid Imaging: PET-MRI
Positron emission tomography (PET)/computed tomography (CT) with 2-deoxy-2-[18F]fluoro-d-glucose (FDG) is widely used for initial staging and treatment response assessment of numerous gastrointestinal malignancies. Hybrid PET/MRI scanners, which simultaneously acquire PET data and MRI data, have the potential to provide accurate whole-body staging in a single examination. Nuclear medicine techniques, in particular hybrid and molecular imaging, could offer additional information for disease characterization of neoplastic diseases. Furthermore, specific advances in nuclear. medicine techniques may be able to assess the metabolic disease activity and to explore inflammatory pathways, providing a rational guide for the newest biological treatments, especially in Crohn' s disease .
6. Advanced GI imaging techniques: Acquisition, Analysis, and Post Processing
Artificial intelligence (AI) is a revolutionary and still unexplored diagnostic tool. Its application in the medical field, and particularly in radiological imaging, is becoming increasingly popular, allowing, for example, image classification, reconstruction, and resolution enhancement . The use of AI in MRI is becoming increasingly popular, as it can reduce image acquisition times by providing very high-resolution images . Two of the most developed techniques that have helped to speed up image acquisition are parallel imaging and compressed sensing. With these techniques, it is possible to collect basic information from multiple coils and then reconstruct images from a smaller sample of data . It is therefore likely that AI will improve the MRI assessment of normal and abnormal bowel by improving the spatial and temporal resolutions.Many new AI tools can recognise complex patterns in data, information, and images, allowing both qualitative and quantitative assessment of radiological features.Among the most promising methods are deep learning methods, which are able to detect undersampled data and also allow the conversion of low-resolution data into high-resolution data . Preliminary studies show the potential of a variational network to classify many different anatomical regions and achieve the diagnostic accuracy of conventional methods .
Texture analysis (TA) is a post-processing technique that can be applied to cross-sectional data to facilitate the analysis of heterogeneity within selected image regions. The derived image series contains features highlighted at different spatial scales, ranging from fine to coarse textures. Histogram quantification then generates the following parameters: mean of pixels within the ROI, standard deviation, symmetry of distribution, mean of positive pixels, kurtosis (distribution “accuracy” or “sharpness”), and entropy (with increasing irregularity or complexity indicated by a higher entropy value). Crohn’s disease activity is associated with increased contrast-enhanced mural signal intensity, reflecting underlying angiogenesis within the bowel wall. Examining this signal beyond simple mean intensity using the texture analysis provided new insights into the underlying vasculopathy. Recently the texture analysis of contrast-enhanced T1-weighted images was correlated to the presence or absence of histological parameters of hypoxia or angiogenesis in Crohn’s disease lesions. Other studies have evaluated the use of MRI delta texture analysis (D-TA) as a methodological element capable of predicting the frequency of complete pathological responses and, consequently, the outcome of patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy (C-RT) followed by radical surgery, with good preliminary results.
Artificial intelligence (AI) can be promising in IBD. Assessment of location, extension, inflammatory activity, and severity of intestinal lesions in Crohn's disease is in fact a complex process, requiring the association an integration of data derived from endoscopic and imaging methods with histological and biochemical investigations, and clinica data. Artificial intelligence (AI) is able to transform the practice of medicine and the management of inflammatory bowel disease (IBD) by replicating the judgement of expert clinicians and uncovering powerful insights by analyzing volumes of data too large and complex for humans to perceive. Intelligence requires the ability to acquire, store, and logically organize information. The emergence of artificial intelligence in IBD has been made possible by the availability of large volumes of digitized medical data and the computational methods required to analyze complex data analysis models, collectively referred to as machine learning (ML). In ML, input data is annotated, labelled, or classified, and can be clinical outcomes, expert measurements, or even physiological processes. ML methods quantify the relationships between the input data and the outcome as a model; this process is called training. Apromising AI MRI models have been developed to improve the loco-regional assessment of advanced rectal cancer as well, to improve the accuracy in loco-regional staging, detection of metastatic lymph nodes, and assessment of response to neoadjuvant chemotherapy. With continued improvements in deep learning algorithms and the combination of MRI with deep learning image recognition, it will be possible to more accurately predict patient prognosis and response to therapy.
In the evaluation of most functional and inflammatory gastrointestinal diseases, especially in young patients, MRI is nowadays considered the primary diagnostic tool, being highly accurate and radiation free. Gastrointestinal MRI has now become a reliable and primary diagnostic tool for the evaluation of most inflammatory and neoplastic gastrointestinal diseases, as it provides in-depth multiparametric evaluation of morphological, pathological, or functional changes in both inflammatory and neoplastic diseases like no other diagnostic modality. The development of postprocessing and artificial intelligence is the new and evolving frontier to improve the diagnostic power of MRI, with increasing levels of sensitivity and specificity.