
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Oreste Gallo | -- | 2686 | 2023-07-30 18:47:41 | | | |
| 2 | Lindsay Dong | Meta information modification | 2686 | 2023-07-31 05:08:59 | | |
Video Upload Options
Laryngeal cancer is a relatively common neoplasm of head and neck, whose management hinges on a combination of treatments such as surgery, radiotherapy and chemotherapy. Tumor recurrences may present important differences from the primary tumor that largely depend on previous treatments. The immune system plays a crucial role in the natural course of the disease, owing the capability to influence its behavior through a complex interaction of mechanisms. For this reason, the use of immunotherapy in addition to standard therapies is increasingly gaining importance nowadays and the selection of patients who can benefit the most from this treatment can help optimizing its success. However, conventional treatments can induce relevant changes in the host immune response, thus affecting tumor progression and patient outcome. This fact should be taken into account when planning immune-based treatments like immune checkpoint inhibitors.
1. Introduction
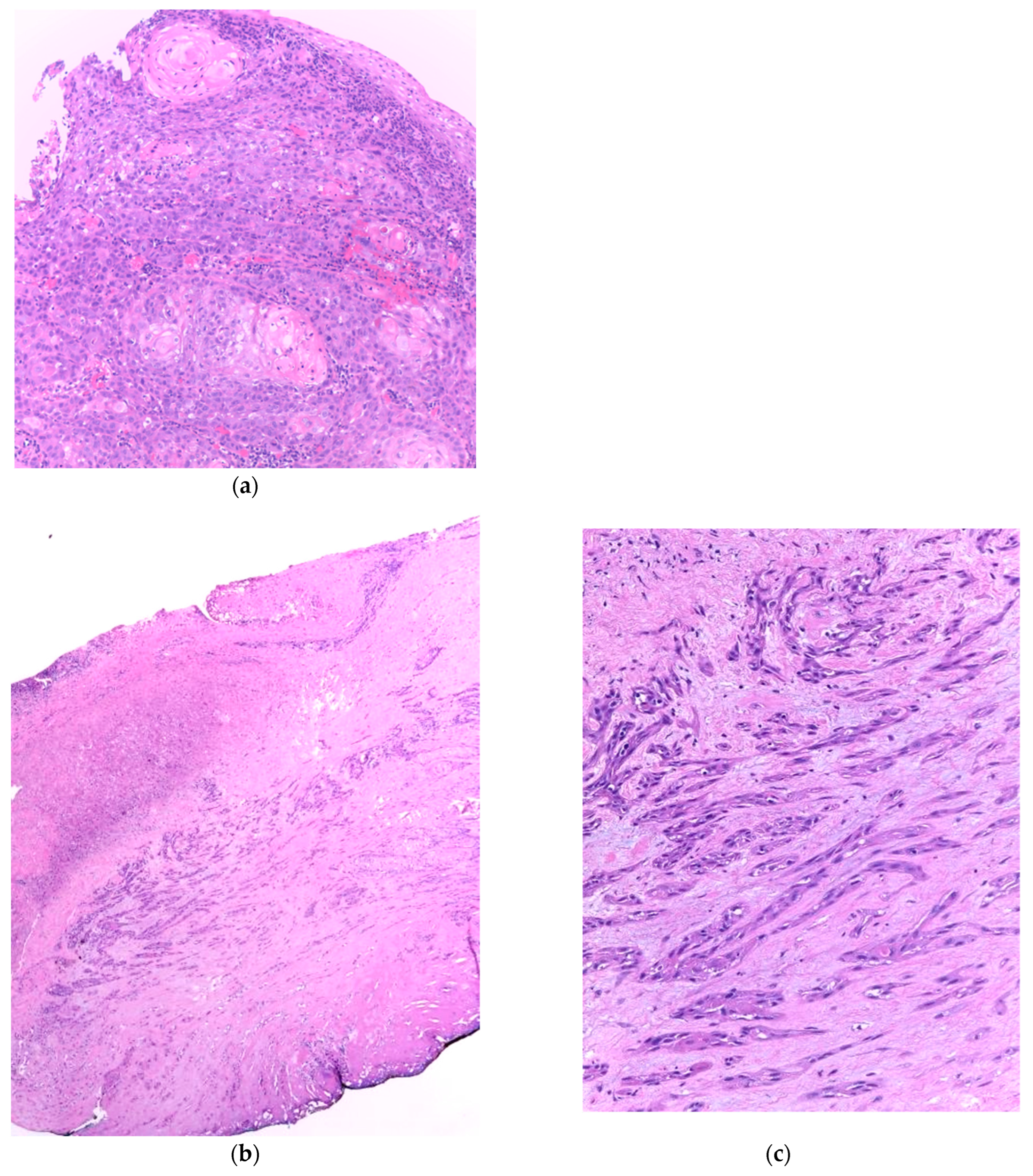
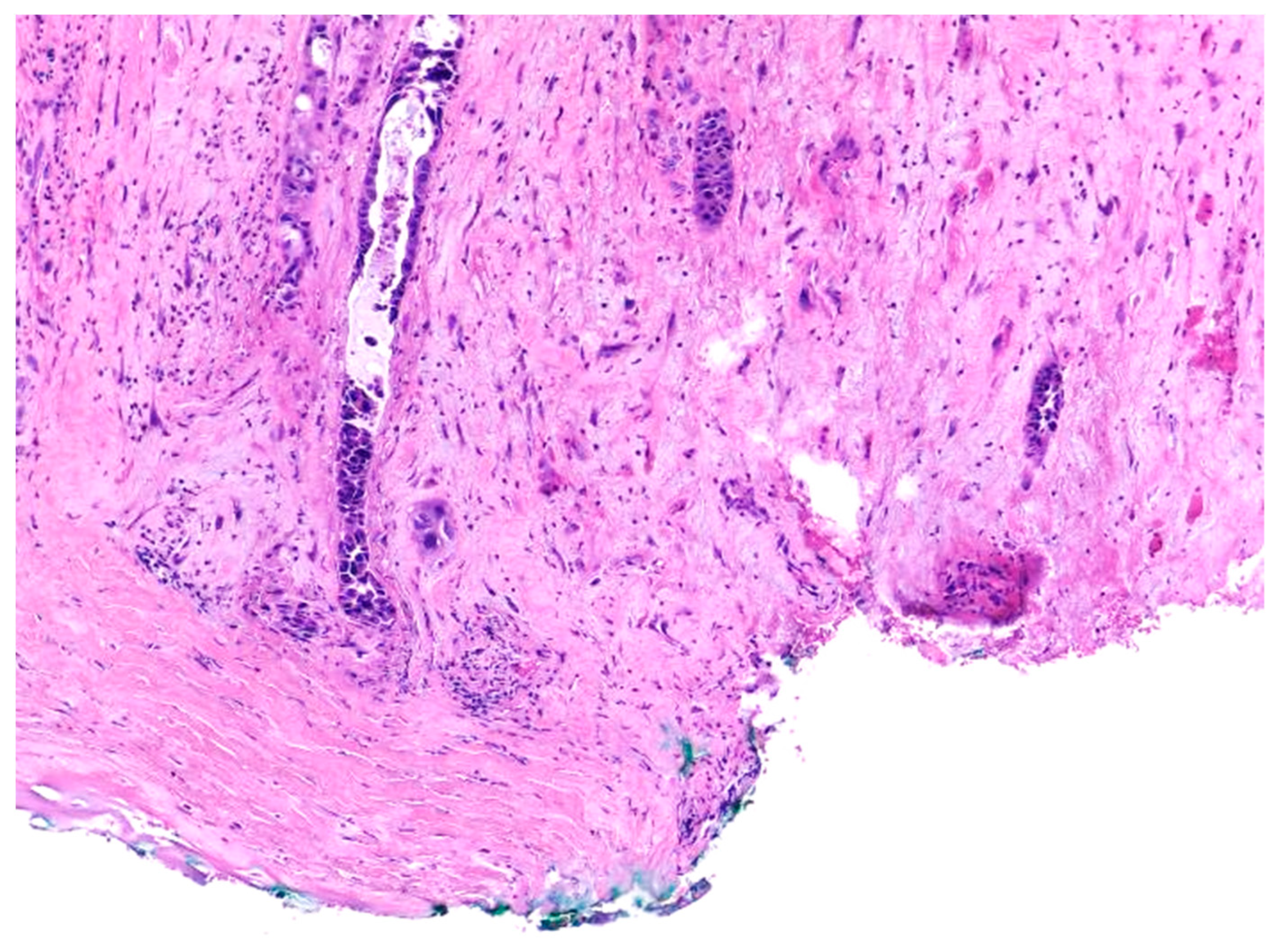
2. Histopathologic Changes of Laryngeal Anatomic Structures after Radiotherapy
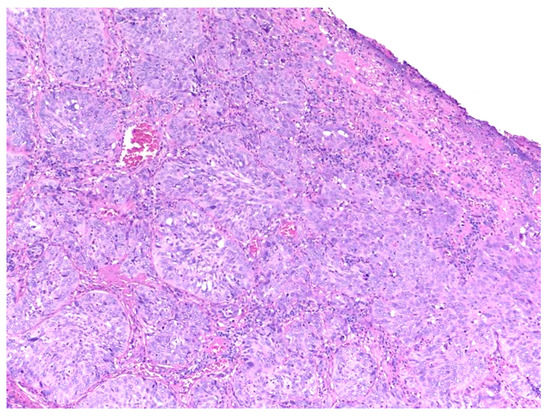
3. Recurrent Squamous Cell Carcinoma: Histopathologic Changes in Postradiotherapy Recurrence vs. Postsurgical Recurrence
4. Treatment-Induced Modifications of the Tumor Microenvironment
4.1. Radiotherapy Effects on TME
4.2. Chemotherapy and Cetuximab Effects on the TME
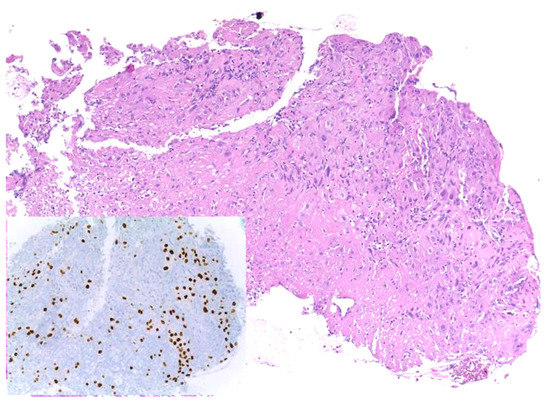
5. Modifications of PDL1 Expression in Recurrences
6. Conclusions
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- ECIS—European Cancer Information System. Available online: https://ecis.jrc.ec.europa.eu (accessed on 2 February 2023).
- Chatenoud, L.; Garavello, W.; Pagan, E.; Bertuccio, P.; Gallus, S.; La Vecchia, C.; Negri, E.; Bosetti, C. Laryngeal cancer mortality trends in European countries. Int. J. Cancer 2016, 138, 833–842.
- Locatello, L.G.; Bruno, C.; Gallo, O. Early glottic cancer recurrence: A critical review on its current management. Crit. Rev. Oncol. Hematol. 2021, 160, 103298.
- Lee, M.Y.; Belfiglio, M.; Zeng, J.; Fleming, C.W.; Koyfman, S.; Joshi, N.P.; Lamarre, E.; Prendes, B.; Scharpf, J.; Lorenz, R.R.; et al. Primary Total Laryngectomy versus Organ Preservation for Locally Advanced T3/T4a Laryngeal Cancer. Laryngoscope 2022, 133, 1122–1131.
- Magnes, T.; Wagner, S.; Kiem, D.; Weiss, L.; Rinnerthaler, G.; Greil, R.; Melchardt, T. Prognostic and Predictive Factors in Advanced Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2021, 22, 4981.
- Locatello, L.G.; Cannavicci, A.; Gallo, O. Prognostic impact of initial treatment in surgically salvaged recurrences of early glottic cancer. Laryngoscope 2019, 129, 2328–2333.
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–426.
- Langley, R.E.; Bump, E.A.; Quartuccio, S.G.; Medeiros, D.; Braunhut, S.J. Radiation-induced apoptosis in microvascular endothelial cells. Br. J. Cancer 1997, 75, 666–672.
- Abouyared, M.; Kerr, D.A.; Burroway, B.; Sabra, J.; Sargi, Z.; Nicolli, E.; Leibowitz, J. Abnormal Microvasculature in Laryngectomy Mucosal Margins may be Associated with Increased Risk of Fistula. Head Neck Pathol. 2019, 13, 364–370.
- Fayardo, L.F. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol. 2005, 44, 13–22.
- Berg, E.E.; Kolachala, V.; Branski, R.C.; Muller, S.; Johns, M.M. Pathologic effects of external-beam irradiation on human vocal folds. Ann. Otol. Rhinol. Laryngol. 2011, 120, 748–754.
- Johns, M.M.; Kolachala, V.; Berg, E.; Muller, S.; Creighton, F.X.; Branski, R.C. Radiation fibrosis of the vocal fold: From man to mouse. Laryngoscope 2012, 122 (Suppl. 5), S107–S125.
- Tanigami, Y.; Kawai, Y.; Kaba, S.; Uozumi, R.; Ohnishi, H.; Kita, T.; Omori, K.; Kishimoto, Y. Establishment of a radiation-induced vocal fold fibrosis mouse model. Biochem. Biophys Res. Commun. 2022, 601, 31–37.
- Pandya, J.A.; Srikant, N.; Boaz, K.; Manaktala, N.; Kapila, S.N.; Yinti, S.R. Post-radiation changes in oral tissues - An analysis of cancer irradiation cases. South Asian J. Cancer 2014, 3, 159–162.
- Qiao, L.; Chen, Y.; Liang, N.; Xie, J.; Deng, G.; Chen, F.; Wang, X.; Liu, F.; Li, Y.; Zhang, J. Targeting Epithelial-to-Mesenchymal Transition in Radioresistance: Crosslinked Mechanisms and Strategies. Front. Oncol. 2022, 12, 775238.
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15.
- Luo, W. Nasopharyngeal carcinoma ecology theory: Cancer as multidimensional spatio-temporal “unity of ecology and evolution” pathological ecosystem. Theranostics 2023, 13, 1607–1631.
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal 2020, 18, 59.
- Qian, J.M.; Schoenfeld, J.D. Radiotherapy and Immunotherapy for Head and Neck Cancer: Current Evidence and Challenges. Front. Oncol. 2021, 10, 608772.
- Curry, J.M.; Sprandio, J.; Cognetti, D.; Luginbuhl, A.; Bar-ad, V.; Pribitkin, E.; Tuluc, M. Tumor microenvironment in head and neck squamous cell carcinoma. Semin. Oncol. 2014, 41, 217–234.
- Rad, H.S.; Shiravand, Y.; Radfar, P.; Ladwa, R.; Perry, C.; Han, X.; Warkiani, M.E.; Adams, M.N.; Hughes, B.G.; O’Byrne, K.; et al. Understanding the tumor microenvironment in head and neck squamous cell carcinoma. Clin. Transl. Immunol. 2022, 11, e1397.
- Wang, G.; Zhang, M.; Cheng, M.; Wang, X.; Li, K.; Chen, J.; Chen, Z.; Chen, S.; Chen, J.; Xiong, G.; et al. Tumor microenvironment in head and neck squamous cell carcinoma: Functions and regulatory mechanisms. Cancer Lett. 2021, 507, 55–69.
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1, e89829.
- Esteban, F.; Concha, A.; Delgado, M.; Pérez-Ayala, M.; Ruiz-Cabello, F.; Garrido, F. Lack of MHC class I antigens and tumour aggressiveness of the squamous cell carcinoma of the larynx. Br. J. Cancer 1990, 62, 1047–1051.
- Concha-Benavente, F.; Ferris, R.L. Reversing rajR Mediated Immunoescape by Targeted Monoclonal Antibody Therapy. Front. Pharmacol. 2017, 8, 332.
- Xiao, C.; Song, F.; Zheng, Y.L.; Lv, J.; Wang, Q.F.; Xu, N. Exosomes in Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 894.
- McLaughlin, M.; Patin, E.C.; Pedersen, M.; Wilkins, A.; Dillon, M.T.; Melcher, A.A.; Harrington, K.J. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 2020, 20, 203–217.
- Arina, A.; Beckett, M.; Fernandez, C.; Zheng, W.; Pitroda, S.; Chmura, S.J.; Luke, J.J.; Forde, M.; Hou, Y.; Burnette, B.; et al. Tumor-reprogrammed resident T cells resist radiation to control tumors. Nat. Commun. 2019, 10, 3959.
- Kroemer, G.; Galluzzi, L.; Keep, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72.
- Zhang, C.; Liang, Z.; Ma, S.; Liu, X. Radiotherapy and Cytokine Storm: Risk and Mechanism. Front. Oncol. 2021, 11, 670464.
- Green, D.R.; Ferguson, T.; Zitvogel, L.; Kroemer, G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 2009, 9, 353–363.
- Golden, E.B.; Frances, D.; Pellicciotta, I.; Demaria, S.; Helen Barcellos-Hoff, M.; Formenti, S.C. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 2014, 3, e28518.
- Golden, E.B.; Apetoh, L. Radiotherapy and immunogenic cell death. Semin. Radiat. Oncol. 2015, 25, 11–17.
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870.
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; de Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271.
- Sharabi, A.B.; Nirschl, C.J.; Kochel, C.M.; Nirschl, T.R.; Francica, B.J.; Velarde, E.; Deweese, T.L.; Drake, C.G. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol. Res. 2015, 3, 345–355.
- Knops, A.M.; South, A.; Rodeck, U.; Martinez-Outschoorn, U.; Harshyne, L.A.; Johnson, J.; Luginbuhl, A.J.; Curry, J.M. Cancer-Associated Fibroblast Density, Prognostic Characteristics, and Recurrence in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Front. Oncol. 2020, 10, 565306.
- Leduc, C.; Adam, J.; Louvet, E.; Sourisseau, T.; Dorvault, N.; Bernard, M.; Maingot, E.; Faivre, L.; Cassin-Kuo, M.S.; Boissier, E.; et al. TPF induction chemotherapy increases PD-L1 expression in tumour cells and immune cells in head and neck squamous cell carcinoma. ESMO Open 2018, 3, e000257.
- Fiedler, M.; Weber, F.; Hautmann, M.G.; Bohr, C.; Reichert, T.E.; Ettl, T. Infiltrating immune cells are associated with radiosensitivity and favorable survival in head and neck cancer treated with definitive radiotherapy. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2020, 129, 612–620.
- Koukourakis, I.M.; Gkegka, A.G.; Xanthopoulou, E.; Nanos, C.; Giatromanolaki, A.; Koukourakis, M.I. Prognostic and Predictive Relevance of Tumor-Infiltrating Lymphocytes in Squamous Cell Head-Neck Cancer Patients Treated with Radical Radiotherapy/Chemo-Radiotherapy. Curr. Oncol. 2022, 29, 4274–4284.
- Hudson, K.; Cross, N.; Jordan-Mahy, N.; Leyland, R. The Extrinsic and Intrinsic Roles of PD-L1 and Its Receptor PD-1: Implications for Immunotherapy Treatment. Front. Immunol. 2020, 11, 568931.
- Birtalan, E.; Danos, K.; Gurbi, B.; Brauswetter, D.; Halasz, J.; Kalocsane Piurko, V.; Acs, B.; Antal, B.; Mihalyi, R.; Pato, A.; et al. Expression of PD-L1 on Immune Cells Shows Better Prognosis in Laryngeal, Oropharygeal, and Hypopharyngeal Cancer. Appl. Immunohistochem. Mol. Morphol. 2018, 26, e79–e85.
- Fasano, M.; Corte, C.M.D.; Liello, R.D.; Viscardi, G.; Sparano, F.; Iacovino, M.L.; Paragliola, F.; Piccolo, A.; Napolitano, S.; Martini, G.; et al. Immunotherapy for head and neck cancer: Present and future. Crit. Rev. Oncol. Hematol. 2022, 174, 103679.
- Taverna, C.; Franchi, A. Role of Surgical Pathologist for Detection of Immunooncologic Predictive Factors in Head and Neck Cancer. Adv. Anat. Pathol. 2023, 30, 167–173.
- Samanta, D.; Park, Y.; Ni, X.; Li, H.; Zahnow, C.A.; Gabrielson, E.; Pan, F.; Semenza, G.L. Chemotherapy induces enrichment of CD47+/CD73+/PDL1+ immune evasive triple-negative breast cancer cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1239–E1248.
- Zhang, P.; Su, D.M.; Liang, M.; Fu, J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol. Immunol. 2008, 45, 1470–1476.
- Peng, J.; Hamanishi, J.; Matsumura, N.; Abiko, K.; Murat, K.; Baba, T.; Yamaguchi, K.; Horikawa, N.; Hosoe, Y.; Murphy, S.K.; et al. Chemotherapy Induces Programmed Cell Death-Ligand 1 Overexpression via the Nuclear Factor-κB to Foster an Immunosuppressive Tumor Microenvironment in Ovarian Cancer. Cancer Res. 2015, 75, 5034–5045.
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695.
- Ock, C.Y.; Kim, S.; Keam, B.; Kim, S.; Ahn, Y.O.; Chung, E.J.; Kim, J.H.; Kim, T.M.; Kwon, S.K.; Jeon, Y.K.; et al. Changes in programmed death-ligand 1 expression during cisplatin treatment in patients with head and neck squamous cell carcinoma. Oncotarget 2017, 8, 97920–97927.




