Laryngeal cancer is a relatively common neoplasm of head and neck, whose management hinges on a combination of treatments such as surgery, radiotherapy and chemotherapy. Tumor recurrences may present important differences from the primary tumor that largely depend on previous treatments. The immune system plays a crucial role in the natural course of the disease, owing the capability to influence its behavior through a complex interaction of mechanisms. For this reason, the use of immunotherapy in addition to standard therapies is increasingly gaining importance nowadays and the selection of patients who can benefit the most from this treatment can help optimizing its success. However, conventional treatments can induce relevant changes in the host immune response, thus affecting tumor progression and patient outcome. This fact should be taken into account when planning immune-based treatments like immune checkpoint inhibitors.
1. Introduction
Laryngeal cancer is the second most common cancer of the upper aerodigestive tract, accounting for approximately 20% of all head and neck malignancies [
1]. The age standardized incidence rates in Europe for the year 2020 were 9.8 × 100,000 and 1.4 × 100,000, while mortality rates were 4.8 × 100,000 and 0.5 × 100,000 for males and females, respectively [
2]. High tobacco exposure and alcohol consumption are the two major well-established risk factors, whereas HPV infection seems to play a marginal role at this anatomic site [
3]. Laryngeal cancer is treated using a combination of chemotherapy, radiation, and surgical techniques, depending on the histologic type, biology, location, and stage, as well as patient and other factors. Early glottic cancer can be treated either with curative radiotherapy (RT) or with surgery, ensuring a comparable overall survival. RT is often preferred as primary treatment because it grants a better functional integrity of the larynx, and the risk of local recurrence is comparable with that of surgical treatments [
4]. Treatment for locally advanced T3 and T4 laryngeal cancers includes total laryngectomy with or without postoperative RT, but since the introduction of organ preservation techniques, including RT with or without chemotherapy, the number of total laryngectomies performed has drastically reduced [
5]. However, the incidence of local recurrence can be as high as 35–50% [
6,
7], and salvage surgery is generally performed in these cases.
In recent years, the tumor immune microenvironment has been the object of extensive studies, and as a result, immune check point inhibitors (CPI) have been introduced in the treatment of recurrent/metastatic head and neck squamous carcinoma as valid alternative to standard chemotherapy. In general, the clinical benefits of these treatments have been quite variable, hence the need to select those patients who may obtain the maximal efficacy through the identification of predictive biomarkers. Moreover, among the critical issues that must be considered in the evaluation of the efficacy of CPI as well as in the assessment of predictive biomarkers are the changes in the tumor microenvironment that occur after primary treatment in recurrent/persistent SCCs.
2. Histopathologic Changes of Laryngeal Anatomic Structures after Radiotherapy
Among the several anatomic components, the most relevant changes induced by radiotherapy involve the vasculature [
8]. The early effects on blood vessels include detachment of endothelial cells from the basement membrane and apoptosis [
9], and this damage is greater in small capillaries of the tumor bed because their wall lacks a pericytic layer and it is mainly formed by the endothelium [
9]. These vessels are often dilated and may be occluded by thrombosis due to the prothrombotic state created by radiation and the endothelial damage [
9]. Small- and medium- sized arteries develop subendothelial or adventitial fibrosis, hyalinization of the media, and accumulation of lipid-laden macrophages in the intima, a picture that is virtually indistinguishable from atherosclerosis. Overall, this vascular damage results in hypoxia of the tumor microenvironment. Accordingly, radiation-induced microvascular damage in the irradiated larynx includes the presence of telangiectatic capillaries as well as the thickening and hyalinization of the arteriolar wall [
10].
A constant delayed tissue change of radiotherapy is subepithelial fibrosis, which consists of areas of dense acellular or paucicellular collagen with variable extension and severity [
11]. Laryngeal mucosa is expanded by dense collagen deposition, and fibrin may also be detected in the stroma in between collagen fibers and fibroblasts [
10]. In their study of 20 irradiated vocal folds from 13 patients, Berg et al. found increased collagen and muscle fiber disorganization in the irradiated specimens as compared to the controls, together with a significant increase of hyaluronic acid and fibronectin tissue content, and a decrease of laminin [
12]. This is accompanied by increased transcription of markers for fibrosis, oxidative stress, inflammation, glycosaminoglycan production, and apoptosis [
13]. The mechanisms involved in the development of vocal fold fibrosis have been investigated in murine models [
13,
14], which show similar histologic and biochemical changes to irradiated human vocal folds. Transcriptional analysis revealed upregulated expressions of TGF-beta1, which is responsible for the fibrotic changes and induces myofibroblast differentiation, and iNOS at six months. Conversely, the expressions of Acta2, Col1a1, Col3a1, and MMP8 were downregulated, indicating reduced collagen turnover [
14].
Both the lining epithelium and salivary-type glands of the laryngeal mucosa are affected by radiation injury. In the acute phase, necrosis of the epithelium predominates, although ischemia resulting from vascular damage may also cause delayed necrosis [
11]. Epithelial atrophy is a delayed effect, and histologically consists of thinning of the surface epithelium, loss of salivary gland-type acini, and sialometaplasia (squamous metaplasia) of the acini and ducts in the remaining glands.
3. Recurrent Squamous Cell Carcinoma: Histopathologic Changes in Postradiotherapy Recurrence vs. Postsurgical Recurrence
The changes induced by radiation in normal tissues can be detected in specimens from salvage surgery or after recurrence of head and neck squamous cell carcinoma (HNSCC) as well. However, they combine with important modifications induced by the tumor itself. Unfortunately, systematic histopathologic studies describing the tumor changes in persistent/recurrent laryngeal SCC after radiotherapy or chemotherapy in comparison with persistent/recurrent SCC after conservative surgeries are lacking. Pandya et al. examined 27 cases of oral SCC that recurred after radiation therapy within an average span of 11 months and compared their histologic features with those of 26 non-irradiated cases of oral SCC [
16]. As expected, irradiated carcinomas presented significantly increased fibrinous exudates, necrosis, and vessel wall thickening in comparison with nonirradiated cases [
17]. Moreover, intrinsic changes in the histologic features of the irradiated tumors included a decrease of the degree of keratinisation and inflammation, whereas nuclear pleomorphism was significantly increased [
16].
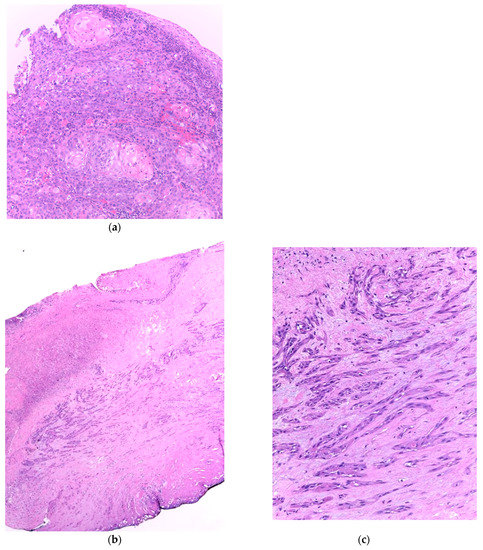
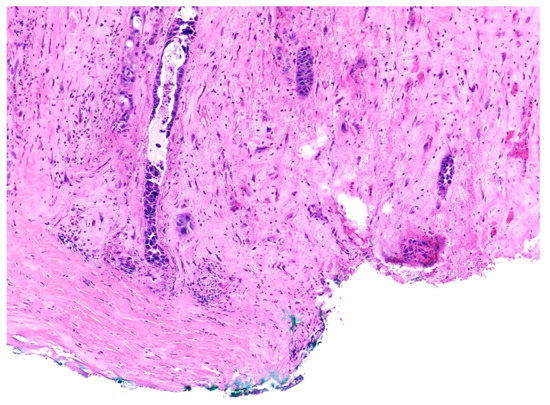
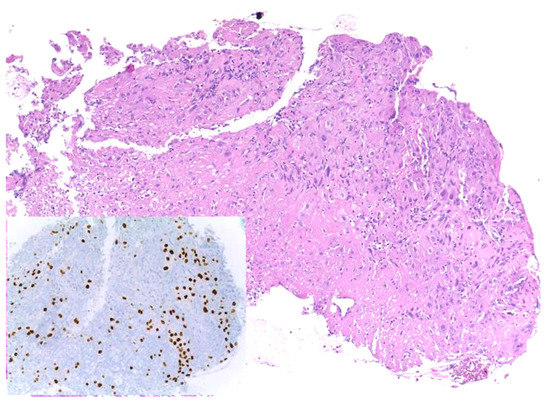
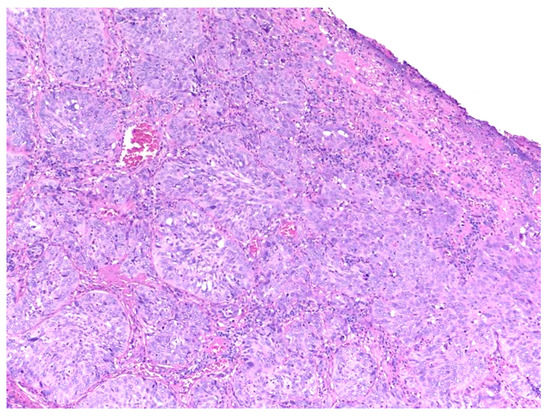
Similar changes can be observed in recurrent/persistent postradiation laryngeal SCCs. Figure 1 illustrates a postradiation recurrent SCC of the vocal cord. The tumor shows no connection with the surface epithelium and consists of cords and a small island of tumor cells within a fibrotic stroma. Although the significance of these findings for the biology of the tumor remains to be fully determined, they nevertheless have an impact on the evaluation of resection margins (Figure 2), as well as on the interpretation of histopathologic findings in biopsies, where neoplastic cells may be difficult to identify in small superficial samples, or if they are set within necrosis or fibrosis (Figure 3). In comparison, postsurgical recurrences of laryngeal SCC consist of irregular infiltrative tumor islands, but necrosis, acellular fibrosis with collagenization, inflammation, and the characteristic damage to vessels are usually absent (Figure 4).
Figure 1. (a). Incisional biopsy of a moderately differentiated squamous cell carcinoma of the vocal cord. The tumor consists of islands of atypical squamous cells with areas of keratinization. There is a light inflammatory infiltrate beneath the surface epithelium. (b) Recurrent squamous cell carcinoma of the vocal cord following radiotherapy. (c) The surface epithelium is attenuated or absent and there is fibrosis of the superficial mucosa. The tumor is visible in the mid part of the section and consists of elongated nests and small groups of neoplastic cells separated by fibrotic stroma.
Figure 2. Recurrent squamous cell carcinoma of the vocal cord following radiotherapy. The histologic section shows the deep margin of a specimen of vocal cord resection, inked in green. The tumor consists of small, separated nests of tumor cells and it is thus impossible to assess the margin status with certainty.
Figure 3. Recurrent squamous cell carcinoma of the vocal cord following radiotherapy. In this biopsy specimen, tumor cells are obscured by necrosis and fibrosis. They are highlighted by the nuclear immunohistochemical staining for P40 (inset).
Figure 4. Recurrent laryngeal squamous cell carcinoma following surgery. The tumor presents with cohesive irregular nests of neoplastic cells.
4. Treatment-Induced Modifications of the Tumor Microenvironment
The TME encompasses a dynamic ensemble of elements comprising immune cells, nonimmune cells, and extracellular components, taking active part in the milieu where neoplastic cells develop [
23]. Cells involved in TME accomplish a dual role in cancer imbalance, simultaneously favoring and hampering its progression in a tricky biological dialogue [
19]. The immune cells include myeloid derived stem cells (MDSCs), regulatory T-cells (T-regs), tumor-infiltrating lymphocytes (TILs), TAMs, and dendritic cells (DCs). Nonimmune cells are mainly represented by CAFs. TME balance hinges on a constant interplay between immune cells, CAFs, and neoplastic cells which is modulated by several factors such as cytokines, chemokines, growth factors, extracellular matrix, and exosomes [
24]. A tumor can be regarded as a complex ecosystem in which neoplastic cells interact with TME components, undergoing a process of coevolution in which they acquire a temporally and spatially heterogeneous phenotype with selective advantages such as the capability for immune evasion [
19].
TME of laryngeal SCC has shown evidence of peculiar features which are responsible for a significant decrease in antitumoral response compared with SCCs of other districts [
28,
29,
30,
31]. The immunosuppressive environment of laryngeal cancer could be partly attributed to selective events following genetic alterations induced by alcohol and tobacco consumption [
29]. Indeed, tobacco smoking has shown a meaningful association with immunosuppression and lowering of cytotoxic activity in TME [
33]. Suppression of immune activity in HNSCC TME takes place through several mechanisms, including downregulation of HLA, which impairs neoplastic cells recognition by T-cells [
25], upregulation of epidermal growth factor (EGFR) with impairment of an efficient immune activation [
27], and production of tumor-derived exosomes inducing CD8+ T-cells apoptosis [
26,
28,
29,
30,
31].
4.1. Radiotherapy Effects on TME
Radiotherapy can profoundly affect TME of laryngeal cancer, exerting both immunosuppressive and immunostimulatory effects [
28,
29,
30,
31,
46]. Radiation exposure has been traditionally associated with immunosuppressive effects, due to the high susceptibility of immune cells to ionizing radiations, despite some differences among lymphocytic subsets in their radiosensitivities. Indeed, circulating CD8+ T-cells seem to be the most radiosensitive, while preexistent intratumoral T-cells are more radioresistant and are meant to perform the antitumoral effect [
28]. Of note, T-regs hold a marked radioresistance, fairly contributing to the suppression of immune response following radiations [
47]. However, radiotherapy enhances immune suppression in TME also by the recruitment of immunoregulatory cells, namely TAM M2, T-regs, and MDSCs, whose immunoregulatory functions undergo further modulation due to radiation effects [
8,
28].
Mechanisms contributing to this complex process are numerous and involve different pathways [
28,
46]. Firstly, radiation induces the immunogenic cell death (ICD) of cancer cells, which is a recently described cell death modality responsible for the activation of host immune response against dying cells antigens [
31,
47,
49]. In detail, radiations induce ICD by killing neoplastic cells which subsequently release the danger-associated molecular patterns (DAMPs), the latter eliciting an antigen-specific immune response. DAMPs include several types of molecules such as calreticulin, which is translocated from the endoplasmic reticulum to the cell surface and represents an ‘eat me’ signal for the DCs. Among others, they also include HMGB1 and ATP, which are released in the extracellular milieu. All these molecules account for danger signals, in turn favoring DCs-associated cross-priming of CD8+ CTLs [
28,
46,
48,
49,
50]. Once activated, CD8+ T-cells target neoplastic cells both inside the irradiated field and at distant sites from the neoplastic burden, configuring a peculiar immune-mediated phenomenon that has been named the abscopal effect [
51,
52,
53]. Other mechanisms contributing to the abscopal effect have been investigated in some in vitro and in vivo experiments on various tumor cell lines. For example, the increase of MHC class I presentation occurs as a direct effect of ionizing radiations in a dose-dependent manner, which could enhance antitumoral CTLs response [
54,
55].
Besides the immune compartment, stromal cells have also shown susceptibility to radiations, being influenced by the overall radiation-induced increase of proinflammatory cytokines such as IL-1 beta, IL-6, IL-8, and, most importantly, TGF-beta [
8,
46]. The mainly affected stromal elements of TME are CAFs. They showed loss of mobility and tumor-invasive capability along with prolonged survival, potentially due to the enhancement of focal contacts mediated by integrins alfa2, alfa5, and beta1, whose expression turned out to be increased by radiation [
8]. CAFs are responsible for several immunosuppressive effects on TME, and have been linked to higher clinical stages and local recurrences in HNSCCs [
8,
32].
4.2. Chemotherapy and Cetuximab Effects on the TME
Along with radiation-induced modifications of TME in HNSCCs recurrences, chemotherapy’s effects on the antitumoral immune response are worth mentioning as well. In advanced HNSCCs, it has been demonstrated that TPF (docetaxel, cisplatin, and fluorouracil) induction chemotherapy increases TILs CD8+ and Foxp3+, which is a marker of T-regs. In particular, CD8+ TILs showed an increased density after chemotherapy, and Foxp3+ TILs underwent an increasing, yet not statistically significant, trend. Noteworthily, there has also been observed a higher CD8+/Foxp3+ TILs ratio after treatment, which is predictive of a good prognosis and better response to immune checkpoint inhibitors [
36]. To this respect, it has been demonstrated that high CD8+ TILs infiltration is significantly associated with better outcome in terms of disease-free survival (DFS) and tumor relapses in advanced laryngeal cancers treated with definitive chemoradiation [
61,
62].
5. Modifications of PDL1 Expression in Recurrences
Programmed death-ligand 1 (PD-L1), also known as B7-H1, is a transmembrane protein belonging to the B7 superfamily, and it is mainly expressed on neoplastic cells and mononuclear immune cells. It binds to the PD-1 receptor expressed on T-cells, activating an inhibitory pathway that leads to their anergy, thus inducing a suppression of the antitumoral immune response [
64]. It has been found in the tumor microenvironment of several solid cancers, including a significant subset of HNSCCs, where it has been detected both on tumor cells and tumor infiltrating mononuclear cells (TIMCs) [
65]. Following the approval of immune checkpoint inhibitors pembrolizumab and nivolumab by FDA and EMA in the treatment of recurrent and metastatic HNSCCs, PD-L1 evaluation gained a pivotal importance in the diagnostic algorithm of HNSCCs, but its assessment still suffers from a lack of solid standardization [
66,
67].
Considering the effects of chemo/radiotherapy on PD-L1 expression in HNSCCs, and particularly laryngeal tumors, is of utmost importance, since most of the patients eligible for immunotherapy have been previously treated with neoadjuvant therapy, therefore possibly coming across these chemo/radio-induced PD-L1 fluctuations. Upregulation of PD-L1 following exposure to different kinds of chemoagents has already been described in other solid tumors such as breast and ovarian cancers [
69,
70,
71].
RT increases IFN type I secretion, which, albeit enhancing the activation of the antitumoral immune response, promotes the expression of PD-L1 on neoplastic cells, thus implying a concomitant suppression of immune activity. An in vivo study on mice has shown that administration of ionizing radiations on tumor microenvironment upregulates PD-L1 expression on tumor cells, DCs, and, less intensely, on macrophages [
73]. Upregulation of PD-L1 has been demonstrated by Ock et al. both in vitro and in vivo on HNSCCs tumor cells exposed to cisplatin. Overall, they observed a treatment-induced variation of PD-L1 expression in 37.1% of cases, with a strong deviation in cisplatin-treated samples. Moreover, the most striking alteration was recorded in pretreatment negative samples that showed a positivization for PD-L1 in 69.2% of cases following cisplatin treatment. Interestingly, they also demonstrated an activation of the MEK pathway in association with PD-L1 upregulation, suggesting that regulation of MEK protein could exert a role in PD-L1 modulation [
41].
6. Conclusions
In summary, recurrent laryngeal SCC presents profound histopathologic differences, both from the primary tumor and, more importantly, according to the type of primary treatment. The modifications induced by RT in the tumor are more striking than those seen in postsurgical recurrences, and concern both the tumor cells and the microenvironment. They are strictly related to the tissue changes induced by RT (necrosis, fibrosis, modifications of the tumor vessels), and besides being a potential source of diagnostic problems, they subtend profound changes of the tumor immune microenvironment. Indeed, RT, CHT, and other systemic treatments can modify the TME of laryngeal SCC, exerting both immunosuppressive and immunostimulatory effects. Overall, the increases in immunoregulatory cells and immune checkpoints such as CTLA-4, TIM-3, PD-1, and PD-L1 induced by RT and CHT strongly support the use of ICI in recurrent/persistent laryngeal SCC.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15123259