Mycotoxins are small organic molecules synthesized as secondary metabolites by certain fungal species that may contaminate various agriculture commodities such as cereals, corn, nuts, spices, soybeans and coffee beans, among others. Aflatoxins (AFB
1, B
2, G
1 and G
2), ochratoxin A (OTA), citrinin, patulin, trichothecenes (mainly deoxynivalenol (DON), T2-toxin (T
2) and HT2-toxin (HT
2)), fumonisins (FB
1, FB
2 and FB
3) and zearalenone (ZEA) are the most prevalent mycotoxins in cereal crops [
1]. Mycotoxin contamination of cereal grains remains the main food safety issue. These toxins are produced primarily by fungi genera
Aspergillus,
Fusarium,
Penicillium, and
Alternaria [
2]. Aflatoxins are generated by
A. flavus and A. parasiticus, while ochratoxin A can be produced by
Aspergillus spp. [
3] and
Penicillium spp. [
4]. Fumonisins, trichothecenes and ZEA are produced by
Fusarium spp. and are collectively called fusarium mycotoxins. One mycotoxin can be produced by several fungi species and some mold species can produce multiple mycotoxins; nevertheless, toxic fungal development is not always accompanied by mycotoxin production [
5]. The fusarium mycotoxins may cause significant pre-harvest grain loss, while aflatoxins and OTA are mostly occurring during storage due to improper postharvest handling and storage [
6,
7].
Mycotoxins are hazardous to both human and animal health [
8]. They can cause acute toxicity and chronic diseases depending on the dosages. Acute intoxication occurs when highly contaminated food/feed is ingested in large quantities, which often results in death, as happened in Western India in 1974, Kenya from 1981 to 2006, Tanzania in 2016, Taiwan and Malaysia [
9,
10,
11,
12,
13]. Long-term exposure to mycotoxins at doses slightly above the regulation limits can lead to chronic diseases such as liver cancer caused by AFs in Africa and China [
14,
15,
16,
17,
18,
19], kidney diseases caused by OTA in Europe and Egypt, and esophageal cancer caused by FUMs in Africa and some regions of China [
20,
21,
22,
23]. There has been a wide variety of toxic effects in both animals and humans from oral intake of mycotoxin-contaminated foods/feeds, such as immunosuppression, genotoxic, teratogenic or cancerous mutagenesis.
Among the mycotoxins that commonly occurred in cereal grains, aflatoxins, including AFB
1, AFB
2, AFG
1, AFG
2 and AFM
1, are considered the most toxic and have been proven to be human carcinogens; thereby, they are classified as group 1 carcinogens, while FB
1, FB
2 and OTA are carcinogenic in tested animals, but there is not sufficient evidence about their carcinogenicity in human, and thus they are classified as group 2B carcinogens [
24]. Despite health impacts, it has been shown that these contaminants can cost billions of dollars every year. In underdeveloped countries, aflatoxins have impacted around 4.5 billion individuals and aflatoxicosis is ranked sixth among the top 10 health threats [
25]. Zearalenone (ZEA) has been reported to have immunotoxic, hepatotoxic and xenogenic effects [
26]. Trichothecenes are divided into four groups: types A, B, C and D, with type A and B trichothecenes being the most prominent toxins in barley, wheat, oats and maize. Studies have shown that type A trichothecenes are more toxic than type B, while type B trichothecenes are present in contaminated cereals at a higher concentration than trichothecenes A [
4,
14]. The A group includes but is not limited to T-2 and HT-2 toxins, while the B group mainly includes deoxynivalenol (DON), nivalenol (NIV), 3-acetyldeoxynivalenol (3Ac-DON), 15-acetyldeoxynivalenol (15Ac-DON) and fusarenone X [
4,
27].
2. Aflatoxins and Their Producing Fungi
Aflatoxins are synthesized by
Aspergillus flavus and
A. parasiticus. A. flavus produces B aflatoxins, while
A. parasiticus produces both B and G forms [
3]. Aflatoxins B
1, B
2, G
1 and G
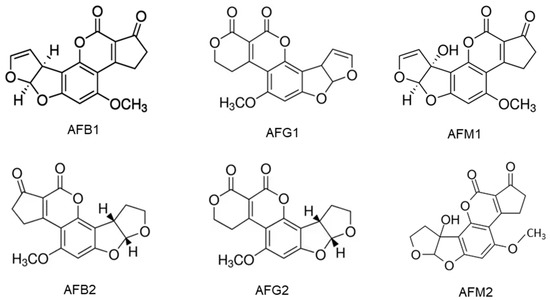
2 are naturally biosynthesized and frequently detected in cereal grains, particularly, maize, while the hydroxylated metabolites of AFB
1 and AFB
2 are aflatoxin M
1 (AFM
1) and M
2 (AFM
2) which are present in the meat, eggs, milk and cheese produced from animals which ingested aflatoxin-contaminated feed [
30]. AFM
1 is the major metabolite of AFB
1 in milk from nursing humans and animals that consume AFB
1-contaminated food or feed [
4]. The chemical structures of AFs are shown in
Figure 1. They are slightly soluble in water with a solubility of 10–20 µg/mL and completely soluble in moderately polar solvents such as chloroform, menthol and dimethyl sulfoxide [
24]. The low hydrophilicity and high hydrophobicity of AFs enable them to bind to cell membrane lipids easily.
Figure 1. Chemical structures of aflatoxins B1, B2, G1, G2, M1 and M2.
Aflatoxins (AFs) are cytotoxic and genotoxic. AFB
1, AFG
1 and AFM
1 are carcinogenic when ingested orally via the diet or delivered by gavage. The evidence for the carcinogenicity of AFB
2 and AFG
2 is insufficient. The AFB
1 is more toxic than AFG
1 in liver carcinogenicity but AFG
1 induced a higher incidence of kidney tumors than AFB
1. The AFB
1 is about 10 times more potent than AFM
1 in causing liver carcinogenicity [
31]. The AFB
1 is genotoxic and participates in the extrahepatic cycle, leading to chromosomal abnormalities, micronucleus formation, sister chromatid exchange, unscheduled DNA synthesis and DNA strand breakage [
32]. The damage to DNA ultimately leads to the development of cancer. A human cell line study shows that AFB
1 and AFG
1, their precursors, as well as their metabolites aflatoxicol (AFL) and AFM
1 are genotoxic [
33]. Studies with poultry have found that the AFB
1 and its metabolites mainly target the liver, where the toxin is metabolized mainly by CYP1A2 and CYP3A4 and causes numerous mutations, particularly in the p53 tumor suppressor gene [
32]. This should be at least partially responsible for the high liver cancer incidence in some regions of the world where people are frequently exposed to food products contaminated with AFs.
3. Ochratoxins and Their Producing Fungi
Ochratoxins (OTs) are produced by certain
Aspergillus species and some
Penicillium species, including
A. ochraceus,
A. alliaceus,
A. auricomus,
A. carbonarius,
A. glaucus,
A. melleus,
A. niger,
P. nordicum and
P. verrucosum among them [
4,
34]. The main forms of OTs are ochratoxin A, B and C, which differ in chemical structure and toxicity. Ochratoxin B (OTB) is a non-chlorinated form of OTA and ochratoxin C (OTC) is an ethyl ester of OTA formed in the presence of rumen fluid [
8]. Among these ochratoxins, OTA is the most prevalent and toxic followed by OTC and OTB [
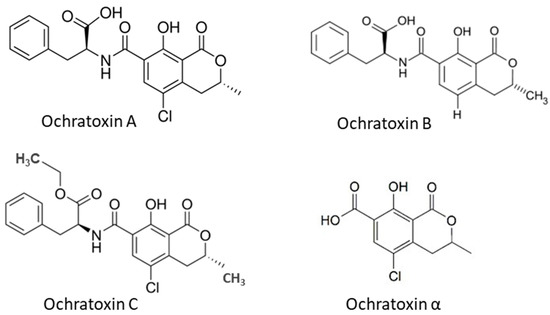
35]. OTα is a non-toxic metabolite of OTA. The OTA has been found in several types of cereals, including corn, wheat, barley, rye, rice and other plant products such as coffee beans, dried fruits and spices. The chemical structures of OTs (
Figure 2) show that all OTs contain a non-polar end and several polar groups on the side chain; thus, they can bind to membrane lipids and proteins such as plasma albumin [
22,
36].
Figure 2. Chemical structure of ochratoxins occurred in cereal grains.
OTA is at least ten times more toxic than OTB and OTC. The OTA is nephrotoxic to all animal species and humans, and it has been related to the Balkan endemic nephropathy [
37,
38]. In addition, the OTA is also known to be hepatotoxic, immunotoxic, neurotoxic, teratogenic and carcinogenic, involving multiple mechanisms [
35,
39]. A single high dose or multiple lower doses of OTA in rats inhibits protein synthesis, mitochondrial respiration and ATP formation. It also enhances lipid peroxidation and the formation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) [
39,
40]. High levels of ROS result in decreased activity of cellular antioxidant enzymes, thus leading to oxidative stress and further inflammatory diseases [
40]. Elevated levels of RNS induce nitrosative stress associated with DNA damage, tissue toxicity, cancer and inflammatory conditions, which may be responsible for cell injury and death [
41].
4. Zearalenone and Its Producing Fungi
ZEA is produced by fungi of the genus
Fusarium spp., including
F. graminearum,
F. culmorum,
F. cerealis,
F. equiseti,
F. crookwellense,
F. semitectum,
F. verticillioides,
F. sporotrichioides,
F. oxysporum and
F. acuminatum [
26], but mainly
F. graminearum and
F. culmorum [
42]. Corn has been described as the most vulnerable food to ZEA contamination. Other food crops, including wheat, barley, oat and rye, have also been found with this toxin. ZEA is immunotoxic, hepatotoxic and xenogenic [
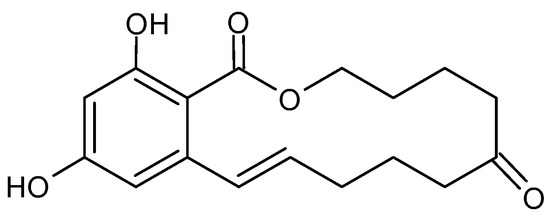
26]. The chemical structure of ZEA consists of a resorcinol moiety fused to a 14-member macrocyclic lactone ring with a trans double bond, two hydroxyl groups, two ketones and one methyl branch (
Figure 3), which allow it to be easily absorbed through the gastrointestinal tract, to interact with proteins and lipids in the biological system to exert its toxicity [
43,
44]. The ZEA induces histopathological changes in the liver, with the subsequent development of liver cancer; it exerts hematotoxic effects by disturbing blood coagulation and modifying blood parameters. ZEA and its derivatives are non-steroids but have estrogenic activity in mammalian animals. They bind to estrogen receptors of cells and inhibit the secretion of steroid hormones, interfere with the estrogen response in the pre-ovulatory phase, and inhibit follicle maturation in mammals; this leads to disorders of the hormonal balance of the body, and, subsequently, many diseases of the reproductive system [
43,
44]. Higher concentrations of ZEA cause permanent estrus, pseudo-pregnancy and infertility in animals [
44]. The detail toxicity of ZEA in humans and animals has been extensively reviewed by Ropejko and Twarużek [
26].
Figure 3. Chemical structure of Zearalenone.
5. Fumonisins and Their Producing Fungi
Fumonisins (FUMs) are produced in cereals by different species of
Fusarium including
F. verticillioides,
F. proliferatum and
F. fujikuroi, and other related species; they are common contaminants of maize and to a lesser extent of wheat and other cereals [
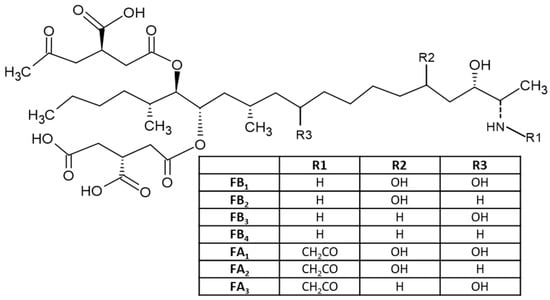
45]. Fumonisins consist of a long hydrocarbon backbone chain similar to that of sphinganine. Six forms of fumonisin including FA
1, FA
2, FB
1, FB
2, FB
3 and FB
4 have been identified, with FB
1 being the most toxic [
4]. All FUMs are water soluble and thus are polar [
24], which is determined by their chemical structures (
Figure 4). These toxins are cytotoxic and carcinogenic to animals at relatively high concentrations. FB
1, the major and most-studied fumonisin, is nephrotoxic and hepatotoxic in several animal species and has been classified as a possible carcinogen to humans (Group 2B) [
24]. FB
1 has been reported to cause leukoencephalomalacia (LEM) in horses, pulmonary edema syndrome (PES) in pigs and liver cancer in rats and hepatotoxic to horses, pigs, rats and vervet monkeys. The FB
1 is cytotoxic to mammalian cell cultures and phytotoxic to several plants. FUMs have also been reported to be linked to human esophageal cancer in Transkei, China and South Africa [
20,
21,
22,
23,
46].
Figure 4. Chemical structure of different fumonisins.
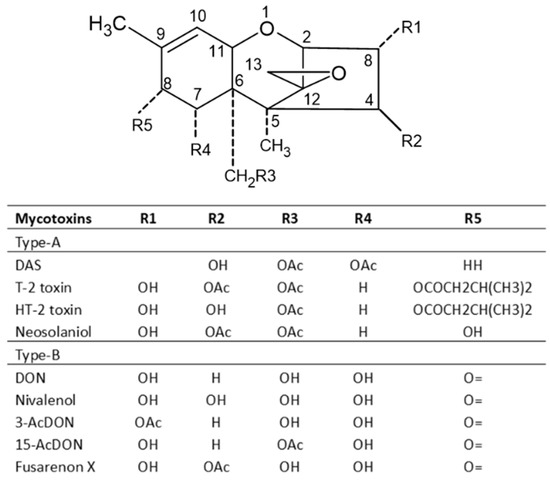
6. Trichothecenes and Their Producing Fungi
Trichothecenes are several groups of mycotoxins produced by fungi of the
Fusarium genus [
14]. They are divided into four groups: types A, B, C and D. The type A group mainly consists of T-2 and HT-2 toxins, diacetoxy- and monoacetoxy-scirpenol (DAS and MAS) and neosolaniol (NEO). The B group mainly includes DON, nivalenol (NIV), fusarenone X and DON derivatives 3Ac-DON and 15Ac-DON [
4,
27]. Type C trichothecenes contain a C-7/C-8 epoxide (e.g., crotocin), while type D trichothecenes have an additional ring linking the C-4 and C-15 position (e.g., roridin A, verrucarin A and satratoxin H) [
47]. Type A and B trichothecenes are the most common in barley, wheat, oats and maize. Studies have shown that the toxicity of type A trichothecenes is greater than that of type B but, fortunately, the concentrations of type A trichothecenes present in contaminated cereals are much lower than that of trichothecenes B [
4]. Among trichothecene mycotoxins, the T-2 toxin is the most toxic and it is considered an immunosuppressive, cytotoxic, lymphocytic and carcinogenic mycotoxin in mammalian cells. The toxicity of the T-2 toxin is extensively reviewed by Janik et al. (2021) [
48]. The chemical structures of trichothecenes are depicted in
Figure 5 [
49], which indicates that DON and NIV are more polar, while the T-2 toxin is hydrophobic, and the polarity of HT-2 toxin is between DON/NIV and T-2 toxin. This may contribute to the higher toxicity of T-2 toxin. However, T-2 toxin levels in food and feed are not regulated in many countries including the US.
Figure 5. Chemical structures of major trichothecene mycotoxins.
Type A trichothecenes are manly produced by strains of
F. sporotrichioides and
F. poae, while type B trichothecenes toxins are mainly produced by strains of
F. culmorum and
F. graminearum [
50]. Milder climatic conditions without high humidity favor the production of type A trichothecenes [
51]. In the European maize growing areas, this toxin is usually detected in corn red ear rot. Oral exposure to T-2 toxin can lead to a fatal condition, known as alimentary toxic aleukia (ATA) with radiation poisoning-like symptoms [
52]. DON and other Type B trichothecenes are not as potent in mammalian systems compared with T-2 toxin, but can still be lethal at high enough concentrations. DON is also known as ‘vomitoxin’ for its ability to cause diarrhea and emesis. DON and its derivatives cause a variety of maladies, including anorexia, feed refusal in livestock, growth retardation, leukocytosis, hemorrhage and adverse effects on reproduction and development. The toxicity of DON and its derivatives to animals is in the order of 15Ac-DON > 3Ac-DON > DON > DON-3Glc [
2,
53].