Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jianmei Yu | -- | 2306 | 2023-08-03 22:33:44 | | | |
| 2 | Jason Zhu | Meta information modification | 2306 | 2023-08-07 04:53:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yu, J.; Pedroso, I.R. Common Mycotoxins in Cereal Grains and Producing Fungi. Encyclopedia. Available online: https://encyclopedia.pub/entry/47643 (accessed on 08 February 2026).
Yu J, Pedroso IR. Common Mycotoxins in Cereal Grains and Producing Fungi. Encyclopedia. Available at: https://encyclopedia.pub/entry/47643. Accessed February 08, 2026.
Yu, Jianmei, Ivana Ramos Pedroso. "Common Mycotoxins in Cereal Grains and Producing Fungi" Encyclopedia, https://encyclopedia.pub/entry/47643 (accessed February 08, 2026).
Yu, J., & Pedroso, I.R. (2023, August 03). Common Mycotoxins in Cereal Grains and Producing Fungi. In Encyclopedia. https://encyclopedia.pub/entry/47643
Yu, Jianmei and Ivana Ramos Pedroso. "Common Mycotoxins in Cereal Grains and Producing Fungi." Encyclopedia. Web. 03 August, 2023.
Copy Citation
Cereal grains are the most important food staples for human beings and livestock animals. They can be processed into various types of food and feed products such as bread, pasta, breakfast cereals, cake, snacks, beer, complete feed, and pet foods. However, cereal grains are vulnerable to the contamination of soil microorganisms, particularly molds. The toxigenic fungi/molds not only cause quality deterioration and grain loss, but also produce toxic secondary metabolites, mycotoxins, which can cause acute toxicity, death, and chronic diseases such as cancer, immunity suppression, growth impairment, and neural tube defects in humans, livestock animals and pets.
cereal grains
mycotoxins
health impacts
grain storage molds
1. Introduction
Mycotoxins are small organic molecules synthesized as secondary metabolites by certain fungal species that may contaminate various agriculture commodities such as cereals, corn, nuts, spices, soybeans and coffee beans, among others. Aflatoxins (AFB1, B2, G1 and G2), ochratoxin A (OTA), citrinin, patulin, trichothecenes (mainly deoxynivalenol (DON), T2-toxin (T2) and HT2-toxin (HT2)), fumonisins (FB1, FB2 and FB3) and zearalenone (ZEA) are the most prevalent mycotoxins in cereal crops [1]. Mycotoxin contamination of cereal grains remains the main food safety issue. These toxins are produced primarily by fungi genera Aspergillus, Fusarium, Penicillium, and Alternaria [2]. Aflatoxins are generated by A. flavus and A. parasiticus, while ochratoxin A can be produced by Aspergillus spp. [3] and Penicillium spp. [4]. Fumonisins, trichothecenes and ZEA are produced by Fusarium spp. and are collectively called fusarium mycotoxins. One mycotoxin can be produced by several fungi species and some mold species can produce multiple mycotoxins; nevertheless, toxic fungal development is not always accompanied by mycotoxin production [5]. The fusarium mycotoxins may cause significant pre-harvest grain loss, while aflatoxins and OTA are mostly occurring during storage due to improper postharvest handling and storage [6][7].
Mycotoxins are hazardous to both human and animal health [8]. They can cause acute toxicity and chronic diseases depending on the dosages. Acute intoxication occurs when highly contaminated food/feed is ingested in large quantities, which often results in death, as happened in Western India in 1974, Kenya from 1981 to 2006, Tanzania in 2016, Taiwan and Malaysia [9][10][11][12][13]. Long-term exposure to mycotoxins at doses slightly above the regulation limits can lead to chronic diseases such as liver cancer caused by AFs in Africa and China [14][15][16][17][18][19], kidney diseases caused by OTA in Europe and Egypt, and esophageal cancer caused by FUMs in Africa and some regions of China [20][21][22][23]. There has been a wide variety of toxic effects in both animals and humans from oral intake of mycotoxin-contaminated foods/feeds, such as immunosuppression, genotoxic, teratogenic or cancerous mutagenesis.
Among the mycotoxins that commonly occurred in cereal grains, aflatoxins, including AFB1, AFB2, AFG1, AFG2 and AFM1, are considered the most toxic and have been proven to be human carcinogens; thereby, they are classified as group 1 carcinogens, while FB1, FB2 and OTA are carcinogenic in tested animals, but there is not sufficient evidence about their carcinogenicity in human, and thus they are classified as group 2B carcinogens [24]. Despite health impacts, it has been shown that these contaminants can cost billions of dollars every year. In underdeveloped countries, aflatoxins have impacted around 4.5 billion individuals and aflatoxicosis is ranked sixth among the top 10 health threats [25]. Zearalenone (ZEA) has been reported to have immunotoxic, hepatotoxic and xenogenic effects [26]. Trichothecenes are divided into four groups: types A, B, C and D, with type A and B trichothecenes being the most prominent toxins in barley, wheat, oats and maize. Studies have shown that type A trichothecenes are more toxic than type B, while type B trichothecenes are present in contaminated cereals at a higher concentration than trichothecenes A [4][14]. The A group includes but is not limited to T-2 and HT-2 toxins, while the B group mainly includes deoxynivalenol (DON), nivalenol (NIV), 3-acetyldeoxynivalenol (3Ac-DON), 15-acetyldeoxynivalenol (15Ac-DON) and fusarenone X [4][27].
The cereal grains can be contaminated by these mycotoxins in the field (before harvest) or during post-harvest handling and storage. Some cereals often contain more than one mycotoxin. To protect consumers from mycotoxicoses, many countries have established or adopted regulations to limit exposure to mycotoxins. However, the adopted standards among different nations or multilateral agencies vary widely due largely to the level of economic development and the susceptibility of a nation’s crops to contamination [28]. Studies indicate that the economic costs of standard enforcement and the loss of trade opportunities resulting from the differences in allowable levels of mycotoxins in grains and grain-derived products are substantial. In developing countries, improving food safety along Western standards may result in considerable costs, thus unaffordable high food prices for low-income populations [29].
2. Aflatoxins and Their Producing Fungi
Aflatoxins are synthesized by Aspergillus flavus and A. parasiticus. A. flavus produces B aflatoxins, while A. parasiticus produces both B and G forms [3]. Aflatoxins B1, B2, G1 and G2 are naturally biosynthesized and frequently detected in cereal grains, particularly, maize, while the hydroxylated metabolites of AFB1 and AFB2 are aflatoxin M1 (AFM1) and M2 (AFM2) which are present in the meat, eggs, milk and cheese produced from animals which ingested aflatoxin-contaminated feed [30]. AFM1 is the major metabolite of AFB1 in milk from nursing humans and animals that consume AFB1-contaminated food or feed [4]. The chemical structures of AFs are shown in Figure 1. They are slightly soluble in water with a solubility of 10–20 µg/mL and completely soluble in moderately polar solvents such as chloroform, menthol and dimethyl sulfoxide [24]. The low hydrophilicity and high hydrophobicity of AFs enable them to bind to cell membrane lipids easily.
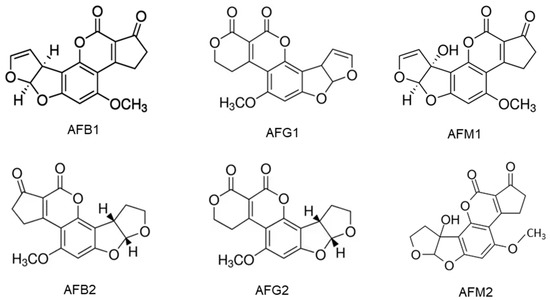
Figure 1. Chemical structures of aflatoxins B1, B2, G1, G2, M1 and M2.
Aflatoxins (AFs) are cytotoxic and genotoxic. AFB1, AFG1 and AFM1 are carcinogenic when ingested orally via the diet or delivered by gavage. The evidence for the carcinogenicity of AFB2 and AFG2 is insufficient. The AFB1 is more toxic than AFG1 in liver carcinogenicity but AFG1 induced a higher incidence of kidney tumors than AFB1. The AFB1 is about 10 times more potent than AFM1 in causing liver carcinogenicity [31]. The AFB1 is genotoxic and participates in the extrahepatic cycle, leading to chromosomal abnormalities, micronucleus formation, sister chromatid exchange, unscheduled DNA synthesis and DNA strand breakage [32]. The damage to DNA ultimately leads to the development of cancer. A human cell line study shows that AFB1 and AFG1, their precursors, as well as their metabolites aflatoxicol (AFL) and AFM1 are genotoxic [33]. Studies with poultry have found that the AFB1 and its metabolites mainly target the liver, where the toxin is metabolized mainly by CYP1A2 and CYP3A4 and causes numerous mutations, particularly in the p53 tumor suppressor gene [32]. This should be at least partially responsible for the high liver cancer incidence in some regions of the world where people are frequently exposed to food products contaminated with AFs.
3. Ochratoxins and Their Producing Fungi
Ochratoxins (OTs) are produced by certain Aspergillus species and some Penicillium species, including A. ochraceus, A. alliaceus, A. auricomus, A. carbonarius, A. glaucus, A. melleus, A. niger, P. nordicum and P. verrucosum among them [4][34]. The main forms of OTs are ochratoxin A, B and C, which differ in chemical structure and toxicity. Ochratoxin B (OTB) is a non-chlorinated form of OTA and ochratoxin C (OTC) is an ethyl ester of OTA formed in the presence of rumen fluid [8]. Among these ochratoxins, OTA is the most prevalent and toxic followed by OTC and OTB [35]. OTα is a non-toxic metabolite of OTA. The OTA has been found in several types of cereals, including corn, wheat, barley, rye, rice and other plant products such as coffee beans, dried fruits and spices. The chemical structures of OTs (Figure 2) show that all OTs contain a non-polar end and several polar groups on the side chain; thus, they can bind to membrane lipids and proteins such as plasma albumin [22][36].
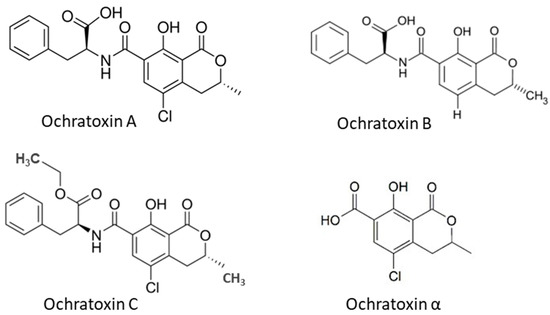
Figure 2. Chemical structure of ochratoxins occurred in cereal grains.
OTA is at least ten times more toxic than OTB and OTC. The OTA is nephrotoxic to all animal species and humans, and it has been related to the Balkan endemic nephropathy [37][38]. In addition, the OTA is also known to be hepatotoxic, immunotoxic, neurotoxic, teratogenic and carcinogenic, involving multiple mechanisms [35][39]. A single high dose or multiple lower doses of OTA in rats inhibits protein synthesis, mitochondrial respiration and ATP formation. It also enhances lipid peroxidation and the formation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) [39][40]. High levels of ROS result in decreased activity of cellular antioxidant enzymes, thus leading to oxidative stress and further inflammatory diseases [40]. Elevated levels of RNS induce nitrosative stress associated with DNA damage, tissue toxicity, cancer and inflammatory conditions, which may be responsible for cell injury and death [41].
4. Zearalenone and Its Producing Fungi
ZEA is produced by fungi of the genus Fusarium spp., including F. graminearum, F. culmorum, F. cerealis, F. equiseti, F. crookwellense, F. semitectum, F. verticillioides, F. sporotrichioides, F. oxysporum and F. acuminatum [26], but mainly F. graminearum and F. culmorum [42]. Corn has been described as the most vulnerable food to ZEA contamination. Other food crops, including wheat, barley, oat and rye, have also been found with this toxin. ZEA is immunotoxic, hepatotoxic and xenogenic [26]. The chemical structure of ZEA consists of a resorcinol moiety fused to a 14-member macrocyclic lactone ring with a trans double bond, two hydroxyl groups, two ketones and one methyl branch (Figure 3), which allow it to be easily absorbed through the gastrointestinal tract, to interact with proteins and lipids in the biological system to exert its toxicity [43][44]. The ZEA induces histopathological changes in the liver, with the subsequent development of liver cancer; it exerts hematotoxic effects by disturbing blood coagulation and modifying blood parameters. ZEA and its derivatives are non-steroids but have estrogenic activity in mammalian animals. They bind to estrogen receptors of cells and inhibit the secretion of steroid hormones, interfere with the estrogen response in the pre-ovulatory phase, and inhibit follicle maturation in mammals; this leads to disorders of the hormonal balance of the body, and, subsequently, many diseases of the reproductive system [43][44]. Higher concentrations of ZEA cause permanent estrus, pseudo-pregnancy and infertility in animals [44]. The detail toxicity of ZEA in humans and animals has been extensively reviewed by Ropejko and Twarużek [26].
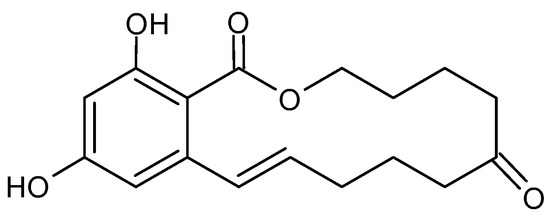
Figure 3. Chemical structure of Zearalenone.
5. Fumonisins and Their Producing Fungi
Fumonisins (FUMs) are produced in cereals by different species of Fusarium including F. verticillioides, F. proliferatum and F. fujikuroi, and other related species; they are common contaminants of maize and to a lesser extent of wheat and other cereals [45]. Fumonisins consist of a long hydrocarbon backbone chain similar to that of sphinganine. Six forms of fumonisin including FA1, FA2, FB1, FB2, FB3 and FB4 have been identified, with FB1 being the most toxic [4]. All FUMs are water soluble and thus are polar [24], which is determined by their chemical structures (Figure 4). These toxins are cytotoxic and carcinogenic to animals at relatively high concentrations. FB1, the major and most-studied fumonisin, is nephrotoxic and hepatotoxic in several animal species and has been classified as a possible carcinogen to humans (Group 2B) [24]. FB1 has been reported to cause leukoencephalomalacia (LEM) in horses, pulmonary edema syndrome (PES) in pigs and liver cancer in rats and hepatotoxic to horses, pigs, rats and vervet monkeys. The FB1 is cytotoxic to mammalian cell cultures and phytotoxic to several plants. FUMs have also been reported to be linked to human esophageal cancer in Transkei, China and South Africa [20][21][22][23][46].
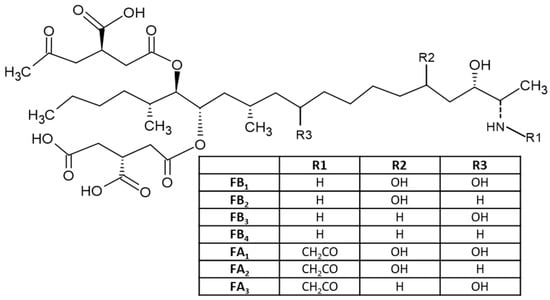
Figure 4. Chemical structure of different fumonisins.
6. Trichothecenes and Their Producing Fungi
Trichothecenes are several groups of mycotoxins produced by fungi of the Fusarium genus [14]. They are divided into four groups: types A, B, C and D. The type A group mainly consists of T-2 and HT-2 toxins, diacetoxy- and monoacetoxy-scirpenol (DAS and MAS) and neosolaniol (NEO). The B group mainly includes DON, nivalenol (NIV), fusarenone X and DON derivatives 3Ac-DON and 15Ac-DON [4][27]. Type C trichothecenes contain a C-7/C-8 epoxide (e.g., crotocin), while type D trichothecenes have an additional ring linking the C-4 and C-15 position (e.g., roridin A, verrucarin A and satratoxin H) [47]. Type A and B trichothecenes are the most common in barley, wheat, oats and maize. Studies have shown that the toxicity of type A trichothecenes is greater than that of type B but, fortunately, the concentrations of type A trichothecenes present in contaminated cereals are much lower than that of trichothecenes B [4]. Among trichothecene mycotoxins, the T-2 toxin is the most toxic and it is considered an immunosuppressive, cytotoxic, lymphocytic and carcinogenic mycotoxin in mammalian cells. The toxicity of the T-2 toxin is extensively reviewed by Janik et al. (2021) [48]. The chemical structures of trichothecenes are depicted in Figure 5 [49], which indicates that DON and NIV are more polar, while the T-2 toxin is hydrophobic, and the polarity of HT-2 toxin is between DON/NIV and T-2 toxin. This may contribute to the higher toxicity of T-2 toxin. However, T-2 toxin levels in food and feed are not regulated in many countries including the US.
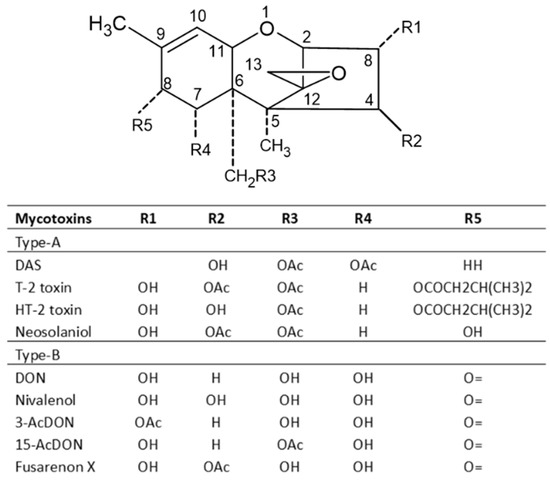
Figure 5. Chemical structures of major trichothecene mycotoxins.
Type A trichothecenes are manly produced by strains of F. sporotrichioides and F. poae, while type B trichothecenes toxins are mainly produced by strains of F. culmorum and F. graminearum [50]. Milder climatic conditions without high humidity favor the production of type A trichothecenes [51]. In the European maize growing areas, this toxin is usually detected in corn red ear rot. Oral exposure to T-2 toxin can lead to a fatal condition, known as alimentary toxic aleukia (ATA) with radiation poisoning-like symptoms [52]. DON and other Type B trichothecenes are not as potent in mammalian systems compared with T-2 toxin, but can still be lethal at high enough concentrations. DON is also known as ‘vomitoxin’ for its ability to cause diarrhea and emesis. DON and its derivatives cause a variety of maladies, including anorexia, feed refusal in livestock, growth retardation, leukocytosis, hemorrhage and adverse effects on reproduction and development. The toxicity of DON and its derivatives to animals is in the order of 15Ac-DON > 3Ac-DON > DON > DON-3Glc [2][53].
References
- Malachová, A.; Stránská, M.; Václavíková, M.; Elliott, C.T.; Black, C.; Meneely, J.; Hajšlová, J.; Ezekiel, C.N.; Schuhmacher, R.; Krska, R. Advanced LC–MS-based methods to study the co-occurrence and metabolization of multiple mycotoxins in cereals and cereal-based food. Anal. Bioanal. Chem. 2018, 410, 801–825.
- Freire, L.; Sant’ana, A.S. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205.
- Pitt, J.; Taniwaki, M.H.; Cole, M. Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement of Food Safety Objectives. Food Control 2013, 32, 205–215.
- Patriarca, A.; Pinto, V.F. Prevalence of mycotoxins in foods and decontamination. Curr. Opin. Food Sci. 2017, 14, 50–60.
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144.
- Yogendrarajah, P.; Jacxsens, L.; De Saeger, S.; De Meulenaer, B. Co-occurrence of multiple mycotoxins in dry chilli (Capsicum annum L.) samples from the markets of Sri Lanka and Belgium. Food Control 2014, 46, 26–34.
- Neme, K.; Mohammed, A. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategies. A review. Food Control 2017, 78, 412–425.
- De Ruyck, K.; De Boevre, M.; Huybrechts, I.; De Saeger, S. Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review. Mutat. Res.-Rev. Mutat. Res. 2015, 766, 32–41.
- Reddy, B.N.; Raghavender, C.R. Outbreaks of Aflatoxicoses in India. Afr. J. Food Agric. Nutr. Dev. 2007, 7, 1–15.
- Obura, A. Aflatoxicosis: Evidence from Kenya. In Aflatoxins: Finding Solutions for Improved Food Safety; Unnevehr, L.J., Grace, D., Eds.; International Food Policy Research Institute: Washington, DC, USA, 2013.
- Probst, C.; Njapau, H.; Cotty, P.J. Outbreak of an Acute Aflatoxicosis in Kenya in 2004: Identification of the Causal Agent. Appl. Environ. Microbiol. 2007, 73, 2762–2764.
- Kamala, A.; Shirima, C.; Jani, B.; Bakari, M.; Sillo, H.; Rusibamayila, N.; De Saeger, S.; Kimanya, M.; Gong, Y.; Simba, A.; et al. Outbreak of an acute aflatoxicosis in Tanzania during 2016. World Mycotoxin J. 2018, 11, 311–320.
- Marasas, W.F.; Kellerman, T.S.; Gelderblom, W.C.; Coetzer, J.A.; Thiel, P.G.; Van Der Lugt, J.J. Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J. Veter.-Res. 1988, 55, 197–203.
- Wu, Q.; Dohnal, V.; Kuca, K.; Yuan, Z. Trichothecenes: Structure-Toxic Activity Relationships. Curr. Drug Metab. 2013, 14, 641–660.
- Rheeder, J.; Marasas, W.; Theil, P.; Sydenham, E.; Shephard, G.; Van Schalkwyk, D. Fusarium moniliformeand Fumonisins in Corn in Relation to Human Esophageal Cancer in Transkei. Phytopathology 1992, 82, 353–357.
- Xue, K.S.; Tang, L.; Sun, G.; Wang, S.; Hu, X.; Wang, J.-S. Mycotoxin exposure is associated with increased risk of esophageal squamous cell carcinoma in Huaian area, China. BMC Cancer 2019, 19, 12–18.
- Chen, C.; Riley, R.T.; Wu, F. Dietary Fumonisin and Growth Impairment in Children and Animals: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1448–1464.
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Huang, T.; Yu, J.; Tang, L.; Gao, W.; Wang, J.S. Fumonisin B1 contamination of home-grown corn in high-risk areas for esophageal and liver cancer in China. Food Addit. Contam. 2007, 24, 181–185.
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Zhang, Y.; Xie, Y.; Zhang, H.; Tang, L.; Wang, J.S. Co-contamination of aflatoxin B1 and fumonisin B1 in food and human dietary exposure in three areas of China. Food Addit. Contam. 2011, 28, 461–470.
- Marasas, W.F.O. Fumonisins: Their implications for human and animal health. Nat. Toxins 1995, 3, 193–198.
- Gelderblom, W.C.A.; Semple, E.; Marasas, W.F.O.; Farber, E. The cancer-initiating potential of the fumonisin B mycotoxins. Carcinogenesis 1992, 13, 433–437.
- Chu, F.S.; Guo, Y. Simultaneous occurrence of Fumonisin B1 and other mycotoxins in moldy corn collected from the People’s Republic of China in regions with high incidences of esophageal cancer. Appl. Environ. Microbiol. 1994, 60, 847–852.
- Alizadeh, A.M.; Roshandel, G.; Roudbarmohammadi, S.; Roudbary, M.; Sohanaki, H.; Ghiasian, S.A.; Taherkhani, A.; Semnani, S.; Aghasi, M. Fumonisin B1 Contamination of Cereals and Risk of Esophageal Cancer in a High Risk Area in Northeastern Iran. Asian Pac. J. Cancer Prev. 2012, 13, 2625–2628.
- IARC. Monographs on the evaluation of carcinogenic risks to humans: Chemical agents and related occupations. In A Review of Human Carcinogens; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100F, pp. 224–248.
- Wild, C.P. Aflatoxin Exposure in Developing Countries: The Critical Interface of Agriculture and Health. Food Nutr. Bull. 2007, 28, S372–S380.
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites—General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35.
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627.
- Dohlman, E. Mycotoxin hazards and regulations: Impacts on food and animal feed crop trade. In International Trade and Food Safety/AER-828; Jean, C., Buzby, J.C., Eds.; Agricultural Economic Report No. 828; US Department of Agriculture: Washington, DC, USA, 2003; pp. 97–108. Available online: https://www.ers.usda.gov/webdocs/publications/41603/15644_aer828_1_.pdf?v=42055 (accessed on 14 December 2021).
- van Veen, T.W.S. International trade and food safety in developing countries. Food Control 2005, 16, 491–496.
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2017, 7, 2170.
- Schmale, D.G.; Munkvold, G.P. Mycotoxins in Crops: A Threat to Human and Domestic Animal Health. Plant Health Instr. 2009, 1, 10.
- Ülger, T.G.; Uçar, A.; Çakıroğlu, F.P.; Yilmaz, S. Genotoxic effects of mycotoxins. Toxicon 2020, 185, 104–113.
- Theumer, M.; Henneb, Y.; Khoury, L.; Snini, S.; Tadrist, S.; Canlet, C.; Puel, O.; Oswald, I.; Audebert, M. Genotoxicity of aflatoxins and their precursors in human cells. Toxicol. Lett. 2018, 287, 100–107.
- Kuiper-Goodman, T.; Scott, P.M. Risk assessment of the mycotoxin ochratoxin A. Biomed. Environ. Sci. BES 1989, 2, 179–248.
- Heussner, A.H.; Bingle, L.E.H. Comparative Ochratoxin Toxicity: A Review of the Available Data. Toxins 2015, 7, 4253–4282.
- Kőszegi, T.; Poór, M.; Manderville, R.A.; Pfohl-Leszkowicz, A. Ochratoxin A: Molecular Interactions, Mechanisms of Toxicity and Prevention at the Molecular Level. Toxins 2016, 8, 111.
- Fuchs, R.; Peraica, M. Ochratoxin A in human kidney diseases. Food Addit. Contam. 2005, 22, 53–57.
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137.
- Gupta, R.C.; Srivastava, A.; Lall, R. Chapter 72—Ochratoxins and Citrinin. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1019–1027.
- Longobardi, C.; Ferrara, G.; Andretta, E.; Montagnaro, S.; Damiano, S.; Ciarcia, R. Ochratoxin A and Kidney Oxidative Stress: The Role of Nutraceuticals in Veterinary Medicine—A Review. Toxins 2022, 14, 398.
- Lonkar, P.; Dedon, P.C. Reactive species and DNA damage in chronic inflammation: Reconciling chemical mechanisms and biological fates. Int. J. Cancer 2010, 128, 1999–2009.
- Zhang, G.-L.; Feng, Y.-L.; Song, J.-L.; Zhou, X.-S. Zearalenone: A Mycotoxin with Different Toxic Effect in Domestic and Laboratory Animals’ Granulosa Cells. Front. Genet. 2018, 9, 667.
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56.
- Shier, W.T.; Shier, A.C.; Xie, W.; Mirocha, C.J. Structure-activity relationships for human estrogenic activity in zearalenone mycotoxins. Toxicon Off. J. Int. Soc. Toxinol. 2001, 39, 1435–1438.
- WHO. World Health Organization Food Safety Digest—Fumonisins. 2018. Available online: https://www.who.int/foodsafety/FSDigest_Fumonisins_EN.pdf (accessed on 11 December 2021).
- Persson, E.C.; Sewram, V.; Evans, A.A.; London, W.T.; Volkwyn, Y.; Shen, Y.-J.; Van Zyl, J.A.; Chen, G.; Lin, W.; Shephard, G.S.; et al. Fumonisin B1 and risk of hepatocellular carcinoma in two Chinese cohorts. Food Chem. Toxicol. 2012, 50, 679–683.
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From Simple to Complex Mycotoxins. Toxins 2011, 3, 802–814.
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Stela, M.; Bijak, M. T-2 Toxin—The Most Toxic Trichothecene Mycotoxin: Metabolism, Toxicity, and Decontamination Strategies. Molecules 2021, 26, 6868.
- Jakovac-Strajn, B.; Tavčar-Kalcher, G. A Method Using Gas Chromatography—Mass Spectrometry for the Detection of Mycotoxins from Trichothecene Groups A and B in Grains. In Gas Chromatography in Plant Science, Wine Technology, Toxicology and Some Specific Applications; IntechOpen: London, UK, 2012; pp. 225–244.
- Polak-Śliwińska, M.; Paszczyk, B. Trichothecenes in Food and Feed, Relevance to Human and Animal Health and Methods of Detection: A Systematic Review. Molecules 2021, 26, 454.
- Audenaert, K.; Vanheule, A.; Höfte, M.; Haesaert, G. Deoxynivalenol: A Major Player in the Multifaceted Response of Fusarium to Its Environment. Toxins 2014, 6, 1–19.
- Foroud, N.A.; Baines, D.; Gagkaeva, T.Y.; Thakor, N.; Badea, A.; Steiner, B.; Bürstmayr, M.; Bürstmayr, H. Trichothecenes in Cereal Grains—An Update. Toxins 2019, 11, 634.
- Broekaert, N.; Devreese, M.; Demeyere, K.; Berthiller, F.; Michlmayr, H.; Varga, E.; Adam, G.; Meyer, E.; Croubels, S. Comparative in vitro cytotoxicity of modified deoxynivalenol on porcine intestinal epithelial cells. Food Chem. Toxicol. 2016, 95, 103–109.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
07 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




