
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Federico Nencioni | -- | 1847 | 2023-07-27 17:13:14 | | | |
| 2 | Wendy Huang | Meta information modification | 1847 | 2023-07-28 09:18:49 | | |
Video Upload Options
Metal ions are fundamental to guarantee the regular physiological activity of the human organism. They are involved in several biological processes such as electron transfer, oxygen transport, the maintenance of osmotic pressure, and the regulation of DNA transcription. Metals such as iron, cobalt, selenium, copper, zinc, and manganese are essential for human life and are usually required in trace amounts. On the other hand, aluminum, mercury, arsenic, and others are considered non-essential metals since they possess no biological function. The importance of metals in the human organism is so fundamental that several pathologies, among which are neurodegenerative diseases (NDs), are related to a common phenomenon known as metal dyshomeostasis.
1. Introduction
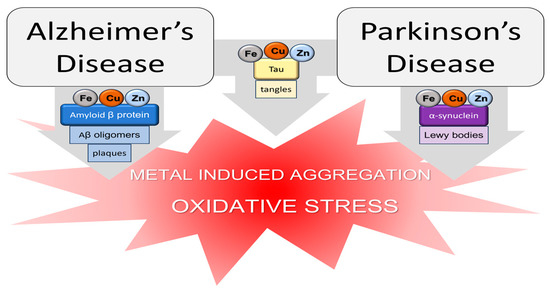
2. Zinc, Copper, and Iron
3. Manganese, Nickel, and Aluminum
References
- Wang, Z.; Pierson, R.; Heymsfield, S. The Five-Level Model: A New Approach to Organizing Body-Composition Research. Am. J. Clin. Nutr. 1992, 56, 19–28.
- Andrews, N.C. Disorders of Iron Metabolism. N. Engl. J. Med. 1999, 341, 1986–1995.
- Moustakas, M. The Role of Metal Ions in Biology, Biochemistry and Medicine. Materials 2021, 14, 549.
- Farina, M.; Avila, D.S.; da Rocha, J.B.T.; Aschner, M. Metals, Oxidative Stress and Neurodegeneration: A Focus on Iron, Manganese and Mercury. Neurochem. Int. 2013, 62, 575–594.
- Slobodian, M.R.; Petahtegoose, J.D.; Wallis, A.L.; Levesque, D.C.; Merritt, T.J.S. The Effects of Essential and Non-Essential Metal Toxicity in the Drosophila Melanogaster Insect Model: A Review. Toxics 2021, 9, 269.
- Lachowicz, J.I.; Lecca, L.I.; Meloni, F.; Campagna, M. Metals and Metal-Nanoparticles in Human Pathologies: From Exposure to Therapy. Molecules 2021, 26, 6639.
- Chen, P.; Miah, M.R.; Aschner, M. Metals and Neurodegeneration. F1000Research 2016, 5, 366.
- Cicero, C.E.; Mostile, G.; Vasta, R.; Rapisarda, V.; Signorelli, S.S.; Ferrante, M.; Zappia, M.; Nicoletti, A. Metals and Neurodegenerative Diseases. A Systematic Review. Environ. Res. 2017, 159, 82–94.
- Shah, H.; Dehghani, F.; Ramezan, M.; Gannaban, R.B.; Haque, Z.F.; Rahimi, F.; Abbasi, S.; Shin, A.C. Revisiting the Role of Vitamins and Minerals in Alzheimer’s Disease. Antioxidants 2023, 12, 415.
- Yamauchi, O.; Odani, A.; Takani, M. Metal–Amino Acid Chemistry. Weak Interactions and Related Functions of Side Chain Groups. J. Chem. Soc. Dalton Trans. 2002, 34, 3411–3421.
- Liu, X.; Wu, M.; Li, C.; Yu, P.; Feng, S.; Li, Y.; Zhang, Q. Interaction Structure and Affinity of Zwitterionic Amino Acids with Important Metal Cations (Cd2+, Cu2+, Fe3+, Hg2+, Mn2+, Ni2+ and Zn2+) in Aqueous Solution: A Theoretical Study. Molecules 2022, 27, 2407.
- Liu, Z.; Chen, S.; Qiao, F.; Zhang, X. Interaction of Peptide Backbones and Transition Metal Ions: 1. an IM-MS and DFT Study of the Binding Pattern, Structure and Fragmentation of Pd(II)/Ni(II)-Polyalanine Complexes. Int. J. Mass. Spectrom. 2019, 438, 87–96.
- Di Natale, C.; De Benedictis, I.; De Benedictis, A.; Marasco, D. Metal–Peptide Complexes as Promising Antibiotics to Fight Emerging Drug Resistance: New Perspectives in Tuberculosis. Antibiotics 2020, 9, 337.
- Witkowska, D.; Rowińska-Żyrek, M. Biophysical Approaches for the Study of Metal-Protein Interactions. J. Inorg. Biochem. 2019, 199, 110783.
- Guo, C.; Cheng, M.; Gross, M.L. Protein-Metal-Ion Interactions Studied by Mass Spectrometry-Based Footprinting with Isotope-Encoded Benzhydrazide. Anal. Chem. 2019, 91, 1416–1423.
- Wu, G. Amino Acids: Metabolism, Functions, and Nutrition. Amino Acids 2009, 37, 1–17.
- Morris, R.; Black, K.A.; Stollar, E.J. Uncovering Protein Function: From Classification to Complexes. Essays Biochem. 2022, 66, 255–285.
- Potocki, S.; Rowinska-Zyrek, M.; Witkowska, D.; Pyrkosz, M.; Szebesczyk, A.; Krzywoszynska, K.; Kozlowski, H. Metal Transport and Homeostasis within the Human Body: Toxicity Associated with Transport Abnormalities. Curr. Med. Chem. 2012, 19, 2738–2759.
- Gaggelli, E.; Kozlowski, H.; Valensin, D.; Valensin, G. Copper Homeostasis and Neurodegenerative Disorders (Alzheimer’s, Prion, and Parkinson’s Diseases and Amyotrophic Lateral Sclerosis). Chem. Rev. 2006, 106, 1995–2044.
- Kozlowski, H.; Janicka-Klos, A.; Brasun, J.; Gaggelli, E.; Valensin, D.; Valensin, G. Copper, Iron, and Zinc Ions Homeostasis and Their Role in Neurodegenerative Disorders (Metal Uptake, Transport, Distribution and Regulation). Coord. Chem. Rev. 2009, 253, 2665–2685.
- Kozlowski, H.; Luczkowski, M.; Remelli, M.; Valensin, D. Copper, Zinc and Iron in Neurodegenerative Diseases (Alzheimer’s, Parkinson’s and Prion Diseases). Coord. Chem. Rev. 2012, 256, 2129–2141.
- Liu, Y.; Nguyen, M.; Robert, A.; Meunier, B. Metal Ions in Alzheimer’s Disease: A Key Role or Not? Acc. Chem. Res. 2019, 52, 2026–2035.
- Wang, L.; Yin, Y.-L.; Liu, X.-Z.; Shen, P.; Zheng, Y.-G.; Lan, X.-R.; Lu, C.-B.; Wang, J.-Z. Current Understanding of Metal Ions in the Pathogenesis of Alzheimer’s Disease. Transl. Neurodegener. 2020, 9, 10.
- Foley, P.B.; Hare, D.J.; Double, K.L. A Brief History of Brain Iron Accumulation in Parkinson Disease and Related Disorders. J. Neural Transm. Vienna Austria 1996 2022, 129, 505–520.
- Balachandran, R.C.; Mukhopadhyay, S.; McBride, D.; Veevers, J.; Harrison, F.E.; Aschner, M.; Haynes, E.N.; Bowman, A.B. Brain Manganese and the Balance between Essential Roles and Neurotoxicity. J. Biol. Chem. 2020, 295, 6312–6329.
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464.
- Meneghini, R. Iron Homeostasis, Oxidative Stress, and DNA Damage. Free Radic. Biol. Med. 1997, 23, 783–792.
- Barnham, K.J.; Bush, A.I. Metals in Alzheimer’s and Parkinson’s Diseases. Curr. Opin. Chem. Biol. 2008, 12, 222–228.
- Swartz, H.M.; Sarna, T.; Zecca, L. Modulation by Neuromelanin of the Availability and Reactivity of Metal Ions. Ann. Neurol. 1992, 32, S69–S75.
- Liu, Y.; Hong, L.; Kempf, V.R.; Wakamatsu, K.; Ito, S.; Simon, J.D. Ion-Exchange and Adsorption of Fe(III) by Sepia Melanin. Pigment. Cell Res. 2004, 17, 262–269.
- Zecca, L.; Pietra, R.; Goj, C.; Mecacci, C.; Radice, D.; Sabbioni, E. Iron and Other Metals in Neuromelanin, Substantia Nigra, and Putamen of Human Brain. J. Neurochem. 1994, 62, 1097–1101.
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese Is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108.
- Myhre, O.; Utkilen, H.; Duale, N.; Brunborg, G.; Hofer, T. Metal Dyshomeostasis and Inflammation in Alzheimer’s and Parkinson’s Diseases: Possible Impact of Environmental Exposures. Oxid. Med. Cell. Longev. 2013, 2013, e726954.
- Li, B.; Xia, M.; Zorec, R.; Parpura, V.; Verkhratsky, A. Astrocytes in Heavy Metal Neurotoxicity and Neurodegeneration. Brain Res. 2021, 1752, 147234.
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public. Health 2020, 17, 679.
- Babić Leko, M.; Langer Horvat, L.; Španić Popovački, E.; Zubčić, K.; Hof, P.R.; Šimić, G. Metals in Alzheimer’s Disease. Biomedicines 2023, 11, 1161.
- Brewer, G.J.; Kanzer, S.H.; Zimmerman, E.A.; Molho, E.S.; Celmins, D.F.; Heckman, S.M.; Dick, R. Subclinical Zinc Deficiency in Alzheimer’s Disease and Parkinson’s Disease. Am. J. Alzheimers Dis. Dementiasr 2010, 25, 572–575.
- Ahmed, S.S.S.J.; Santosh, W. Metallomic Profiling and Linkage Map Analysis of Early Parkinson’s Disease: A New Insight to Aluminum Marker for the Possible Diagnosis. PLoS ONE 2010, 5, e11252.
- Giacoppo, S.; Galuppo, M.; Calabrò, R.S.; D’Aleo, G.; Marra, A.; Sessa, E.; Bua, D.G.; Potortì, A.G.; Dugo, G.; Bramanti, P.; et al. Heavy Metals and Neurodegenerative Diseases: An Observational Study. Biol. Trace Elem. Res. 2014, 161, 151–160.
- Zhao, H.-W.; Lin, J.; Wang, X.-B.; Cheng, X.; Wang, J.-Y.; Hu, B.-L.; Zhang, Y.; Zhang, X.; Zhu, J.-H. Assessing Plasma Levels of Selenium, Copper, Iron and Zinc in Patients of Parkinson’s Disease. PLoS ONE 2013, 8, e83060.
- Yadav, J.; Verma, A.K.; Ahmad, M.K.; Garg, R.K.; Shiuli; Mahdi, A.A.; Srivastava, S. Metals Toxicity and Its Correlation with the Gene Expression in Alzheimer’s Disease. Mol. Biol. Rep. 2021, 48, 3245–3252.
- Paglia, G.; Miedico, O.; Cristofano, A.; Vitale, M.; Angiolillo, A.; Chiaravalle, A.E.; Corso, G.; Di Costanzo, A. Distinctive Pattern of Serum Elements During the Progression of Alzheimer’s Disease. Sci. Rep. 2016, 6, 22769.
- Wang, Z.-X.; Tan, L.; Wang, H.-F.; Ma, J.; Liu, J.; Tan, M.-S.; Sun, J.-H.; Zhu, X.-C.; Jiang, T.; Yu, J.-T. Serum Iron, Zinc, and Copper Levels in Patients with Alzheimer’s Disease: A Replication Study and Meta-Analyses. J. Alzheimers Dis. 2015, 47, 565–581.
- Koç, E.R.; Ilhan, A.; Zübeyde Aytürk, A.; Acar, B.; Gürler, M.; Altuntaş, A.; Karapirli, M.; Bodur, A.S. A Comparison of Hair and Serum Trace Elements in Patients with Alzheimer Disease and Healthy Participants. Turk. J. Med. Sci. 2015, 45, 1034–1039.
- Rembach, A.; Doecke, J.D.; Roberts, B.R.; Watt, A.D.; Faux, N.G.; Volitakis, I.; Pertile, K.K.; Rumble, R.L.; Trounson, B.O.; Fowler, C.J.; et al. Longitudinal Analysis of Serum Copper and Ceruloplasmin in Alzheimer’s Disease. J. Alzheimers Dis. 2013, 34, 171–182.
- Alsadany, M.A.; Shehata, H.H.; Mohamad, M.I.; Mahfouz, R.G. Histone Deacetylases Enzyme, Copper, and IL-8 Levels in Patients With Alzheimer’s Disease. Am. J. Alzheimers Dis. Other Demen. 2013, 28, 54–61.
- Mariani, S.; Ventriglia, M.; Simonelli, I.; Donno, S.; Bucossi, S.; Vernieri, F.; Melgari, J.-M.; Pasqualetti, P.; Rossini, P.M.; Squitti, R. Fe and Cu Do Not Differ in Parkinson’s Disease: A Replication Study plus Meta-Analysis. Neurobiol. Aging 2013, 34, 632–633.
- Crespo, Â.C.; Silva, B.; Marques, L.; Marcelino, E.; Maruta, C.; Costa, S.; Timóteo, Â.; Vilares, A.; Couto, F.S.; Faustino, P.; et al. Genetic and Biochemical Markers in Patients with Alzheimer’s Disease Support a Concerted Systemic Iron Homeostasis Dysregulation. Neurobiol. Aging 2014, 35, 777–785.
- Fukushima, T.; Tan, X.; Luo, Y.; Kanda, H. Relationship between Blood Levels of Heavy Metals and Parkinson’s Disease in China. Neuroepidemiology 2010, 34, 18–24.
- Dexter, D.T.; Carayon, A.; Javoy-Agid, F.; Agid, Y.; Wells, F.R.; Daniel, S.E.; Lees, A.J.; Jenner, P.; Marsden, C.D. Alterations in the Levels of Iron, Ferritin and Other Trace Metals in Parkinson’s Disease and Other Neurodegenerative Diseases Affecting the Basal Ganglia. Brain 1991, 114, 1953–1975.
- Babić Leko, M.; Jurasović, J.; Nikolac Perković, M.; Španić, E.; Sekovanić, A.; Orct, T.; Lukinović Škudar, V.; Bačić Baronica, K.; Kiđemet-Piskač, S.; Vogrinc, Ž.; et al. The Association of Essential Metals with APOE Genotype in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82, 661–672.
- Hozumi, I.; Hasegawa, T.; Honda, A.; Ozawa, K.; Hayashi, Y.; Hashimoto, K.; Yamada, M.; Koumura, A.; Sakurai, T.; Kimura, A.; et al. Patterns of Levels of Biological Metals in CSF Differ among Neurodegenerative Diseases. J. Neurol. Sci. 2011, 303, 95–99.
- Squitti, R.; Ventriglia, M.; Simonelli, I.; Bonvicini, C.; Costa, A.; Perini, G.; Binetti, G.; Benussi, L.; Ghidoni, R.; Koch, G.; et al. Copper Imbalance in Alzheimer’s Disease: Meta-Analysis of Serum, Plasma, and Brain Specimens, and Replication Study Evaluating ATP7B Gene Variants. Biomolecules 2021, 11, 960.
- Miller, L.M.; Wang, Q.; Telivala, T.P.; Smith, R.J.; Lanzirotti, A.; Miklossy, J. Synchrotron-Based Infrared and X-Ray Imaging Shows Focalized Accumulation of Cu and Zn Co-Localized with β-Amyloid Deposits in Alzheimer’s Disease. J. Struct. Biol. 2006, 155, 30–37.
- Dong, J.; Atwood, C.S.; Anderson, V.E.; Siedlak, S.L.; Smith, M.A.; Perry, G.; Carey, P.R. Metal Binding and Oxidation of Amyloid-Beta within Isolated Senile Plaque Cores: Raman Microscopic Evidence. Biochemistry 2003, 42, 2768–2773.
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, Iron and Zinc in Alzheimer’s Disease Senile Plaques. J. Neurol. Sci. 1998, 158, 47–52.
- Smith, M.A.; Harris, P.L.R.; Sayre, L.M.; Perry, G. Iron Accumulation in Alzheimer Disease Is a Source of Redox-Generated Free Radicals. Proc. Natl. Acad. Sci. USA 1997, 94, 9866–9868.
- Liu, B.; Moloney, A.; Meehan, S.; Morris, K.; Thomas, S.E.; Serpell, L.C.; Hider, R.; Marciniak, S.J.; Lomas, D.A.; Crowther, D.C. Iron Promotes the Toxicity of Amyloid β Peptide by Impeding Its Ordered Aggregation. J. Biol. Chem. 2011, 286, 4248–4256.
- Sóvágó, I.; Várnagy, K.; Kállay, C.; Grenács, Á. Interactions of Copper(II) and Zinc(II) Ions with the Peptide Fragments of Proteins Related to Neurodegenerative Disorders: Similarities and Differences. Curr. Med. Chem. 2023, 30, 4050–4071.
- Nath, A.K.; Dey, S.G. Simultaneous Binding of Heme and Cu with Amyloid β Peptides: Active Site and Reactivities. Dalton Trans. 2022, 51, 4986–4999.
- Stefaniak, E.; Bal, W. CuII Binding Properties of N-Truncated Aβ Peptides: In Search of Biological Function. Inorg. Chem. 2019, 58, 13561–13577.
- Arena, G.; Rizzarelli, E. Zn2+ Interaction with Amyloid-Β: Affinity and Speciation. Mol. Basel Switz. 2019, 24, 2796.
- Atrián-Blasco, E.; Conte-Daban, A.; Hureau, C. Mutual Interference of Cu and Zn Ions in Alzheimer’s Disease: Perspectives at the Molecular Level. Dalton Trans. 2017, 46, 12750–12759.
- Wärmländer, S.K.T.S.; Österlund, N.; Wallin, C.; Wu, J.; Luo, J.; Tiiman, A.; Jarvet, J.; Gräslund, A. Metal Binding to the Amyloid-β Peptides in the Presence of Biomembranes: Potential Mechanisms of Cell Toxicity. J. Biol. Inorg. Chem. JBIC Publ. Soc. Biol. Inorg. Chem. 2019, 24, 1189–1196.
- De Gregorio, G.; Biasotto, F.; Hecel, A.; Luczkowski, M.; Kozlowski, H.; Valensin, D. Structural Analysis of Copper(I) Interaction with Amyloid β Peptide. J. Inorg. Biochem. 2019, 195, 31–38.
- Trapani, G.; Satriano, C.; La Mendola, D. Peptides and Their Metal Complexes in Neurodegenerative Diseases: From Structural Studies to Nanomedicine Prospects. Curr. Med. Chem. 2018, 25, 715–747.
- Ahmadi, S.; Zhu, S.; Sharma, R.; Wilson, D.J.; Kraatz, H.-B. Interaction of Metal Ions with Tau Protein. The Case for a Metal-Mediated Tau Aggregation. J. Inorg. Biochem. 2019, 194, 44–51.
- Binolfi, A.; Quintanar, L.; Bertoncini, C.W.; Griesinger, C.; Fernández, C.O. Bioinorganic Chemistry of Copper Coordination to Alpha-Synuclein: Relevance to Parkinson’s Disease. Coord. Chem. Rev. 2012, 256, 2188–2201.
- Valensin, D.; Dell’Acqua, S.; Kozlowski, H.; Casella, L. Coordination and Redox Properties of Copper Interaction with α-Synuclein. J. Inorg. Biochem. 2016, 163, 292–300.
- González, N.; Arcos-López, T.; König, A.; Quintanar, L.; Menacho Márquez, M.; Outeiro, T.F.; Fernández, C.O. Effects of Alpha-Synuclein Post-Translational Modifications on Metal Binding. J. Neurochem. 2019, 150, 507–521.
- Atrián-Blasco, E.; Gonzalez, P.; Santoro, A.; Alies, B.; Faller, P.; Hureau, C. Cu and Zn Coordination to Amyloid Peptides: From Fascinating Chemistry to Debated Pathological Relevance. Coord. Chem. Rev. 2018, 375, 38–55.
- Leal, S.S.; Botelho, H.M.; Gomes, C.M. Metal Ions as Modulators of Protein Conformation and Misfolding in Neurodegeneration. Coord. Chem. Rev. 2012, 256, 2253–2270.
- Gamez, P.; Caballero, A.B. Copper in Alzheimer’s Disease: Implications in Amyloid Aggregation and Neurotoxicity. AIP Adv. 2015, 5, 092503.
- Faller, P.; Hureau, C.; La Penna, G. Metal Ions and Intrinsically Disordered Proteins and Peptides: From Cu/Zn Amyloid-β to General Principles. Acc. Chem. Res. 2014, 47, 2252–2259.
- DeToma, A.S.; Salamekh, S.; Ramamoorthy, A.; Lim, M.H. Misfolded Proteins in Alzheimer’s Disease and Type II Diabetes. Chem. Soc. Rev. 2012, 41, 608–621.
- Ke, P.C.; Sani, M.-A.; Ding, F.; Kakinen, A.; Javed, I.; Separovic, F.; Davis, T.P.; Mezzenga, R. Implications of Peptide Assemblies in Amyloid Diseases. Chem. Soc. Rev. 2017, 46, 6492–6531.
- Viles, J.H. Metal Ions and Amyloid Fiber Formation in Neurodegenerative Diseases. Copper, Zinc and Iron in Alzheimer’s, Parkinson’s and Prion Diseases. Coord. Chem. Rev. 2012, 256, 2271–2284.
- Miller, Y.; Ma, B.; Nussinov, R. Zinc Ions Promote Alzheimer Abeta Aggregation via Population Shift of Polymorphic States. Proc. Natl. Acad. Sci. USA 2010, 107, 9490–9495.
- Sharma, A.K.; Pavlova, S.T.; Kim, J.; Kim, J.; Mirica, L.M. The Effect of Cu2+ and Zn2+ on the Aβ42 Peptide Aggregation and Cellular Toxicity. Metallomics 2013, 5, 1529–1536.
- Bush, A.I.; Pettingell, W.H.; Multhaup, G.; d Paradis, M.; Vonsattel, J.P.; Gusella, J.F.; Beyreuther, K.; Masters, C.L.; Tanzi, R.E. Rapid Induction of Alzheimer A Beta Amyloid Formation by Zinc. Science 1994, 265, 1464–1467.
- Carboni, E.; Lingor, P. Insights on the Interaction of Alpha-Synuclein and Metals in the Pathophysiology of Parkinson’s Disease. Metallomics 2015, 7, 395–404.
- Drew, S.C. The N Terminus of α-Synuclein Forms CuII-Bridged Oligomers. Chem. Eur. J. 2015, 21, 7111–7118.
- Li, W.-J.; Jiang, H.; Song, N.; Xie, J.-X. Dose- and Time-Dependent α-Synuclein Aggregation Induced by Ferric Iron in SK-N-SH Cells. Neurosci. Bull. 2010, 26, 205–210.
- Rasia, R.M.; Bertoncini, C.W.; Marsh, D.; Hoyer, W.; Cherny, D.; Zweckstetter, M.; Griesinger, C.; Jovin, T.M.; Fernández, C.O. Structural Characterization of Copper(II) Binding to α-Synuclein: Insights into the Bioinorganic Chemistry of Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2005, 102, 4294–4299.
- Li, X.; Du, X.; Ni, J. Zn2+ Aggravates Tau Aggregation and Neurotoxicity. Int. J. Mol. Sci. 2019, 20, 487.
- Zubčić, K.; Hof, P.R.; Šimić, G.; Jazvinšćak Jembrek, M. The Role of Copper in Tau-Related Pathology in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 572308.
- Ahmadi, S.; Wu, B.; Song, R.; Zhu, S.; Simpson, A.; Wilson, D.J.; Kraatz, H.-B. Exploring the Interactions of Iron and Zinc with the Microtubule Binding Repeats R1 and R4. J. Inorg. Biochem. 2020, 205, 110987.
- Soragni, A.; Zambelli, B.; Mukrasch, M.D.; Biernat, J.; Jeganathan, S.; Griesinger, C.; Ciurli, S.; Mandelkow, E.; Zweckstetter, M. Structural Characterization of Binding of Cu(II) to Tau Protein. Biochemistry 2008, 47, 10841–10851.
- Balogh, B.D.; Szakács, B.; Di Natale, G.; Tabbì, G.; Pappalardo, G.; Sóvágó, I.; Várnagy, K. Copper (II) Binding Properties of an Octapeptide Fragment from the R3 Region of Tau Protein: A Combined Potentiometric, Spectroscopic and Mass Spectrometric Study. J. Inorg. Biochem. 2021, 217, 111358.
- Bacchella, C.; Gentili, S.; Bellotti, D.; Quartieri, E.; Draghi, S.; Baratto, M.C.; Remelli, M.; Valensin, D.; Monzani, E.; Nicolis, S.; et al. Binding and Reactivity of Copper to R1 and R3 Fragments of Tau Protein. Inorg. Chem. 2020, 59, 274–286.
- Jing, J.; Tu, G.; Yu, H.; Huang, R.; Ming, X.; Zhan, H.; Zhan, F.; Xue, W. Copper (Cu2+) Ion-Induced Misfolding of Tau Protein R3 Peptide Revealed by Enhanced Molecular Dynamics Simulation. Phys. Chem. Chem. Phys. 2021, 23, 11717–11726.
- Rivers-Auty, J.; Tapia, V.S.; White, C.S.; Daniels, M.J.D.; Drinkall, S.; Kennedy, P.T.; Spence, H.G.; Yu, S.; Green, J.P.; Hoyle, C.; et al. Zinc Status Alters Alzheimer’s Disease Progression through NLRP3-Dependent Inflammation. J. Neurosci. 2021, 41, 3025–3038.
- Singh, I.; Sagare, A.P.; Coma, M.; Perlmutter, D.; Gelein, R.; Bell, R.D.; Deane, R.J.; Zhong, E.; Parisi, M.; Ciszewski, J.; et al. Low Levels of Copper Disrupt Brain Amyloid-β Homeostasis by Altering Its Production and Clearance. Proc. Natl. Acad. Sci. USA 2013, 110, 14771–14776.
- Sparks, D.L.; Schreurs, B.G. Trace Amounts of Copper in Water Induce β-Amyloid Plaques and Learning Deficits in a Rabbit Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2003, 100, 11065–11069.
- Prasanthi, J.R.P.; Schrag, M.; Dasari, B.; Marwarha, G.; Dickson, A.; Kirsch, W.M.; Ghribi, O. Deferiprone Reduces Amyloid-β and Tau Phosphorylation Levels but Not Reactive Oxygen Species Generation in Hippocampus of Rabbits Fed a Cholesterol-Enriched Diet. J. Alzheimers Dis. 2012, 30, 167–182.
- Chen, P.; Chakraborty, S.; Mukhopadhyay, S.; Lee, E.; Paoliello, M.M.B.; Bowman, A.B.; Aschner, M. Manganese Homeostasis in the Nervous System. J. Neurochem. 2015, 134, 601–610.
- Shen, X.; Liu, J.; Fujita, Y.; Liu, S.; Maeda, T.; Kikuchi, K.; Obara, T.; Takebe, A.; Sayama, R.; Takahashi, T.; et al. Iron Treatment Inhibits Aβ42 Deposition in Vivo and Reduces Aβ42/Aβ40 Ratio. Biochem. Biophys. Res. Commun. 2019, 512, 653–658.
- Alimonti, A.; Ristori, G.; Giubilei, F.; Stazi, M.A.; Pino, A.; Visconti, A.; Brescianini, S.; Monti, M.S.; Forte, G.; Stanzione, P.; et al. Serum Chemical Elements and Oxidative Status in Alzheimer’s Disease, Parkinson Disease and Multiple Sclerosis. NeuroToxicology 2007, 28, 450–456.
- Guilarte, T.R. Manganese Neurotoxicity: New Perspectives from Behavioral, Neuroimaging, and Neuropathological Studies in Humans and Non-Human Primates. Front. Aging Neurosci. 2013, 5, 23.
- Hirsch, E.C.; Brandel, J.-P.; Galle, P.; Javoy-Agid, F.; Agid, Y. Iron and Aluminum Increase in the Substantia Nigra of Patients with Parkinson’s Disease: An X-Ray Microanalysis. J. Neurochem. 1991, 56, 446–451.
- Altschuler, E. Aluminum-Containing Antacids as a Cause of Idiopathic Parkinson’s Disease. Med. Hypotheses 1999, 53, 22–23.
- Zatta, P.; Zambenedetti, P.; Milanese, M. Activation of Monoamine Oxidase Type-B by Aluminum in Rat Brain Homogenate. NeuroReport 1999, 10, 3645–3648.
- Baum, L.; Chan, I.H.S.; Cheung, S.K.-K.; Goggins, W.B.; Mok, V.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; Woo, J.; et al. Serum Zinc Is Decreased in Alzheimer’s Disease and Serum Arsenic Correlates Positively with Cognitive Ability. BioMetals 2010, 23, 173–179.
- Bhattacharjee, S.; Zhao, Y.; Hill, J.M.; Culicchia, F.; Kruck, T.P.A.; Percy, M.E.; Pogue, A.I.; Walton, J.R.; Lukiw, W.J. Selective Accumulation of Aluminum in Cerebral Arteries in Alzheimer’s Disease (AD). J. Inorg. Biochem. 2013, 126, 35–37.
- González-Domínguez, R.; García-Barrera, T.; Gómez-Ariza, J.L. Characterization of Metal Profiles in Serum during the Progression of Alzheimer’s Disease. Metallomics 2014, 6, 292–300.
- Smorgon, C.; Mari, E.; Atti, A.R.; Dalla Nora, E.; Zamboni, P.F.; Calzoni, F.; Passaro, A.; Fellin, R. Trace Elements and Cognitive Impairment: An Elderly Cohort Study. Arch. Gerontol. Geriatr. 2004, 38, 393–402.
- Du, K.; Liu, M.; Pan, Y.; Zhong, X.; Wei, M. Association of Serum Manganese Levels with Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 231.
- Koseoglu, E.; Koseoglu, R.; Kendirci, M.; Saraymen, R.; Saraymen, B. Trace Metal Concentrations in Hair and Nails from Alzheimer’s Disease Patients: Relations with Clinical Severity. J. Trace Elem. Med. Biol. 2017, 39, 124–128.
- Szabo, S.T.; Harry, G.J.; Hayden, K.M.; Szabo, D.T.; Birnbaum, L. Comparison of Metal Levels between Postmortem Brain and Ventricular Fluid in Alzheimer’s Disease and Nondemented Elderly Controls. Toxicol. Sci. 2016, 150, 292–300.
- Gorantla, N.V.; Das, R.; Balaraman, E.; Chinnathambi, S. Transition Metal Nickel Prevents Tau Aggregation in Alzheimer’s Disease. Int. J. Biol. Macromol. 2020, 156, 1359–1365.
- Tong, Y.; Yang, H.; Tian, X.; Wang, H.; Zhou, T.; Zhang, S.; Yu, J.; Zhang, T.; Fan, D.; Guo, X.; et al. High Manganese, A Risk for Alzheimer’s Disease: High Manganese Induces Amyloid-β Related Cognitive Impairment. J. Alzheimers Dis. 2014, 42, 865–878.
- Walton, J.R.; Wang, M.-X. APP Expression, Distribution and Accumulation Are Altered by Aluminum in a Rodent Model for Alzheimer’s Disease. J. Inorg. Biochem. 2009, 103, 1548–1554.
- Bouras, C.; Giannakopoulos, P.; Good, P.F.; Hsu, A.; Hof, P.R.; Perl, D.P. A Laser Microprobe Mass Analysis of Brain Aluminum and Iron in Dementia Pugilistica: Comparison with Alzheimer’s Disease. Eur. Neurol. 2007, 38, 53–58.
- Rondeau, V.; Jacqmin-Gadda, H.; Commenges, D.; Helmer, C.; Dartigues, J.-F. Aluminum and Silica in Drinking Water and the Risk of Alzheimer’s Disease or Cognitive Decline: Findings from 15-Year Follow-up of the PAQUID Cohort. Am. J. Epidemiol. 2009, 169, 489–496.
- Zhang, Q.L.; Jia, L.; Jiao, X.; Guo, W.L.; Ji, J.W.; Yang, H.L.; Niu, Q. APP/PS1 Transgenic Mice Treated with Aluminum: An Update of Alzheimer’s Disease Model. Int. J. Immunopathol. Pharmacol. 2012, 25, 49–58.




