You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chitra Vinnakota | -- | 3610 | 2023-07-28 02:48:27 | | | |
| 2 | Lindsay Dong | Meta information modification | 3610 | 2023-07-28 03:03:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Vinnakota, C.; Hudson, M.R.; Jones, N.C.; Sundram, S.; Hill, R.A. Roles of GluN2D NMDA Receptor Subunit in Schizophrenia. Encyclopedia. Available online: https://encyclopedia.pub/entry/47374 (accessed on 23 December 2025).
Vinnakota C, Hudson MR, Jones NC, Sundram S, Hill RA. Roles of GluN2D NMDA Receptor Subunit in Schizophrenia. Encyclopedia. Available at: https://encyclopedia.pub/entry/47374. Accessed December 23, 2025.
Vinnakota, Chitra, Matthew R. Hudson, Nigel C. Jones, Suresh Sundram, Rachel A. Hill. "Roles of GluN2D NMDA Receptor Subunit in Schizophrenia" Encyclopedia, https://encyclopedia.pub/entry/47374 (accessed December 23, 2025).
Vinnakota, C., Hudson, M.R., Jones, N.C., Sundram, S., & Hill, R.A. (2023, July 28). Roles of GluN2D NMDA Receptor Subunit in Schizophrenia. In Encyclopedia. https://encyclopedia.pub/entry/47374
Vinnakota, Chitra, et al. "Roles of GluN2D NMDA Receptor Subunit in Schizophrenia." Encyclopedia. Web. 28 July, 2023.
Copy Citation
Glutamate N-methyl-D-aspartate receptor (NMDAR) hypofunction has been proposed to underlie schizophrenia symptoms. This theory arose from the observation that administration of NMDAR antagonists, which are compounds that inhibit NMDAR activity, reproduces behavioural and molecular schizophrenia-like phenotypes, including hallucinations, delusions and cognitive impairments in healthy humans and animal models. However, the role of specific NMDAR subunits in these schizophrenia-relevant phenotypes is largely unknown. Mounting evidence implicates the GluN2D subunit of NMDAR in some of these symptoms and pathology.
GluN2D
schizophrenia
NMDA receptor
NMDAR antagonists
1. Schizophrenia
Schizophrenia is a severe, debilitating, chronic neuropsychiatric disorder with a lifetime prevalence of 0.72% [1]. It has a complex and heterogeneous presentation making it difficult to diagnose and to identify a consistent underlying aetiology or pathology. People with schizophrenia demonstrate high inter-individual variability with respect to symptoms, disease course, outcome and response to treatment. Schizophrenia symptoms are divided into three main classes: positive, negative and cognitive deficits. Of the three, positive symptoms are the most easily identifiable and are defined as psychotic features that are not usually present in healthy people, and include hallucinations, delusions and disorganised speech and behaviour [2][3]. Negative symptoms refer to a reduction or disruption in normal emotions and behaviours manifesting as social and emotional withdrawal, apathy and avolition [2][4]. Cognitive symptoms vary in severity amongst people with schizophrenia and include elements such as deficits in verbal memory, working memory, attention, executive functioning, cognitive flexibility and processing speed [5].
Schizophrenia typically has an early onset, with most people being diagnosed in their late adolescence—early adulthood years. The onset of the first psychotic episode is usually preceded by a prodromal period during which symptoms gradually emerge and this period can last several years. An early onset combined with long-term deficits in social, educational and occupational function make this disorder one of the leading causes of chronic disability with significant impacts on the quality of life of patients and their families and caregivers [6][7].
The causes of schizophrenia are not fully understood but are thought to be multifactorial, involving a complex interplay between multiple genetic variants and environmental factors. Genome-wide association studies have identified multiple common variants of small effect spanning over 250 genetic loci, suggesting that schizophrenia is a polygenic disorder in most cases [8][9]. Genes associated with schizophrenia risk are involved in various functions, including the regulation of the postsynaptic membrane, synaptic transmission and neurodevelopmental pathways, including glutamate pathways [9][10][11]. Non-genetic factors that increase lifetime risk for schizophrenia include obstetric complications, advanced paternal age, living in an urban setting, childhood trauma or adversity, cannabis use and first-generation migration [12][13][14][15][16][17][18][19]. Although there have been advances in our understanding of risk factors associated with schizophrenia, the aetiological complexity has made it a challenge to identify the underlying disease mechanisms and find effective cures and preventative strategies.
Currently available pharmacological treatments, chiefly conventional and atypical anti-psychotics, and psychotherapy, have proven clinical utility and can help manage positive symptoms in some people [20][21]. However, antipsychotic medications have limited efficacy and are poorly tolerated, with substantial side effects in approximately 30% of people. Moreover, they usually offer little benefit in improving negative and cognitive symptoms, and these symptom types therefore remain a pressing, unmet medical need [20][22]. The prevalence, burden and current lack of effective treatments for schizophrenia highlight a need to improve our understanding of the underlying mechanisms and neurobiology of the disorder in order to identify and develop better treatments.
2. Glutamatergic Signalling in the Central Nervous System
Glutamate plays a key role in mediating the homeostatic balance between excitation and inhibition in the brain, cortico-cortical neurotransmission, neuronal development, neurodegeneration, nervous system plasticity and learning and memory [23]. Glutamate carries out its actions through its receptors which are divided into two groups: the ionotropic and metabotropic receptors [24][25]. The ionotropic receptors, namely the NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors, are integral membrane proteins composed of four large subunits (>900 residues) and act as ligand-gated cation channels [26]. The metabotropic receptors are G-protein-coupled and activate intracellular biochemical cascades [25][27].
NMDA Receptor Structure and Function
NMDARs are widely distributed and can be found on both neuronal and non-neuronal cells. These receptors play a key role in many physiological processes, including neurodevelopment, synaptogenesis and locomotion, and due to their role as critical mediators of activity-dependent synaptic plasticity, they are especially important for learning, memory formation and other forms of cognition [28][29][30][31][32][33][34]. NMDARs are unique in that they require the concomitant binding of glycine and glutamate along with membrane depolarisation for activation and are thus referred to as ‘molecular coincidence detectors’ [35][36][37]. At resting membrane potential, extracellular magnesium (Mg2+) can be found in the ion channel pore, blocking NMDARs in a voltage-dependent manner. Partial depolarisation of the neuron relieves the blockade, opening the channel. The subsequent Ca2+ influx into the neuron triggers a cascade of events that can influence both local, acute functional synaptic plasticity and, via changes in gene expression, sustained neural plasticity [38].
The heteromeric composition of NMDARs enables different pharmacological, biophysical and functional properties for the receptor, and these heteromers vary in distribution and expression, both regionally and temporally, throughout development. The different subunits that make up the NMDAR are termed GluN1, GluN2 and GluN3. The GluN1 subunit is encoded by a single gene but has eight splice variants [39]. There are four different GluN2 subunits (A–D), encoded by four different genes and two different Glun3 subunits (A and B) [25][40]. A functional NMDAR is typically composed of two GluN1 subunits and two subunits from among the GluN2A-D and GluN3A-B subunits [28][41]. The obligatory GluN1 subunit is ubiquitously expressed throughout the brain and over the lifespan [25][42]. Of the GluN2 subunits, which have a more varied and complex temporal and spatial expression, GluN2A and GluN2B are the predominant subtypes found in the adult human brain, whilst the GluN2C and GluN2D subunits are more highly expressed in the developing brain [28][43]. The subunit composition of NMDARs influences its functional properties, including agonist affinity, Mg2+ block, decay kinetics and modulation by polyamines [44][45]. Given the importance of NMDAR subunits in mediating normal brain function, it is not surprising that the dysfunction of these subunits has been linked to various neurological diseases, including schizophrenia. The NMDAR hypofunction model is one of the most commonly adopted and supported models of schizophrenia and is often employed to study the aetiology and pathology of the disorder as well as for the development of novel treatment strategies.
3. NMDA Receptor Hypothesis of Schizophrenia
From the late 1950s, phencyclidine (PCP) and ketamine have been reported to induce positive, negative and cognitive phenotypes such as psychosis-like dissociative states and neurocognitive disturbances in healthy individuals like those observed in people with schizophrenia [46][47][48][49][50][51]. Furthermore, these drugs exacerbate symptoms, including psychosis in individuals with schizophrenia [49][52][53]. The NMDAR hypothesis was first proposed soon after, in the late 1970s–1980s, when it was found that PCP and ketamine carry out their actions via NMDAR blockade [47][54][55][56].
Although NMDAR hypofunction has been linked to schizophrenia symptoms, the precise underlying mechanisms are still unclear. One hypothesis is that it is primarily the dysfunction of NMDARs on GABAergic interneurons, rather than more widespread NMDAR dysfunction, which contributes to the molecular, physiological and behavioural characteristics of schizophrenia [57][58]. GABAergic interneurons are stimulated by postsynaptic NMDAR activation and, in turn, synapse onto excitatory glutamatergic pyramidal cells in a negative feedback loop. GABAergic interneurons connect to hundreds of pyramidal cells in this manner, enabling them to coordinate synchronised network activity throughout the brain, including the hippocampus. The activity of these glutamatergic pyramidal cells, in turn, drives downstream striatal dopaminergic neurons (Figure 1) [59][60].
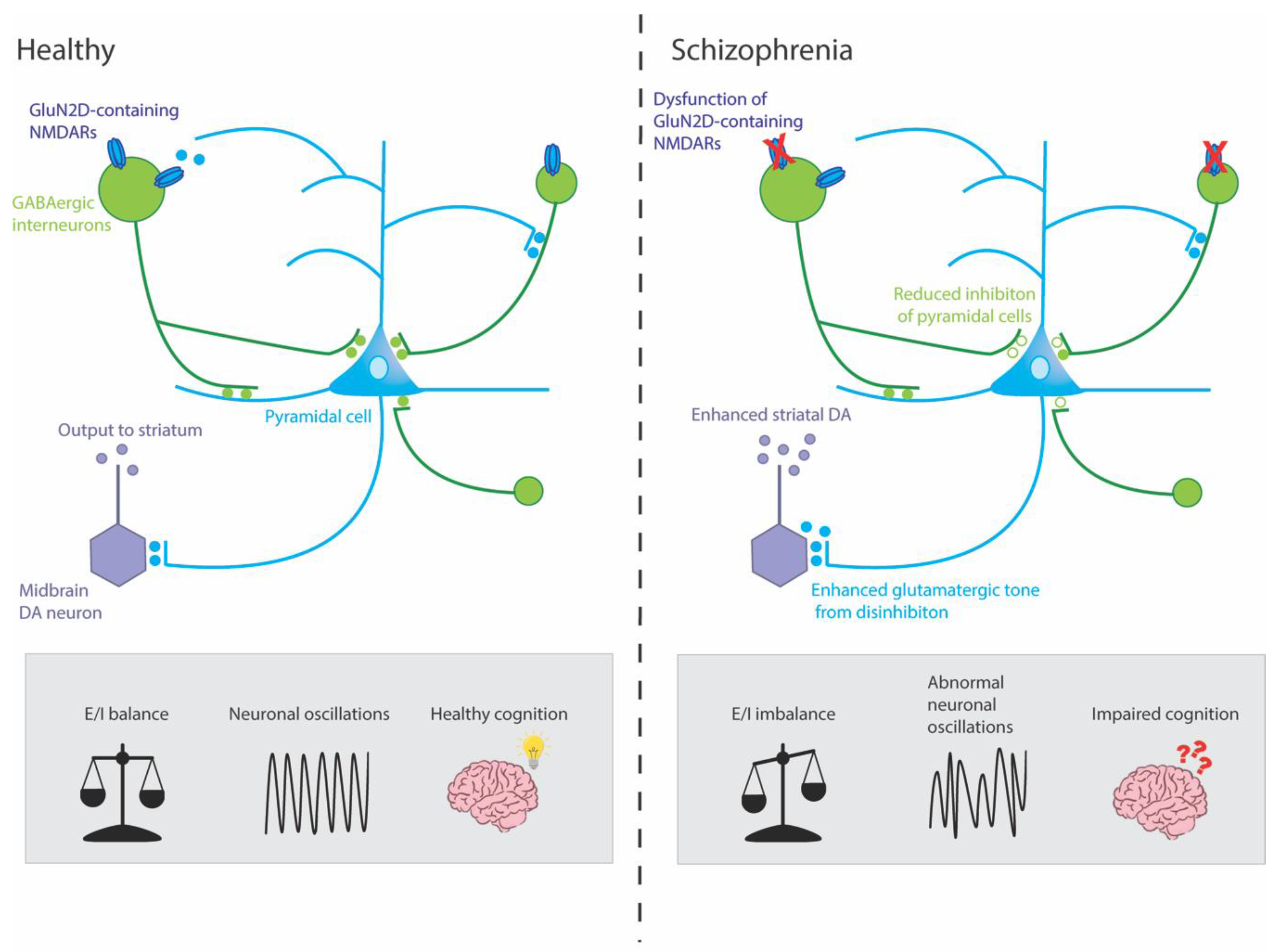
Figure 1. Mechanisms by which dysfunction of GluN2D-containing NMDARs could potentially result in symptoms of schizophrenia. This simplified circuit shows that, in healthy individuals, GluN2D-containing NMDARs stimulate GABAergic interneurons on which they are expressed, which, in turn, mediate inhibition and coordinate the synchronized firing of networks of excitatory pyramidal neurons. These pyramidal neurons, in turn, stimulate dopaminergic (DA) neurons in the midbrain (via the ventral striatum and ventral pallidum, not shown) which project to the associative striatum. In healthy individuals, this circuit is under homeostatic balance, leading to gamma oscillations and healthy cognition. In schizophrenia, hypofunction of the GluN2D-containing NMDARs could lead to GABAergic interneurons increasing excitation of pyramidal neurons by reducing inhibition (disinhibition) onto pyramidal neurons. The resulting aberrant increase in cortical excitation would lead to abnormal neuronal oscillations and impaired cognition. Moreover, this hyperactivity might lead to an overdrive in midbrain DA neurons and enhanced striatal DA, which has been linked to the positive symptoms of schizophrenia.
4. GluN2D Subunit
4.1. GluN2D Receptor Subunit Expression and Distribution
The GluN2D (common names: NMDAR2D, NR2D, GluRε4) subunit is encoded by the GRIN2D gene, which consists of 13 exons, spanning 49.3kB, and is located on chromosome 19q13.1-qter in the human genome [25]. The GRIN2D gene has two potential splice isoforms (NR2D-1 and NR2D-2), the longest of which contains 1356 amino acids [25][61]. In rodents, GluN2D has been extensively characterized; expression levels are first detected between embryonic day (E)15 and 18 during late-embryogenesis, with levels peaking by post-natal day (P)7–10 [62][63]. The GluN2D mRNA and protein expression levels decrease gradually after the early neonatal phase until late adolescence (P40–50), when they reach their relatively low steady-state expression level [44][62][63][64]. During the embryonic and early neonatal phases, the expression of GluN2D is widespread and detected in several regions, including the spinal cord, midbrain nuclei, diencephalon (thalamus, hypothalamus), certain basal ganglia nuclei (substantia nigra and subthalamic nucleus), retina, olfactory bulb, cerebellum, cerebral cortex and hippocampus [43][44][61][62][65]. The ubiquitous distribution of the GluN2D subunit during the early phases of life makes this subunit particularly interesting amongst the NMDAR subunits as it suggests that GluN2D plays a critical role in modulating circuit connectivity and function during neurodevelopment. This could be of significance when considering a disorder such as schizophrenia that is thought to have its origins in early development [66][67].
As the rodent ages and GluN2D expression levels reduce, it becomes more localized to distinct cell subtypes [44][62][63]. This is especially apparent in the hippocampus and cortex, where multiple studies have shown that GluN2D is enriched in parvalbumin (PV)-containing GABAergic interneurons in mature rodents, whereas its expression and activity on glutamatergic pyramidal cells decreases [65][68][69][70][71]. Electrophysiological analyses from the adult mouse medial prefrontal cortex (mPFC) showed that a GluN2C/D positive allosteric modulator, CIQ(+), increased the intrinsic excitability of interneurons and enhanced excitatory postsynaptic currents (EPSCs) from interneurons, whilst not having any effect on the surrounding pyramidal cells [69].
In humans, the spatial and temporal expression of GluN2D is thought to be consistent with that reported in rodents; however, it is yet to be well characterised. In human foetal brains, GluN2D mRNA is abundantly expressed and is one of the predominant NMDAR subunits expressed between gestational weeks 8 and 20 [72]. In contrast, a study of neurologically normal, adult human post-mortem brains reported only moderate expression of GluN2D mRNA in the prefrontal, parietal and motor cortices, where instead the major subtypes expressed were the GluN2A and GluN2B subunits [73]. The study also reported that whilst GluN2D expression was low within most neurons in the hippocampus, expression was moderately intense within a small subset of hippocampal neurons, particularly in the hilus, a region containing many interneurons [73].
Multiple studies examining the subunit composition of NMDARs in the central nervous system suggest that while the majority of GluN2D subunits are associated with the GluN1 subunit, the GluN2D subunit also forms heteromeric assemblies with GluN2A and/or GluN2B subunits in different brain regions and neuronal subpopulations [74][75][76].
4.2. GluN2D Receptor Subunit Function
Glutamate displays 5–6 times greater potency at GluN2D-containing NMDARs than GluN2A- or GluN2B-containing NMDARs [25][77]. Similarly, GluN1 agonists such as glycine are most potent when the GluN2 subunit in the NMDAR is GluN2D [78]. GluN2D-contaning NMDARs also have a weak Mg2+ block that is 10-fold lower than that of GluN2A- or GluN2B-containing receptors and are also reported to have a slightly lower Ca2+ permeability [79][80]. The resistance to Mg2+ block suggests that neurons expressing the GluN2D subunit may be more responsive to synaptic glutamate release. The non-competitive NMDAR antagonist ketamine is more potent at, and shows approximately five-fold selectivity for GluN2D-containing receptors, compared with GluN2A or GluN2B subunits [81]. This, combined with the fact that GluN2D-containing receptors are predominantly expressed on interneurons, may suggest that the GluN2D-containing receptors might be involved in the cortical disinhibition induced by certain NMDAR antagonists such as ketamine [69][82].
GluN2D-containing receptors play a role in both presynaptic and postsynaptic neurotransmission. Studies have reported GluN2D-containing NMDARs on interneurons in the hippocampus and neocortex where they play a key role in postsynaptic signalling [70][83]. GluN2D-containing receptors are thought to enable interneurons to synchronize and coordinate the firing of large groups of cortical pyramidal neurons. A recent study showed that tonic activation of these GluN2D-containing NMDARs on developing cortical interneurons is required for proper intrinsic excitability, dendritic arborization, GABAergic synaptogenesis and inhibitory tone onto excitatory pyramidal cells [83]. GluN2D-containing NMDARs have also been reported on the dendrites of neurons in the subthalamic nucleus, on dopaminergic projection neurons in the substantia nigra pars compacta and in the dorsal horn of the spinal cord, where they contribute to the modulation of the indirect pathway, mediate dopamine release to the striatum and play a role in pain perception, respectively [84][85]. GluN2D-containing NMDARs expressed at presynaptic sites are thought to play a modulatory role in the hippocampus and cerebellum. In the hippocampus, GluN2D, along with GluN2B and postsynaptic metabotropic glutamate receptors, have been shown to be critical for the induction of spike-time-dependent LTD [86].
Interestingly, it has been reported that the human GRIN2D gene contains four estrogen-responsive elements which are highly preserved in the rat, suggesting that the GluN2D subunit might be under neuroendocrine control [87]. In line with this, a study using ovariectomised rats found an upregulation of GluN2D mRNA in the hypothalamus following 17β-estradiol treatment [87]. Given the well-established links between fluctuating levels of 17β-estradiol and schizophrenia onset and symptom severity [88], it is intriguing to consider that one of the actions by which 17β-estradiol may exert its effects is via regulation of GluN2D and other NMDAR subunits [89].
5. Alterations to GluN2D in Schizophrenia
There is evidence to suggest that the GluN2D subunit is altered in schizophrenia. A study of approximately 200 Japanese people with schizophrenia found single nucleotide polymorphisms (SNPs) in the gene for the GluN2D receptor that might contribute to schizophrenia susceptibility [90]. They report that specific combinations of four SNPs within the GRIN2D gene were significantly associated with schizophrenia. These specific combinations were found in three pairs of SNPs: INT10SNP–EX13SNP2, EX13SNP2–EX13SNP3 and EX6SNP–EX13SNP2 [90]. A recent mutation-screening study also identified an ultra-rare, loss-of-function splice-site mutation (c.1412G>A) in the exonic region of the GRIN2D gene, which may lead to the creation of a truncated, nonfunctional GluN2D receptor, thereby contributing to schizophrenia risk [91]. The study additionally found four missense mutations in schizophrenia patients in the GRIN2D gene, and although the actual functional impact of these amino acid substitutions was not examined, in silico analysis classified each of the four variants as disease-causing based on their predicted effect on protein function [91]. An in situ hybridization study on post-mortem human tissue reported a 53% increase in the proportion of GluN2D mRNA expression in the PFC of people with schizophrenia [92]. This increase in the proportion of GluN2D expression was not seen in anti-psychotic-treated control brains, proving that the change was specific to schizophrenia and could not be attributed to treatment with anti-psychotics alone [92].
6. Consequences of Loss of GluN2D Function
6.1. Genetic Models
GluN2D-knockout (KO) mice are viable, reproduce and grow normally, and have no overt changes in neuronal histology [93]. Moreover, mRNA levels of the other NMDAR subunits are unaffected in these mice [93]. These mice, however, exhibit unique behavioural phenotypes, including diminished spontaneous motor movements in open-field tests [93][94]. GluN2D-KO mice also display deficits in spatial learning and memory, as well as impaired contextual fear memory, but show no deficits in the novel object recognition task [82][95][96]. Most studies report no abnormalities in motor function as measured by the rotarod test, nor any differences in anxiety when compared with WT mice during the light–dark compartment test and elevated maze test [93][97]. However, Miyamoto et al. report reduced sensitivity to stress induced by the elevated-plus maze, the light–dark compartment test and forced swim tests in these KO mice [94]. The discrepancies in these findings might be due to the differences in the way the tests were performed, which could be sensitive to subtly different anxiety- or fear-related behaviours.
GluN2D-KO mice were reported to have similar basal neural oscillatory power between frequency ranges 30 and 200 Hz when compared with WT mice [98]. However, whilst the administration of the NMDAR antagonists MK-801, ketamine and memantine increased oscillatory power in WT mice, they had very little effect on GluN2D-KO mice, especially at the high gamma frequency range (65–140 Hz) [98].
6.2. Pharmacological Manipulations
Although currently, to the best of our knowledge, there are no GluN2D-selective drugs available, competitive antagonists with 3–10-fold higher selectivity for GluN2C/GluN2D compared with GluN2A/GluN2B-containing NMDARs have been developed. One such compound, (2R*,3S*)-1-(phenanthrenyl-2-carbonyl)piperazine-2,3-dicarboxylic acid (PPDA), resulted in more potent inhibition of LTD than LTP in rat hippocampal slices, suggesting a role for the GluN2D (and GluN2C) NMDAR subunits in hippocampal LTD [99].
7. How Might Alterations to the GluN2D Subunit Contribute to Schizophrenia?
7.1. GluN2D Subunit and Parvalbumin-Positive GABAergic Interneurons
As previously discussed, inhibitory interneurons have been identified as the key locus or point of convergence of the glutamatergic, GABAergic and dopaminergic hypotheses of schizophrenia and are also implicated in the cognitive deficits seen in schizophrenia. Interestingly, in situ hybridization, electrophysiology and immunohistochemistry studies have revealed that GluN2D-containing NMDARs are specifically enriched in the PV-expressing subclass of interneurons in the hippocampus and PFC, two regions that underlie learning and memory function (refer to Section 4.1) [68][69][70][83]. This makes the GluN2D subunit particularly intriguing in the context of schizophrenia as several studies have shown that the hypofunction of NMDARs at fast-spiking PV-containing interneurons is sufficient to produce schizophrenia-like symptoms, including cognitive dysfunction [59][100][101][102].
Despite there being more than 20 different classes of GABAergic interneurons [103], it is the interneurons containing the calcium-binding protein, PV, that have been proposed to be especially important in schizophrenia [104][105]. Not only are PV-containing interneurons crucial for regulating cortical inhibition via the pyramidal neurons they innervate, but also for the generation of synchronous gamma-frequency oscillations [105][106][107][108][109][110]. Gamma oscillations are synchronous electrophysiological brain rhythms in the gamma frequency range (30–80 Hz) that are crucial for information processing and appropriate cortical function and underpin a wide range of cognitive processes, including those disrupted in schizophrenia like working memory [111][112][113]. Abnormal gamma-frequency synchrony is a major pathological characteristic of schizophrenia and underlies cognitive deficits [113]. For example, a recent study found lower-amplitude gamma oscillations in people with schizophrenia while they were performing a working memory task [114].
Cortical pyramidal neurons innervated by PV cells stimulate dopaminergic neurons in the midbrain which project to the associative striatum. The associative striatum includes the rostral and dorsal part of the caudate nuclei and is implicated in the pathophysiology of schizophrenia [115][116][117][118]. The associative striatum is rich in dopamine receptors and dopamine afferents and receptors and is thus thought to be the primary site of action of antipsychotics. As such, any disruption to the GluN2D-containing NMDARs on PV cells could also indirectly affect midbrain dopaminergic neurons and lead to enhanced dopamine release in the striatum which has been linked to the positive symptoms of schizophrenia [119]. Following treatment with ketamine, Yamamoto et al. found an increase in locomotor activity and nitric oxide (NO) synthesis in the dendrites of medium spiny neurons in the dorsal striatum and PFC in WT but not GluN2D-KO mice [120]. Postsynaptic neuronal NO synthesis is functionally coupled to the stimulation of NMDARs. The failure of ketamine to induce an increase in striatal NO synthesis in GluN2D-KO mice provides support for the role of GluN2D-containing receptors in the corticostriatal neuronal circuit.
7.2. GluN2D Subunit and Dopaminergic Neurons
Multiple studies have found that the GluN2D subunit forms functional NMDAR channels in the substantia nigra pars compacta dopaminergic neurons [76][121][122][123]. The substantia nigra plays an essential role in modulating motor movement and reward functions. Interestingly, GluN2D-KO mice have a hypolocomotor phenotype and the hyperlocomotor effects of PCP and ketamine are reduced in GluN2D-KO mice [82][97][120]. The amount of dopamine release in the forebrain following PCP treatment is also reduced in GluN2D-KO mice [97]. This suggests that GluN2D-containing NMDARs might play either a direct or indirect role in modulating dopaminergic function and, consequently, locomotor activity. In people with schizophrenia, there are reports of long-term deficits in basic motor function and control [124][125][126]. Thus, it is possible that dysfunction of the GluN2D subunit could affect burst-firing in these dopaminergic neurons, disrupting their function in the nigrostriatal circuitry, which is hypothesised to underlie motor symptoms in schizophrenia [127].
8. Conclusions
Precipitating factors, including any combination of genetic predisposition and environmental factors like maternal infection or obstetric complications, can lead to NMDAR hypofunction disproportionately at fast-spiking PV-containing interneurons during development. This results in pathological phenotypes including impaired oscillatory activity and neuronal synchrony, cortical disinhibition and dopaminergic dysfunction, ultimately giving rise to the various symptoms of schizophrenia. Disruption of the GluN2D subunit and alteration to GluN2D neurotransmission could be a molecular pathway contributing to the symptomatology of schizophrenia. This is of importance as it may provide new insights into the aetiology of this disorder and might even lead to the development of novel drugs for the treatment of specific schizophrenia symptoms, including cognitive dysfunction.
References
- Saha, S.; Chant, D.; Welham, J.; McGrath, J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005, 2, e141.
- McCutcheon, R.A.; Reis Marques, T.; Howes, O.D. Schizophrenia-An Overview. JAMA Psychiatry 2020, 77, 201–210.
- Kahn, R.; Sommer, I.; Murray, R.; Meyer-Lindenberg, A.; Weinberger, D.; Cannon, T.; O’Donovan, M.; Correl, C.; Kane, J.; van Os, J. Schizophrenia. Nature Reviews Disease Primers. Nov 2015, 12, 15067.
- Galderisi, S.; Mucci, A.; Buchanan, R.W.; Arango, C. Negative symptoms of schizophrenia: New developments and unanswered research questions. Lancet Psychiatry 2018, 5, 664–677.
- McCutcheon, R.A.; Keefe, R.S.; McGuire, P.K. Cognitive impairment in schizophrenia: Aetiology, pathophysiology, and treatment. Mol. Psychiatry, 2023; online ahead of print.
- Vigo, D.; Thornicroft, G.; Atun, R. Estimating the true global burden of mental illness. Lancet Psychiatry 2016, 3, 171–178.
- Velligan, D.I.; Brain, C.; Bouérat Duvold, L.; Agid, O. Caregiver burdens associated with treatment-resistant schizophrenia: A quantitative caregiver survey of experiences, attitudes, and perceptions. Front. Psychiatry 2019, 10, 584.
- Ripke, S.; Neale, B.M.; Corvin, A.; Walters, J.T.; Farh, K.-H.; Holmans, P.A.; Lee, P.; Bulik-Sullivan, B.; Collier, D.A.; Huang, H. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427.
- Trubetskoy, V.; Pardiñas, A.F.; Qi, T.; Panagiotaropoulou, G.; Awasthi, S.; Bigdeli, T.B.; Bryois, J.; Chen, C.-Y.; Dennison, C.A.; Hall, L.S. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 2022, 604, 502–508.
- Walsh, T.; McClellan, J.M.; McCarthy, S.E.; Addington, A.M.; Pierce, S.B.; Cooper, G.M.; Nord, A.S.; Kusenda, M.; Malhotra, D.; Bhandari, A.; et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science 2008, 320, 539–543.
- Fromer, M.; Roussos, P.; Sieberts, S.K.; Johnson, J.S.; Kavanagh, D.H.; Perumal, T.M.; Ruderfer, D.M.; Oh, E.C.; Topol, A.; Shah, H.R.; et al. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat. Neurosci. 2016, 19, 1442–1453.
- Ursini, G.; Punzi, G.; Chen, Q.; Marenco, S.; Robinson, J.F.; Porcelli, A.; Hamilton, E.G.; Mitjans, M.; Maddalena, G.; Begemann, M.; et al. Convergence of placenta biology and genetic risk for schizophrenia. Nat. Med. 2018, 24, 792–801.
- Malaspina, D.; Harlap, S.; Fennig, S.; Heiman, D.; Nahon, D.; Feldman, D.; Susser, E.S. Advancing paternal age and the risk of schizophrenia. Arch. Gen. Psychiatry 2001, 58, 361–367.
- Fountoulakis, K.N.; Gonda, X.; Siamouli, M.; Panagiotidis, P.; Moutou, K.; Nimatoudis, I.; Kasper, S. Paternal and maternal age as risk factors for schizophrenia: A case–control study. Int. J. Psychiatry Clin. Pract. 2018, 22, 170–176.
- Cantor-Graae, E.; Selten, J.-P. Schizophrenia and migration: A meta-analysis and review. Am. J. Psychiatry 2005, 162, 12–24.
- Varese, F.; Smeets, F.; Drukker, M.; Lieverse, R.; Lataster, T.; Viechtbauer, W.; Read, J.; Van Os, J.; Bentall, R.P. Childhood adversities increase the risk of psychosis: A meta-analysis of patient-control, prospective-and cross-sectional cohort studies. Schizophr. Bull. 2012, 38, 661–671.
- Toulopoulou, T.; Picchioni, M.; Mortensen, P.B.; Petersen, L. IQ, the urban environment, and their impact on future schizophrenia risk in men. Schizophr. Bull. 2017, 43, 1056–1063.
- McKenzie, K.; Murray, A.; Booth, T. Do urban environments increase the risk of anxiety, depression and psychosis? An epidemiological study. J. Affect. Disord. 2013, 150, 1019–1024.
- Vaucher, J.; Keating, B.J.; Lasserre, A.M.; Gan, W.; Lyall, D.M.; Ward, J.; Smith, D.J.; Pell, J.P.; Sattar, N.; Paré, G. Cannabis use and risk of schizophrenia: A Mendelian randomization study. Mol. Psychiatry 2018, 23, 1287–1292.
- Leucht, S.; Corves, C.; Arbter, D.; Engel, R.R.; Li, C.; Davis, J.M. Second-generation versus first-generation antipsychotic drugs for schizophrenia: A meta-analysis. Lancet 2009, 373, 31–41.
- Kane, J.M.; Correll, C.U. Pharmacologic treatment of schizophrenia. Dialogues Clin. Neurosci. 2022, 12, 345–357.
- Keefe, R.S.; Bilder, R.M.; Davis, S.M.; Harvey, P.D.; Palmer, B.W.; Gold, J.M.; Meltzer, H.Y.; Green, M.F.; Capuano, G.; Stroup, T.S. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch. Gen. Psychiatry 2007, 64, 633–647.
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817.
- Willard, S.S.; Koochekpour, S. Glutamate, glutamate receptors, and downstream signaling pathways. Int. J. Biol. Sci. 2013, 9, 948.
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharm. Rev. 2010, 62, 405–496.
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T. Structure, function, and pharmacology of glutamate receptor ion channels. Pharmacol. Rev. 2021, 73, 1469–1658.
- Reiner, A.; Levitz, J. Glutamatergic signaling in the central nervous system: Ionotropic and metabotropic receptors in concert. Neuron 2018, 98, 1080–1098.
- Monyer, H.; Sprengel, R.; Schoepfer, R.; Herb, A.; Higuchi, M.; Lomeli, H.; Burnashev, N.; Sakmann, B.; Seeburg, P.H. Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science 1992, 256, 1217–1221.
- Bye, C.M.; McDonald, R.J. A specific role of hippocampal NMDA receptors and arc protein in rapid encoding of novel environmental representations and a more general long-term consolidation function. Front. Behav. Neurosci. 2019, 13, 8.
- Talpalar, A.E.; Kiehn, O. Glutamatergic mechanisms for speed control and network operation in the rodent locomotor CpG. Front. Neural Circuits 2010, 4, 19.
- Lambot, L.; Rodriguez, E.C.; Houtteman, D.; Li, Y.; Schiffmann, S.N.; Gall, D.; de Kerchove d’Exaerde, A. Striatopallidal neuron NMDA receptors control synaptic connectivity, locomotor, and goal-directed behaviors. J. Neurosci. 2016, 36, 4976–4992.
- Li, N.; Lee, B.; Liu, R.-J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.-Y.; Aghajanian, G.; Duman, R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010, 329, 959–964.
- Hou, G.; Zhang, Z.-W. NMDA receptors regulate the development of neuronal intrinsic excitability through cell-autonomous mechanisms. Front. Cell. Neurosci. 2017, 11, 353.
- Frangeul, L.; Kehayas, V.; Sanchez-Mut, J.V.; Fièvre, S.; Krishna, K.K.; Pouchelon, G.; Telley, L.; Bellone, C.; Holtmaat, A.; Gräff, J. Input-dependent regulation of excitability controls dendritic maturation in somatosensory thalamocortical neurons. Nat. Commun. 2017, 8, 2015.
- Karakas, E.; Furukawa, H. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science 2014, 344, 992–997.
- Furukawa, H.; Singh, S.K.; Mancusso, R.; Gouaux, E. Subunit arrangement and function in NMDA receptors. Nature 2005, 438, 185–192.
- Lerma, J.; Zukin, R.S.; Bennett, M. Glycine decreases desensitization of N-methyl-D-aspartate (NMDA) receptors expressed in Xenopus oocytes and is required for NMDA responses. Proc. Natl. Acad. Sci. USA 1990, 87, 2354–2358.
- Greer, P.L.; Greenberg, M.E. From synapse to nucleus: Calcium-dependent gene transcription in the control of synapse development and function. Neuron 2008, 59, 846–860.
- Sugihara, H.; Moriyoshi, K.; Ishii, T.; Masu, M.; Nakanishi, S. Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem. Biophys. Res. Commun. 1992, 185, 826–832.
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400.
- Schorge, S.; Colquhoun, D. Studies of NMDA receptor function and stoichiometry with truncated and tandem subunits. J. Neurosci. 2003, 23, 1151–1158.
- Henson, M.A.; Roberts, A.C.; Salimi, K.; Vadlamudi, S.; Hamer, R.M.; Gilmore, J.H.; Jarskog, L.F.; Philpot, B.D. Developmental regulation of the NMDA receptor subunits, NR3A and NR1, in human prefrontal cortex. Cereb. Cortex 2008, 18, 2560–2573.
- Tolle, T.; Berthele, A.; Zieglgansberger, W.; Seeburg, P.H.; Wisden, W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. J. Neurosci. 1993, 13, 5009–5028.
- Monyer, H.; Burnashev, N.; Laurie, D.J.; Sakmann, B.; Seeburg, P.H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 1994, 12, 529–540.
- Vicini, S.; Wang, J.F.; Li, J.H.; Zhu, W.J.; Wang, Y.H.; Luo, J.H.; Wolfe, B.B.; Grayson, D.R. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J. Neurophysiol. 1998, 79, 555–566.
- Krystal, J.H.; Karper, L.P.; Seibyl, J.P.; Freeman, G.K.; Delaney, R.; Bremner, J.D.; Heninger, G.R.; Bowers, M.B., Jr.; Charney, D.S. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 1994, 51, 199–214.
- Javitt, D. Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia. Hillside J. Clin. Psychiatry 1987, 9, 12–35.
- Luby, E.D.; Cohen, B.D.; Rosenbaum, G.; Gottlieb, J.S.; Kelley, R. Study of a new schizophrenomimetic drug—Sernyl. AMA Arch. Neurol. Psychiatry 1959, 81, 363–369.
- Luby, E.D.; Gottlieb, J.S.; Cohen, B.D.; Rosenbaum, G.; Domino, E.F. Model psychoses and schizophrenia. Am. J. Psychiatry 1962, 119, 61–67.
- Rosenbaum, G.; Cohen, B.D.; Luby, E.D.; Gottlieb, J.S.; Yelen, D. Comparison of sernyl with other drugs: Simulation of schizophrenic performance with sernyl, LSD-25, and amobarbital (amytal) sodium; I. Attention, motor function, and proprioception. AMA Arch. Gen. Psychiatry 1959, 1, 651–656.
- Cohen, B.D.; Luby, E.D.; Rosenbaum, G.; Gottlieb, J.S. Combined sernyl and sensory deprivation. Compr. Psychiatry 1960, 1, 345–348.
- Itil, T.; Keskiner, A.; Kiremitci, N.; Holden, J. Effect of phencyclidine in chronic schizophrenics. Can. Psychiatr. Assoc. J. 1967, 12, 209–212.
- Lahti, A.C.; Koffel, B.; LaPorte, D.; Tamminga, C.A. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 1995, 13, 9–19.
- Anis, N.A.; Berry, S.C.; Burton, N.R.; Lodge, D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br. J. Pharm. 1983, 79, 565–575.
- Kim, J.; Kornhuber, H.; Schmid-Burgk, W.; Holzmüller, B. Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci. Lett. 1980, 20, 379–382.
- Javitt, D.C.; Zukin, S.R. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry 1991, 148, 1301–1308.
- Nakazawa, K.; Sapkota, K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol. Ther. 2020, 205, 107426.
- Cohen, S.M.; Tsien, R.W.; Goff, D.C.; Halassa, M.M. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr. Res. 2015, 167, 98–107.
- Nakao, K.; Jeevakumar, V.; Jiang, S.Z.; Fujita, Y.; Diaz, N.B.; Pretell Annan, C.A.; Eskow Jaunarajs, K.L.; Hashimoto, K.; Belforte, J.E.; Nakazawa, K. Schizophrenia-like dopamine release abnormalities in a mouse model of NMDA receptor hypofunction. Schizophr. Bull. 2019, 45, 138–147.
- Akgül, G.; McBain, C.J. Diverse roles for ionotropic glutamate receptors on inhibitory interneurons in developing and adult brain. J. Physiol. 2016, 594, 5471–5490.
- Ishii, T.; Moriyoshi, K.; Sugihara, H.; Sakurada, K.; Kadotani, H.; Yokoi, M.; Akazawa, C.; Shigemoto, R.; Mizuno, N.; Masu, M. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J. Biol. Chem. 1993, 268, 2836–2843.
- Akazawa, C.; Shigemoto, R.; Bessho, Y.; Nakanishi, S.; Mizuno, N. Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J. Comp. Neurol. 1994, 347, 150–160.
- Wenzel, A.; Villa, M.; Mohler, H.; Benke, D. Developmental and regional expression of NMDA receptor subtypes containing the NR2D subunit in rat brain. J. Neurochem. 1996, 66, 1240–1248.
- Dunah, A.W.; Yasuda, R.P.; Wang, Y.h.; Luo, J.; Dávila-García, M.I.; Gbadegesin, M.; Vicini, S.; Wolfe, B.B. Regional and ontogenic expression of the NMDA receptor subunit NR2D protein in rat brain using a subunit-specific antibody. J. Neurochem. 1996, 67, 2335–2345.
- Standaert, D.G.; Landwehrmeyer, G.B.; Kerner, J.A.; Penney Jr, J.B.; Young, A.B. Expression of NMDAR2D glutamate receptor subunit mRNA in neurochemically identified interneurons in the rat neostriatum, neocortex and hippocampus. Mol. Brain Res. 1996, 42, 89–102.
- Cannon, M.; Murray, R.M. Neonatal origins of schizophrenia. Arch. Dis. Child. 1998, 78, 1–3.
- Birnbaum, R.; Weinberger, D.R. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat. Rev. Neurosci. 2017, 18, 727–740.
- Engelhardt, J.v.; Bocklisch, C.; Tönges, L.; Herb, A.; Mishina, M.; Monyer, H. GluN2D-containing NMDA receptors-mediate synaptic currents in hippocampal interneurons and pyramidal cells in juvenile mice. Front. Cell Neurosci. 2015, 9, 95.
- Garst-Orozco, J.; Malik, R.; Lanz, T.A.; Weber, M.L.; Xi, H.; Arion, D.; Enwright III, J.F.; Lewis, D.A.; O’Donnell, P.; Sohal, V.S. GluN2D-mediated excitatory drive onto medial prefrontal cortical PV+ fast-spiking inhibitory interneurons. PLoS ONE 2020, 15, e0233895.
- Perszyk, R.E.; DiRaddo, J.O.; Strong, K.L.; Low, C.M.; Ogden, K.K.; Khatri, A.; Vargish, G.A.; Pelkey, K.A.; Tricoire, L.; Liotta, D.C.; et al. GluN2D-Containing N-methyl-d-Aspartate Receptors Mediate Synaptic Transmission in Hippocampal Interneurons and Regulate Interneuron Activity. Mol. Pharm. 2016, 90, 689–702.
- Alsaad, H.A.; DeKorver, N.W.; Mao, Z.; Dravid, S.M.; Arikkath, J.; Monaghan, D.T. In the telencephalon, GluN2C NMDA receptor subunit mRNA is predominately expressed in glial cells and GluN2D mRNA in interneurons. Neurochem. Res. 2019, 44, 61–77.
- Ritter, L.M.; Unis, A.S.; Meador-Woodruff, J.H. Ontogeny of ionotropic glutamate receptor expression in human fetal brain. Dev. Brain Res. 2001, 127, 123–133.
- Scherzer, C.R.; Landwehrmeyer, G.B.; Kerner, J.A.; Counihan, T.J.; Kosinski, C.M.; Standaert, D.G.; Daggett, L.P.; Velicelebi, G.; Penney, J.B.; Young, A.B. Expression of N-methyl-D-aspartate receptor subunit mRNAs in the human brain: Hippocampus and cortex. J. Comp. Neurol. 1998, 390, 75–90.
- Dunah, A.W.; Luo, J.; Wang, Y.-H.; Yasuda, R.P.; Wolfe, B.B. Subunit Composition ofN-Methyl-D-aspartate Receptors in the Central Nervous System that Contain the NR2D Subunit. Mol. Pharmacol. 1998, 53, 429–437.
- Brickley, S.G.; Misra, C.; Mok, M.S.; Mishina, M.; Cull-Candy, S.G. NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J. Neurosci. 2003, 23, 4958–4966.
- Jones, S.; Gibb, A.J. Functional NR2B-and NR2D-containing NMDA receptor channels in rat substantia nigra dopaminergic neurones. J. Physiol. 2005, 569, 209–221.
- Erreger, K.; Geballe, M.T.; Kristensen, A.; Chen, P.E.; Hansen, K.B.; Lee, C.J.; Yuan, H.; Le, P.; Lyuboslavsky, P.N.; Micale, N. Subunit-specific agonist activity at NR2A-, NR2B-, NR2C-, and NR2D-containing N-methyl-D-aspartate glutamate receptors. Mol. Pharmacol. 2007, 72, 907–920.
- Chen, P.E.; Geballe, M.T.; Katz, E.; Erreger, K.; Livesey, M.R.; O’toole, K.K.; Le, P.; Lee, C.J.; Snyder, J.P.; Traynelis, S.F. Modulation of glycine potency in rat recombinant NMDA receptors containing chimeric NR2A/2D subunits expressed in Xenopus laevis oocytes. J. Physiol. 2008, 586, 227–245.
- Kuner, T.; Schoepfer, R. Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. J. Neurosci. 1996, 16, 3549–3558.
- Clarke, R.J.; Johnson, J.W. NMDA receptor NR2 subunit dependence of the slow component of magnesium unblock. J Neurosci 2006, 26, 5825–5834.
- Kotermanski, S.E.; Johnson, J.W. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J. Neurosci. 2009, 29, 2774–2779.
- Sapkota, K.; Mao, Z.; Synowicki, P.; Lieber, D.; Liu, M.; Ikezu, T.; Gautam, V.; Monaghan, D.T. GluN2D N-Methyl-d-Aspartate Receptor Subunit Contribution to the Stimulation of Brain Activity and Gamma Oscillations by Ketamine: Implications for Schizophrenia. J. Pharm. Exp. 2016, 356, 702–711.
- Hanson, E.; Armbruster, M.; Lau, L.A.; Sommer, M.E.; Klaft, Z.-J.; Swanger, S.A.; Traynelis, S.F.; Moss, S.J.; Noubary, F.; Chadchankar, J. Tonic activation of GluN2C/GluN2D-containing NMDA receptors by ambient glutamate facilitates cortical interneuron maturation. J. Neurosci. 2019, 39, 3611–3626.
- Swanger, S.A.; Vance, K.M.; Pare, J.-F.; Sotty, F.; Fog, K.; Smith, Y.; Traynelis, S.F. NMDA receptors containing the GluN2D subunit control neuronal function in the subthalamic nucleus. J. Neurosci. 2015, 35, 15971–15983.
- Pearlstein, E.; Gouty-Colomer, L.-A.; Michel, F.J.; Cloarec, R.; Hammond, C. Glutamatergic synaptic currents of nigral dopaminergic neurons follow a postnatal developmental sequence. Front. Cell Neurosci. 2015, 9, 210.
- Andrade-Talavera, Y.; Duque-Feria, P.; Paulsen, O.; Rodríguez-Moreno, A. Presynaptic spike timing-dependent long-term depression in the mouse hippocampus. Cereb. Cortex. 2016, 26, 3637–3654.
- Watanabe, T.; Inoue, S.; Hiroi, H.; Orimo, A.; Muramatsu, M. NMDA receptor type 2D gene as target for estrogen receptor in the brain. Mol. Brain Res. 1999, 63, 375–379.
- Riecher-Rössler, A.; Häfner, H.; Stumbaum, M.; Maurer, K.; Schmidt, R. Can estradiol modulate schizophrenic symptomatology? Schizophr. Bull. 1994, 20, 203–214.
- McCarthny, C.R.; Du, X.; Wu, Y.C.; Hill, R.A. Investigating the interactive effects of sex steroid hormones and brain-derived neurotrophic factor during adolescence on hippocampal NMDA receptor expression. Int. J. Endocrinol. 2018, 2018, 7231915.
- Makino, C.; Shibata, H.; Ninomiya, H.; Tashiro, N.; Fukumaki, Y. Identification of single-nucleotide polymorphisms in the human N-methyl-D-aspartate receptor subunit NR2D gene, GRIN2D, and association study with schizophrenia. Psychiatr. Genet. 2005, 15, 215–221.
- Yu, Y.; Lin, Y.; Takasaki, Y.; Wang, C.; Kimura, H.; Xing, J.; Ishizuka, K.; Toyama, M.; Kushima, I.; Mori, D. Rare loss of function mutations in N-methyl-D-aspartate glutamate receptors and their contributions to schizophrenia susceptibility. Transl. Psychiatry 2018, 8, 1–9.
- Akbarian, S.; Sucher, N.J.; Bradley, D.; Tafazzoli, A.; Trinh, D.; Hetrick, W.P.; Potkin, S.G.; Sandman, C.A.; Bunney, W.E., Jr.; Jones, E.G. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J. Neurosci. 1996, 16, 19–30.
- Ikeda, K.; Araki, K.; Takayama, C.; Inoue, Y.; Yagi, T.; Aizawa, S.; Mishina, M. Reduced spontaneous activity of mice defective in the ε4 subunit of the NMDA receptor channel. Mol. Brain Res. 1995, 33, 61–71.
- Miyamoto, Y.; Yamada, K.; Noda, Y.; Mori, H.; Mishina, M.; Nabeshima, T. Lower sensitivity to stress and altered monoaminergic neuronal function in mice lacking the NMDA receptor ε4 subunit. J. Neurosci. 2002, 22, 2335–2342.
- Obiang, P.; Macrez, R.; Jullienne, A.; Bertrand, T.; Lesept, F.; Ali, C.; Maubert, E.; Vivien, D.; Agin, V. GluN2D subunit-containing NMDA receptors control tissue plasminogen activator-mediated spatial memory. J. Neurosci. 2012, 32, 12726–12734.
- Ide, S.; Ikekubo, Y.; Mishina, M.; Hashimoto, K.; Ikeda, K. Cognitive impairment that is induced by (R)-ketamine is abolished in NMDA GluN2D receptor subunit knockout mice. Int. J. Neuropsychopharmacol. 2019, 22, 449–452.
- Hagino, Y.; Kasai, S.; Han, W.; Yamamoto, H.; Nabeshima, T.; Mishina, M.; Ikeda, K. Essential role of NMDA receptor channel ε4 subunit (GluN2D) in the effects of phencyclidine, but not methamphetamine. PLoS ONE 2010, 5, e13722.
- Mao, Z.; He, S.; Mesnard, C.; Synowicki, P.; Zhang, Y.; Chung, L.; Wiesman, A.I.; Wilson, T.W.; Monaghan, D.T. NMDA receptors containing GluN2C and GluN2D subunits have opposing roles in modulating neuronal oscillations; potential mechanism for bidirectional feedback. Brain Res. 2020, 1727, 146571.
- Hrabetova, S.; Serrano, P.; Blace, N.; Heong, W.T.; Skifter, D.A.; Jane, D.E.; Monaghan, D.T.; Sacktor, T.C. Distinct NMDA receptor subpopulations contribute to long-term potentiation and long-term depression induction. J. Neurosci. 2000, 20, RC81.
- Bygrave, A.M.; Kilonzo, K.; Kullmann, D.M.; Bannerman, D.M.; Kätzel, D. Can N-methyl-D-aspartate receptor hypofunction in schizophrenia be localized to an individual cell type? Front. Psychiatry 2019, 10, 835.
- Bygrave, A.M.; Masiulis, S.; Nicholson, E.; Berkemann, M.; Barkus, C.; Sprengel, R.; Harrison, P.J.; Kullmann, D.M.; Bannerman, D.M.; Kätzel, D. Knockout of NMDA-receptors from parvalbumin interneurons sensitizes to schizophrenia-related deficits induced by MK-801. Transl. Psychiatry 2016, 6, e778.
- Korotkova, T.; Fuchs, E.C.; Ponomarenko, A.; von Engelhardt, J.; Monyer, H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 2010, 68, 557–569.
- DeFelipe, J.; López-Cruz, P.L.; Benavides-Piccione, R.; Bielza, C.; Larrañaga, P.; Anderson, S.; Burkhalter, A.; Cauli, B.; Fairén, A.; Feldmeyer, D. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat. Rev. Neurosci. 2013, 14, 202–216.
- Kaar, S.J.; Angelescu, I.; Marques, T.R.; Howes, O.D. Pre-frontal parvalbumin interneurons in schizophrenia: A meta-analysis of post-mortem studies. J. Neural Transm. 2019, 126, 1637–1651.
- Chung, D.W.; Fish, K.N.; Lewis, D.A. Pathological basis for deficient excitatory drive to cortical parvalbumin interneurons in schizophrenia. Am. J. Psychiatry 2016, 173, 1131–1139.
- Nakazawa, K.; Zsiros, V.; Jiang, Z.; Nakao, K.; Kolata, S.; Zhang, S.; Belforte, J.E. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 2012, 62, 1574–1583.
- Uhlhaas, P.J.; Singer, W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010, 11, 100–113.
- Gonzalez-Burgos, G.; Cho, R.Y.; Lewis, D.A. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol. Psychiatry 2015, 77, 1031–1040.
- Antonoudiou, P.; Tan, Y.L.; Kontou, G.; Upton, A.L.; Mann, E.O. Parvalbumin and somatostatin interneurons contribute to the generation of hippocampal gamma oscillations. J. Neurosci. 2020, 40, 7668–7687.
- Kriener, B.; Hu, H.; Vervaeke, K. Parvalbumin interneuron dendrites enhance gamma oscillations. Cell Rep. 2022, 39, 110948.
- Buzsaki, G.; Wang, X.J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225.
- Alekseichuk, I.; Turi, Z.; de Lara, G.A.; Antal, A.; Paulus, W. Spatial working memory in humans depends on theta and high gamma synchronization in the prefrontal cortex. Curr. Biol. 2016, 26, 1513–1521.
- Uhlhaas, P.J.; Singer, W. Oscillations and neuronal dynamics in schizophrenia: The search for basic symptoms and translational opportunities. Biol. Psychiatry 2015, 77, 1001–1009.
- Chen, C.-M.A.; Stanford, A.D.; Mao, X.; Abi-Dargham, A.; Shungu, D.C.; Lisanby, S.H.; Schroeder, C.E.; Kegeles, L.S. GABA level, gamma oscillation, and working memory performance in schizophrenia. NeuroImage Clin. 2014, 4, 531–539.
- de la Fuente-Sandoval, C.; León-Ortiz, P.; Azcárraga, M.; Stephano, S.; Favila, R.; Díaz-Galvis, L.; Alvarado-Alanis, P.; Ramírez-Bermúdez, J.; Graff-Guerrero, A. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: A longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry 2013, 70, 1057–1066.
- Levitt, J.J.; Nestor, P.G.; Levin, L.; Pelavin, P.; Lin, P.; Kubicki, M.; McCarley, R.W.; Shenton, M.E.; Rathi, Y. Reduced structural connectivity in frontostriatal white matter tracts in the associative loop in schizophrenia. Am. J. Psychiatry 2017, 174, 1102–1111.
- Egerton, A.; Chaddock, C.A.; Winton-Brown, T.T.; Bloomfield, M.A.; Bhattacharyya, S.; Allen, P.; McGuire, P.K.; Howes, O.D. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: Findings in a second cohort. Biol. Psychiatry 2013, 74, 106–112.
- Kegeles, L.S.; Abi-Dargham, A.; Frankle, W.G.; Gil, R.; Cooper, T.B.; Slifstein, M.; Hwang, D.-R.; Huang, Y.; Haber, S.N.; Laruelle, M. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch. Gen. Psychiatry 2010, 67, 231–239.
- McCutcheon, R.A.; Jauhar, S.; Pepper, F.; Nour, M.M.; Rogdaki, M.; Veronese, M.; Turkheimer, F.E.; Egerton, A.; McGuire, P.; Mehta, M.M. The topography of striatal dopamine and symptoms in psychosis: An integrative positron emission tomography and magnetic resonance imaging study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 1040–1051.
- Yamamoto, T.; Nakayama, T.; Yamaguchi, J.; Matsuzawa, M.; Mishina, M.; Ikeda, K.; Yamamoto, H. Role of the NMDA receptor GluN2D subunit in the expression of ketamine-induced behavioral sensitization and region-specific activation of neuronal nitric oxide synthase. Neurosci. Lett. 2016, 610, 48–53.
- Morris, P.G.; Mishina, M.; Jones, S. Altered synaptic and extrasynaptic NMDA receptor properties in substantia nigra dopaminergic neurons from mice lacking the GluN2D subunit. Front. Cell Neurosci. 2018, 12, 354.
- Huang, Z.; Gibb, A.J. Mg2+ block properties of triheteromeric GluN1–GluN2B–GluN2D NMDA receptors on neonatal rat substantia nigra pars compacta dopaminergic neurones. J. Physiol. 2014, 592, 2059–2078.
- Sitzia, G.; Mantas, I.; Zhang, X.; Svenningsson, P.; Chergui, K. NMDA receptors are altered in the substantia nigra pars reticulata and their blockade ameliorates motor deficits in experimental parkinsonism. Neuropharmacology 2020, 174, 108136.
- Byrial, P.; Nyboe, L.; Thomsen, P.H.; Clausen, L. Motor impairments in early onset schizophrenia. Early Interv. Psychiatry 2022, 16, 481–491.
- Nadesalingam, N.; Chapellier, V.; Lefebvre, S.; Pavlidou, A.; Stegmayer, K.; Alexaki, D.; Gama, D.B.; Maderthaner, L.; von Känel, S.; Wüthrich, F. Motor abnormalities are associated with poor social and functional outcomes in schizophrenia. Compr. Psychiatry 2022, 115, 152307.
- Peralta, V.; Campos, M.S.; De Jalón, E.G.; Cuesta, M.J. Motor behavior abnormalities in drug-naïve patients with schizophrenia spectrum disorders. Mov. Disord. 2010, 25, 1068–1076.
- van Hooijdonk, C.F.; van der Pluijm, M.; Bosch, I.; van Amelsvoort, T.A.; Booij, J.; de Haan, L.; Selten, J.-P.; van de Giessen, E. The substantia nigra in the pathology of schizophrenia: A review on post-mortem and molecular imaging findings. Eur. Neuropsychopharmacol. 2023, 68, 57–77.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
584
Revisions:
2 times
(View History)
Update Date:
28 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




