Metal ions are fundamental to guarantee the regular physiological activity of the human organism. They are involved in several biological processes such as electron transfer, oxygen transport, the maintenance of osmotic pressure, and the regulation of DNA transcription. Metals such as iron, cobalt, selenium, copper, zinc, and manganese are essential for human life and are usually required in trace amounts. On the other hand, aluminum, mercury, arsenic, and others are considered non-essential metals since they possess no biological function. The importance of metals in the human organism is so fundamental that several pathologies, among which are neurodegenerative diseases (NDs), are related to a common phenomenon known as metal dyshomeostasis.
- metal ions
- Alzheimer’s disease
- Parkinson’s disease
- metal dyshomeostasis
- zinc
- copper
- iron
- manganese
- nickel
- aluminum
1. Introduction
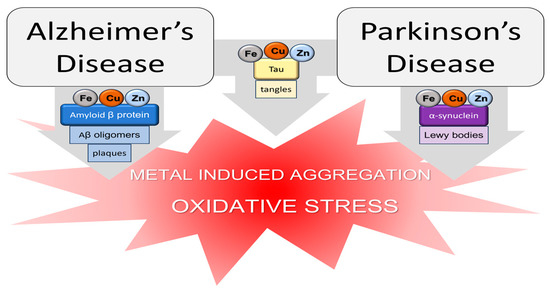
2. Zinc, Copper, and Iron
Zinc, copper, and iron levels in serum, hair, CSF, and the brain have been extensively measured trying to correlate their content with metal dyshomeostasis associated with AD and PD cases [36]. The most applied techniques are atomic absorption, inductively coupled plasma atomic emission spectroscopy (ICP-AES), ICP-mass spectrometry (MS), and ICP-optical emission spectrometry (OES). Serum zinc levels were generally found to be reduced in patients affected by both AD and PD [37][38][39][40][41][42][43]. Decreased Zn concentrations have also been determined in AD hair samples [44]. On the other hand, reduced and increased copper contents have been measured in both AD [39][41][43][45][46] and PD cases [40][47]. Finally, a different behavior is displayed by iron, whose levels are different according to the disease, usually lower [41][42][48] or higher [40][49] in AD and PD patients, respectively. Altered zinc, iron, and copper concentrations have also been found in CSF and post-mortem brains [36][50][51][52][53]. In PD patients, zinc levels are higher in the substantia nigra, caudate nucleus, and lateral putamen [50]. Iron content is higher in the substantia nigra and lower in the globus pallidus [50]. In PD, copper is increased in the putamen and decreased in the substantia nigra [50], while it is decreased in AD brains [53]. The altered metal levels observed in AD also correlate with the presence of Fe and Cu, Zn, in the AD senile plaques, primarily constituted by the aggregated forms of Aβ [54][55][56][57]. Aβ is a well-known amyloidogenic protein associated with AD and it is able to bind copper, zinc, and iron by means of His imidazole, N-terminal amino, and Glu/Asp carboxylate groups [58][59][60][61][62][63][64][65]. In a similar way, the amyloidogenic proteins tau and alpha synuclein, associated with AD and PD, respectively, can steadily coordinate several transition metal ions [66][67][68][69][70] (Figure 1). Metal ions such as Cu and Zn can impact the aggregation of amyloidogenic proteins by affecting the morphologies and kinetics of the aggregates. The scientific community put a lot of effort into understanding the influence of metal ions, primarily copper and zinc, in the aggregation of amyloidogenic proteins [71][72][73][74][75][76][77]. The obtained findings are quite heterogeneous primarily due to the intrinsic complexity of the systems and different experimental conditions and techniques. In general, it is evident that zinc promotes the formation of amorphous Aβ aggregates while copper favors the production of highly cytotoxic oligomers [78][79][80]. As for Aβ, metal ion binding impacts the aggregation of αSyn as well, either showing pro- or anti-aggregatory effects [81][82]. Among all the metal ions, iron and copper are able to influence αSyn aggregation by promoting the formation of multimeric species and αSyn assembly [83][84]. Zinc interaction with the third repeat unit of the microtubule-binding domain of tau (R3tau) leads to the formation of Zn(II)-R3tau aggregates [85]. Such complexes, compared to R3tau, possess higher toxicity towards Neuro-2A (N2A) cells by inducing higher ROS generation in N2A cells. Copper increases the aggregation propensity of tau through its capability to both bind tau and produce ROS [86]. Zn and Fe binding to tau-R1 and R4 was also investigated; Zn(II) and Fe(II) but not Fe(III) coordination was demonstrated by CD and ESI-MS. Both interactions induced conformational changes in R1 and R4 [87]. Copper binding to tau occurs via His residues present in R1, R2, R3, and R4 or at the N-terminal site [88][89][90]. Recent molecular dynamic studies have revealed the misfolding of R3tau upon Cu(II) binding [91]. In addition, the ability of the copper–R3tau complex to promote the oxidation of dopamine has been recently reported [90]. The involvement of zinc, iron, and copper in AD is also supported by in vivo animal studies showing the effects of metal deficiency and/or supplementation in AD mice models [92][93][94][95][96]. For example, a zinc-deficient diet in an APP/PS1 mouse model of AD accelerated memory deficits through the induction of the NLRP3-inflammasome complex [92]. Other studies show that treatment with low levels of Cu(II) in drinking water led to an increase in Aβ production in neuroinflammation [93] and promoted Aβ accumulation, reducing mice’s cognitive functions [94]. Finally, in hypercholesteremia-induced AD rabbits, the administration of Fe(III) chelator deferiprone in drinking water significantly reduced the levels of plasma iron and cholesterol and decreased tau phosphorylation, Aβ40, and Aβ42 but not ROS production in the hippocampus [95]. In contrast, the treatment of an AD mouse model with Fe(II)-containing water markedly reduced Aβ42 deposition, tau phosphorylation, and apoptotic neurons and led to an increase in Aβ40 and a reduction in the Aβ42/Aβ40 ratio [97].3. Manganese, Nickel, and Aluminum
The relevance of Mn, Ni, and Al in both AD and PD is well documented in the literature even if to a lesser extent than the essential Zn, Cu, and Fe ions. In PD, the serum levels of Mn, Ni, and Al are generally higher compared to healthy controls [38][49][98]. Furthermore, acute exposure to Mn can result in manganism, a type of parkinsonism, considered part of the PD etiology [96]. Manganism may be caused by elevated Mn accumulation in the basal ganglia region of the brain [99]. The association between aluminum and PD was suggested by the detection of Al in the Lewy bodies of PD patients, while its value is below the limit of detection in control brains [100]. Such findings are further supported by the higher incidence of ulcer patients that make high use of Al(III)-containing antiacids in PD cases compared to controls [101]. In addition, Al(III) was found to increase monoamine oxidase B and SOD activities in a way similar to what was observed in PD patients [38][102]. As for PD, higher serum levels of Ni [98] and Al [41][103][104][105][106] have been found for AD cases. On the other hand, reduced [42][107] or increased [44][108] Mn serum levels are reported for AD and MCI subjects. Mn content was also lower in the hair and nails of AD cases compared to control subjects [108]. Nickel levels were higher in the post-mortem frontal cortex and ventricular fluid of AD subjects with respect to nondemented elderly controls [109]. At the same time, nickel supplementation in the forms of the NiCl2 and NiCl2-morpholine complex prevented tau aggregation and promoted its degradation with the formation of shorter aggregates [110]. Moreover, in vitro and in vivo investigations on APP/PS1 mice showed dose-dependent neurotoxicity and an increase in Aβ upon Mn(II) treatment [111]. Finally, Al(III) may be implicated in AD pathogenesis via the induction of APP overexpression and the subsequent increase in Aβ and plaque formation in the brain [112]. A laser microprobe mass analysis showed a primary accumulation of Al(III) in the neurofibrillary tangles (NFTs) of AD subjects [113]. A 15-year follow-up study revealed an association between the high consumption of aluminum from drinking water and an increased risk of AD [114]. APP/PS1 transgenic mice, treated with intracerebroventricular microinjections of AlCl3, presented more extensive worsening of cognitive abilities and increases in neural apoptotic rates than APP/PS1 alone and wild-type mice exposed to Al [115].References
- Wang, Z.; Pierson, R.; Heymsfield, S. The Five-Level Model: A New Approach to Organizing Body-Composition Research. Am. J. Clin. Nutr. 1992, 56, 19–28.
- Andrews, N.C. Disorders of Iron Metabolism. N. Engl. J. Med. 1999, 341, 1986–1995.
- Moustakas, M. The Role of Metal Ions in Biology, Biochemistry and Medicine. Materials 2021, 14, 549.
- Farina, M.; Avila, D.S.; da Rocha, J.B.T.; Aschner, M. Metals, Oxidative Stress and Neurodegeneration: A Focus on Iron, Manganese and Mercury. Neurochem. Int. 2013, 62, 575–594.
- Slobodian, M.R.; Petahtegoose, J.D.; Wallis, A.L.; Levesque, D.C.; Merritt, T.J.S. The Effects of Essential and Non-Essential Metal Toxicity in the Drosophila Melanogaster Insect Model: A Review. Toxics 2021, 9, 269.
- Lachowicz, J.I.; Lecca, L.I.; Meloni, F.; Campagna, M. Metals and Metal-Nanoparticles in Human Pathologies: From Exposure to Therapy. Molecules 2021, 26, 6639.
- Chen, P.; Miah, M.R.; Aschner, M. Metals and Neurodegeneration. F1000Research 2016, 5, 366.
- Cicero, C.E.; Mostile, G.; Vasta, R.; Rapisarda, V.; Signorelli, S.S.; Ferrante, M.; Zappia, M.; Nicoletti, A. Metals and Neurodegenerative Diseases. A Systematic Review. Environ. Res. 2017, 159, 82–94.
- Shah, H.; Dehghani, F.; Ramezan, M.; Gannaban, R.B.; Haque, Z.F.; Rahimi, F.; Abbasi, S.; Shin, A.C. Revisiting the Role of Vitamins and Minerals in Alzheimer’s Disease. Antioxidants 2023, 12, 415.
- Yamauchi, O.; Odani, A.; Takani, M. Metal–Amino Acid Chemistry. Weak Interactions and Related Functions of Side Chain Groups. J. Chem. Soc. Dalton Trans. 2002, 34, 3411–3421.
- Liu, X.; Wu, M.; Li, C.; Yu, P.; Feng, S.; Li, Y.; Zhang, Q. Interaction Structure and Affinity of Zwitterionic Amino Acids with Important Metal Cations (Cd2+, Cu2+, Fe3+, Hg2+, Mn2+, Ni2+ and Zn2+) in Aqueous Solution: A Theoretical Study. Molecules 2022, 27, 2407.
- Liu, Z.; Chen, S.; Qiao, F.; Zhang, X. Interaction of Peptide Backbones and Transition Metal Ions: 1. an IM-MS and DFT Study of the Binding Pattern, Structure and Fragmentation of Pd(II)/Ni(II)-Polyalanine Complexes. Int. J. Mass. Spectrom. 2019, 438, 87–96.
- Di Natale, C.; De Benedictis, I.; De Benedictis, A.; Marasco, D. Metal–Peptide Complexes as Promising Antibiotics to Fight Emerging Drug Resistance: New Perspectives in Tuberculosis. Antibiotics 2020, 9, 337.
- Witkowska, D.; Rowińska-Żyrek, M. Biophysical Approaches for the Study of Metal-Protein Interactions. J. Inorg. Biochem. 2019, 199, 110783.
- Guo, C.; Cheng, M.; Gross, M.L. Protein-Metal-Ion Interactions Studied by Mass Spectrometry-Based Footprinting with Isotope-Encoded Benzhydrazide. Anal. Chem. 2019, 91, 1416–1423.
- Wu, G. Amino Acids: Metabolism, Functions, and Nutrition. Amino Acids 2009, 37, 1–17.
- Morris, R.; Black, K.A.; Stollar, E.J. Uncovering Protein Function: From Classification to Complexes. Essays Biochem. 2022, 66, 255–285.
- Potocki, S.; Rowinska-Zyrek, M.; Witkowska, D.; Pyrkosz, M.; Szebesczyk, A.; Krzywoszynska, K.; Kozlowski, H. Metal Transport and Homeostasis within the Human Body: Toxicity Associated with Transport Abnormalities. Curr. Med. Chem. 2012, 19, 2738–2759.
- Gaggelli, E.; Kozlowski, H.; Valensin, D.; Valensin, G. Copper Homeostasis and Neurodegenerative Disorders (Alzheimer’s, Prion, and Parkinson’s Diseases and Amyotrophic Lateral Sclerosis). Chem. Rev. 2006, 106, 1995–2044.
- Kozlowski, H.; Janicka-Klos, A.; Brasun, J.; Gaggelli, E.; Valensin, D.; Valensin, G. Copper, Iron, and Zinc Ions Homeostasis and Their Role in Neurodegenerative Disorders (Metal Uptake, Transport, Distribution and Regulation). Coord. Chem. Rev. 2009, 253, 2665–2685.
- Kozlowski, H.; Luczkowski, M.; Remelli, M.; Valensin, D. Copper, Zinc and Iron in Neurodegenerative Diseases (Alzheimer’s, Parkinson’s and Prion Diseases). Coord. Chem. Rev. 2012, 256, 2129–2141.
- Liu, Y.; Nguyen, M.; Robert, A.; Meunier, B. Metal Ions in Alzheimer’s Disease: A Key Role or Not? Acc. Chem. Res. 2019, 52, 2026–2035.
- Wang, L.; Yin, Y.-L.; Liu, X.-Z.; Shen, P.; Zheng, Y.-G.; Lan, X.-R.; Lu, C.-B.; Wang, J.-Z. Current Understanding of Metal Ions in the Pathogenesis of Alzheimer’s Disease. Transl. Neurodegener. 2020, 9, 10.
- Foley, P.B.; Hare, D.J.; Double, K.L. A Brief History of Brain Iron Accumulation in Parkinson Disease and Related Disorders. J. Neural Transm. Vienna Austria 1996 2022, 129, 505–520.
- Balachandran, R.C.; Mukhopadhyay, S.; McBride, D.; Veevers, J.; Harrison, F.E.; Aschner, M.; Haynes, E.N.; Bowman, A.B. Brain Manganese and the Balance between Essential Roles and Neurotoxicity. J. Biol. Chem. 2020, 295, 6312–6329.
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464.
- Meneghini, R. Iron Homeostasis, Oxidative Stress, and DNA Damage. Free Radic. Biol. Med. 1997, 23, 783–792.
- Barnham, K.J.; Bush, A.I. Metals in Alzheimer’s and Parkinson’s Diseases. Curr. Opin. Chem. Biol. 2008, 12, 222–228.
- Swartz, H.M.; Sarna, T.; Zecca, L. Modulation by Neuromelanin of the Availability and Reactivity of Metal Ions. Ann. Neurol. 1992, 32, S69–S75.
- Liu, Y.; Hong, L.; Kempf, V.R.; Wakamatsu, K.; Ito, S.; Simon, J.D. Ion-Exchange and Adsorption of Fe(III) by Sepia Melanin. Pigment. Cell Res. 2004, 17, 262–269.
- Zecca, L.; Pietra, R.; Goj, C.; Mecacci, C.; Radice, D.; Sabbioni, E. Iron and Other Metals in Neuromelanin, Substantia Nigra, and Putamen of Human Brain. J. Neurochem. 1994, 62, 1097–1101.
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese Is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108.
- Myhre, O.; Utkilen, H.; Duale, N.; Brunborg, G.; Hofer, T. Metal Dyshomeostasis and Inflammation in Alzheimer’s and Parkinson’s Diseases: Possible Impact of Environmental Exposures. Oxid. Med. Cell. Longev. 2013, 2013, e726954.
- Li, B.; Xia, M.; Zorec, R.; Parpura, V.; Verkhratsky, A. Astrocytes in Heavy Metal Neurotoxicity and Neurodegeneration. Brain Res. 2021, 1752, 147234.
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public. Health 2020, 17, 679.
- Babić Leko, M.; Langer Horvat, L.; Španić Popovački, E.; Zubčić, K.; Hof, P.R.; Šimić, G. Metals in Alzheimer’s Disease. Biomedicines 2023, 11, 1161.
- Brewer, G.J.; Kanzer, S.H.; Zimmerman, E.A.; Molho, E.S.; Celmins, D.F.; Heckman, S.M.; Dick, R. Subclinical Zinc Deficiency in Alzheimer’s Disease and Parkinson’s Disease. Am. J. Alzheimers Dis. Dementiasr 2010, 25, 572–575.
- Ahmed, S.S.S.J.; Santosh, W. Metallomic Profiling and Linkage Map Analysis of Early Parkinson’s Disease: A New Insight to Aluminum Marker for the Possible Diagnosis. PLoS ONE 2010, 5, e11252.
- Giacoppo, S.; Galuppo, M.; Calabrò, R.S.; D’Aleo, G.; Marra, A.; Sessa, E.; Bua, D.G.; Potortì, A.G.; Dugo, G.; Bramanti, P.; et al. Heavy Metals and Neurodegenerative Diseases: An Observational Study. Biol. Trace Elem. Res. 2014, 161, 151–160.
- Zhao, H.-W.; Lin, J.; Wang, X.-B.; Cheng, X.; Wang, J.-Y.; Hu, B.-L.; Zhang, Y.; Zhang, X.; Zhu, J.-H. Assessing Plasma Levels of Selenium, Copper, Iron and Zinc in Patients of Parkinson’s Disease. PLoS ONE 2013, 8, e83060.
- Yadav, J.; Verma, A.K.; Ahmad, M.K.; Garg, R.K.; Shiuli; Mahdi, A.A.; Srivastava, S. Metals Toxicity and Its Correlation with the Gene Expression in Alzheimer’s Disease. Mol. Biol. Rep. 2021, 48, 3245–3252.
- Paglia, G.; Miedico, O.; Cristofano, A.; Vitale, M.; Angiolillo, A.; Chiaravalle, A.E.; Corso, G.; Di Costanzo, A. Distinctive Pattern of Serum Elements During the Progression of Alzheimer’s Disease. Sci. Rep. 2016, 6, 22769.
- Wang, Z.-X.; Tan, L.; Wang, H.-F.; Ma, J.; Liu, J.; Tan, M.-S.; Sun, J.-H.; Zhu, X.-C.; Jiang, T.; Yu, J.-T. Serum Iron, Zinc, and Copper Levels in Patients with Alzheimer’s Disease: A Replication Study and Meta-Analyses. J. Alzheimers Dis. 2015, 47, 565–581.
- Koç, E.R.; Ilhan, A.; Zübeyde Aytürk, A.; Acar, B.; Gürler, M.; Altuntaş, A.; Karapirli, M.; Bodur, A.S. A Comparison of Hair and Serum Trace Elements in Patients with Alzheimer Disease and Healthy Participants. Turk. J. Med. Sci. 2015, 45, 1034–1039.
- Rembach, A.; Doecke, J.D.; Roberts, B.R.; Watt, A.D.; Faux, N.G.; Volitakis, I.; Pertile, K.K.; Rumble, R.L.; Trounson, B.O.; Fowler, C.J.; et al. Longitudinal Analysis of Serum Copper and Ceruloplasmin in Alzheimer’s Disease. J. Alzheimers Dis. 2013, 34, 171–182.
- Alsadany, M.A.; Shehata, H.H.; Mohamad, M.I.; Mahfouz, R.G. Histone Deacetylases Enzyme, Copper, and IL-8 Levels in Patients With Alzheimer’s Disease. Am. J. Alzheimers Dis. Other Demen. 2013, 28, 54–61.
- Mariani, S.; Ventriglia, M.; Simonelli, I.; Donno, S.; Bucossi, S.; Vernieri, F.; Melgari, J.-M.; Pasqualetti, P.; Rossini, P.M.; Squitti, R. Fe and Cu Do Not Differ in Parkinson’s Disease: A Replication Study plus Meta-Analysis. Neurobiol. Aging 2013, 34, 632–633.
- Crespo, Â.C.; Silva, B.; Marques, L.; Marcelino, E.; Maruta, C.; Costa, S.; Timóteo, Â.; Vilares, A.; Couto, F.S.; Faustino, P.; et al. Genetic and Biochemical Markers in Patients with Alzheimer’s Disease Support a Concerted Systemic Iron Homeostasis Dysregulation. Neurobiol. Aging 2014, 35, 777–785.
- Fukushima, T.; Tan, X.; Luo, Y.; Kanda, H. Relationship between Blood Levels of Heavy Metals and Parkinson’s Disease in China. Neuroepidemiology 2010, 34, 18–24.
- Dexter, D.T.; Carayon, A.; Javoy-Agid, F.; Agid, Y.; Wells, F.R.; Daniel, S.E.; Lees, A.J.; Jenner, P.; Marsden, C.D. Alterations in the Levels of Iron, Ferritin and Other Trace Metals in Parkinson’s Disease and Other Neurodegenerative Diseases Affecting the Basal Ganglia. Brain 1991, 114, 1953–1975.
- Babić Leko, M.; Jurasović, J.; Nikolac Perković, M.; Španić, E.; Sekovanić, A.; Orct, T.; Lukinović Škudar, V.; Bačić Baronica, K.; Kiđemet-Piskač, S.; Vogrinc, Ž.; et al. The Association of Essential Metals with APOE Genotype in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82, 661–672.
- Hozumi, I.; Hasegawa, T.; Honda, A.; Ozawa, K.; Hayashi, Y.; Hashimoto, K.; Yamada, M.; Koumura, A.; Sakurai, T.; Kimura, A.; et al. Patterns of Levels of Biological Metals in CSF Differ among Neurodegenerative Diseases. J. Neurol. Sci. 2011, 303, 95–99.
- Squitti, R.; Ventriglia, M.; Simonelli, I.; Bonvicini, C.; Costa, A.; Perini, G.; Binetti, G.; Benussi, L.; Ghidoni, R.; Koch, G.; et al. Copper Imbalance in Alzheimer’s Disease: Meta-Analysis of Serum, Plasma, and Brain Specimens, and Replication Study Evaluating ATP7B Gene Variants. Biomolecules 2021, 11, 960.
- Miller, L.M.; Wang, Q.; Telivala, T.P.; Smith, R.J.; Lanzirotti, A.; Miklossy, J. Synchrotron-Based Infrared and X-Ray Imaging Shows Focalized Accumulation of Cu and Zn Co-Localized with β-Amyloid Deposits in Alzheimer’s Disease. J. Struct. Biol. 2006, 155, 30–37.
- Dong, J.; Atwood, C.S.; Anderson, V.E.; Siedlak, S.L.; Smith, M.A.; Perry, G.; Carey, P.R. Metal Binding and Oxidation of Amyloid-Beta within Isolated Senile Plaque Cores: Raman Microscopic Evidence. Biochemistry 2003, 42, 2768–2773.
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, Iron and Zinc in Alzheimer’s Disease Senile Plaques. J. Neurol. Sci. 1998, 158, 47–52.
- Smith, M.A.; Harris, P.L.R.; Sayre, L.M.; Perry, G. Iron Accumulation in Alzheimer Disease Is a Source of Redox-Generated Free Radicals. Proc. Natl. Acad. Sci. USA 1997, 94, 9866–9868.
- Liu, B.; Moloney, A.; Meehan, S.; Morris, K.; Thomas, S.E.; Serpell, L.C.; Hider, R.; Marciniak, S.J.; Lomas, D.A.; Crowther, D.C. Iron Promotes the Toxicity of Amyloid β Peptide by Impeding Its Ordered Aggregation. J. Biol. Chem. 2011, 286, 4248–4256.
- Sóvágó, I.; Várnagy, K.; Kállay, C.; Grenács, Á. Interactions of Copper(II) and Zinc(II) Ions with the Peptide Fragments of Proteins Related to Neurodegenerative Disorders: Similarities and Differences. Curr. Med. Chem. 2023, 30, 4050–4071.
- Nath, A.K.; Dey, S.G. Simultaneous Binding of Heme and Cu with Amyloid β Peptides: Active Site and Reactivities. Dalton Trans. 2022, 51, 4986–4999.
- Stefaniak, E.; Bal, W. CuII Binding Properties of N-Truncated Aβ Peptides: In Search of Biological Function. Inorg. Chem. 2019, 58, 13561–13577.
- Arena, G.; Rizzarelli, E. Zn2+ Interaction with Amyloid-Β: Affinity and Speciation. Mol. Basel Switz. 2019, 24, 2796.
- Atrián-Blasco, E.; Conte-Daban, A.; Hureau, C. Mutual Interference of Cu and Zn Ions in Alzheimer’s Disease: Perspectives at the Molecular Level. Dalton Trans. 2017, 46, 12750–12759.
- Wärmländer, S.K.T.S.; Österlund, N.; Wallin, C.; Wu, J.; Luo, J.; Tiiman, A.; Jarvet, J.; Gräslund, A. Metal Binding to the Amyloid-β Peptides in the Presence of Biomembranes: Potential Mechanisms of Cell Toxicity. J. Biol. Inorg. Chem. JBIC Publ. Soc. Biol. Inorg. Chem. 2019, 24, 1189–1196.
- De Gregorio, G.; Biasotto, F.; Hecel, A.; Luczkowski, M.; Kozlowski, H.; Valensin, D. Structural Analysis of Copper(I) Interaction with Amyloid β Peptide. J. Inorg. Biochem. 2019, 195, 31–38.
- Trapani, G.; Satriano, C.; La Mendola, D. Peptides and Their Metal Complexes in Neurodegenerative Diseases: From Structural Studies to Nanomedicine Prospects. Curr. Med. Chem. 2018, 25, 715–747.
- Ahmadi, S.; Zhu, S.; Sharma, R.; Wilson, D.J.; Kraatz, H.-B. Interaction of Metal Ions with Tau Protein. The Case for a Metal-Mediated Tau Aggregation. J. Inorg. Biochem. 2019, 194, 44–51.
- Binolfi, A.; Quintanar, L.; Bertoncini, C.W.; Griesinger, C.; Fernández, C.O. Bioinorganic Chemistry of Copper Coordination to Alpha-Synuclein: Relevance to Parkinson’s Disease. Coord. Chem. Rev. 2012, 256, 2188–2201.
- Valensin, D.; Dell’Acqua, S.; Kozlowski, H.; Casella, L. Coordination and Redox Properties of Copper Interaction with α-Synuclein. J. Inorg. Biochem. 2016, 163, 292–300.
- González, N.; Arcos-López, T.; König, A.; Quintanar, L.; Menacho Márquez, M.; Outeiro, T.F.; Fernández, C.O. Effects of Alpha-Synuclein Post-Translational Modifications on Metal Binding. J. Neurochem. 2019, 150, 507–521.
- Atrián-Blasco, E.; Gonzalez, P.; Santoro, A.; Alies, B.; Faller, P.; Hureau, C. Cu and Zn Coordination to Amyloid Peptides: From Fascinating Chemistry to Debated Pathological Relevance. Coord. Chem. Rev. 2018, 375, 38–55.
- Leal, S.S.; Botelho, H.M.; Gomes, C.M. Metal Ions as Modulators of Protein Conformation and Misfolding in Neurodegeneration. Coord. Chem. Rev. 2012, 256, 2253–2270.
- Gamez, P.; Caballero, A.B. Copper in Alzheimer’s Disease: Implications in Amyloid Aggregation and Neurotoxicity. AIP Adv. 2015, 5, 092503.
- Faller, P.; Hureau, C.; La Penna, G. Metal Ions and Intrinsically Disordered Proteins and Peptides: From Cu/Zn Amyloid-β to General Principles. Acc. Chem. Res. 2014, 47, 2252–2259.
- DeToma, A.S.; Salamekh, S.; Ramamoorthy, A.; Lim, M.H. Misfolded Proteins in Alzheimer’s Disease and Type II Diabetes. Chem. Soc. Rev. 2012, 41, 608–621.
- Ke, P.C.; Sani, M.-A.; Ding, F.; Kakinen, A.; Javed, I.; Separovic, F.; Davis, T.P.; Mezzenga, R. Implications of Peptide Assemblies in Amyloid Diseases. Chem. Soc. Rev. 2017, 46, 6492–6531.
- Viles, J.H. Metal Ions and Amyloid Fiber Formation in Neurodegenerative Diseases. Copper, Zinc and Iron in Alzheimer’s, Parkinson’s and Prion Diseases. Coord. Chem. Rev. 2012, 256, 2271–2284.
- Miller, Y.; Ma, B.; Nussinov, R. Zinc Ions Promote Alzheimer Abeta Aggregation via Population Shift of Polymorphic States. Proc. Natl. Acad. Sci. USA 2010, 107, 9490–9495.
- Sharma, A.K.; Pavlova, S.T.; Kim, J.; Kim, J.; Mirica, L.M. The Effect of Cu2+ and Zn2+ on the Aβ42 Peptide Aggregation and Cellular Toxicity. Metallomics 2013, 5, 1529–1536.
- Bush, A.I.; Pettingell, W.H.; Multhaup, G.; d Paradis, M.; Vonsattel, J.P.; Gusella, J.F.; Beyreuther, K.; Masters, C.L.; Tanzi, R.E. Rapid Induction of Alzheimer A Beta Amyloid Formation by Zinc. Science 1994, 265, 1464–1467.
- Carboni, E.; Lingor, P. Insights on the Interaction of Alpha-Synuclein and Metals in the Pathophysiology of Parkinson’s Disease. Metallomics 2015, 7, 395–404.
- Drew, S.C. The N Terminus of α-Synuclein Forms CuII-Bridged Oligomers. Chem. Eur. J. 2015, 21, 7111–7118.
- Li, W.-J.; Jiang, H.; Song, N.; Xie, J.-X. Dose- and Time-Dependent α-Synuclein Aggregation Induced by Ferric Iron in SK-N-SH Cells. Neurosci. Bull. 2010, 26, 205–210.
- Rasia, R.M.; Bertoncini, C.W.; Marsh, D.; Hoyer, W.; Cherny, D.; Zweckstetter, M.; Griesinger, C.; Jovin, T.M.; Fernández, C.O. Structural Characterization of Copper(II) Binding to α-Synuclein: Insights into the Bioinorganic Chemistry of Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2005, 102, 4294–4299.
- Li, X.; Du, X.; Ni, J. Zn2+ Aggravates Tau Aggregation and Neurotoxicity. Int. J. Mol. Sci. 2019, 20, 487.
- Zubčić, K.; Hof, P.R.; Šimić, G.; Jazvinšćak Jembrek, M. The Role of Copper in Tau-Related Pathology in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 572308.
- Ahmadi, S.; Wu, B.; Song, R.; Zhu, S.; Simpson, A.; Wilson, D.J.; Kraatz, H.-B. Exploring the Interactions of Iron and Zinc with the Microtubule Binding Repeats R1 and R4. J. Inorg. Biochem. 2020, 205, 110987.
- Soragni, A.; Zambelli, B.; Mukrasch, M.D.; Biernat, J.; Jeganathan, S.; Griesinger, C.; Ciurli, S.; Mandelkow, E.; Zweckstetter, M. Structural Characterization of Binding of Cu(II) to Tau Protein. Biochemistry 2008, 47, 10841–10851.
- Balogh, B.D.; Szakács, B.; Di Natale, G.; Tabbì, G.; Pappalardo, G.; Sóvágó, I.; Várnagy, K. Copper (II) Binding Properties of an Octapeptide Fragment from the R3 Region of Tau Protein: A Combined Potentiometric, Spectroscopic and Mass Spectrometric Study. J. Inorg. Biochem. 2021, 217, 111358.
- Bacchella, C.; Gentili, S.; Bellotti, D.; Quartieri, E.; Draghi, S.; Baratto, M.C.; Remelli, M.; Valensin, D.; Monzani, E.; Nicolis, S.; et al. Binding and Reactivity of Copper to R1 and R3 Fragments of Tau Protein. Inorg. Chem. 2020, 59, 274–286.
- Jing, J.; Tu, G.; Yu, H.; Huang, R.; Ming, X.; Zhan, H.; Zhan, F.; Xue, W. Copper (Cu2+) Ion-Induced Misfolding of Tau Protein R3 Peptide Revealed by Enhanced Molecular Dynamics Simulation. Phys. Chem. Chem. Phys. 2021, 23, 11717–11726.
- Rivers-Auty, J.; Tapia, V.S.; White, C.S.; Daniels, M.J.D.; Drinkall, S.; Kennedy, P.T.; Spence, H.G.; Yu, S.; Green, J.P.; Hoyle, C.; et al. Zinc Status Alters Alzheimer’s Disease Progression through NLRP3-Dependent Inflammation. J. Neurosci. 2021, 41, 3025–3038.
- Singh, I.; Sagare, A.P.; Coma, M.; Perlmutter, D.; Gelein, R.; Bell, R.D.; Deane, R.J.; Zhong, E.; Parisi, M.; Ciszewski, J.; et al. Low Levels of Copper Disrupt Brain Amyloid-β Homeostasis by Altering Its Production and Clearance. Proc. Natl. Acad. Sci. USA 2013, 110, 14771–14776.
- Sparks, D.L.; Schreurs, B.G. Trace Amounts of Copper in Water Induce β-Amyloid Plaques and Learning Deficits in a Rabbit Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2003, 100, 11065–11069.
- Prasanthi, J.R.P.; Schrag, M.; Dasari, B.; Marwarha, G.; Dickson, A.; Kirsch, W.M.; Ghribi, O. Deferiprone Reduces Amyloid-β and Tau Phosphorylation Levels but Not Reactive Oxygen Species Generation in Hippocampus of Rabbits Fed a Cholesterol-Enriched Diet. J. Alzheimers Dis. 2012, 30, 167–182.
- Chen, P.; Chakraborty, S.; Mukhopadhyay, S.; Lee, E.; Paoliello, M.M.B.; Bowman, A.B.; Aschner, M. Manganese Homeostasis in the Nervous System. J. Neurochem. 2015, 134, 601–610.
- Shen, X.; Liu, J.; Fujita, Y.; Liu, S.; Maeda, T.; Kikuchi, K.; Obara, T.; Takebe, A.; Sayama, R.; Takahashi, T.; et al. Iron Treatment Inhibits Aβ42 Deposition in Vivo and Reduces Aβ42/Aβ40 Ratio. Biochem. Biophys. Res. Commun. 2019, 512, 653–658.
- Alimonti, A.; Ristori, G.; Giubilei, F.; Stazi, M.A.; Pino, A.; Visconti, A.; Brescianini, S.; Monti, M.S.; Forte, G.; Stanzione, P.; et al. Serum Chemical Elements and Oxidative Status in Alzheimer’s Disease, Parkinson Disease and Multiple Sclerosis. NeuroToxicology 2007, 28, 450–456.
- Guilarte, T.R. Manganese Neurotoxicity: New Perspectives from Behavioral, Neuroimaging, and Neuropathological Studies in Humans and Non-Human Primates. Front. Aging Neurosci. 2013, 5, 23.
- Hirsch, E.C.; Brandel, J.-P.; Galle, P.; Javoy-Agid, F.; Agid, Y. Iron and Aluminum Increase in the Substantia Nigra of Patients with Parkinson’s Disease: An X-Ray Microanalysis. J. Neurochem. 1991, 56, 446–451.
- Altschuler, E. Aluminum-Containing Antacids as a Cause of Idiopathic Parkinson’s Disease. Med. Hypotheses 1999, 53, 22–23.
- Zatta, P.; Zambenedetti, P.; Milanese, M. Activation of Monoamine Oxidase Type-B by Aluminum in Rat Brain Homogenate. NeuroReport 1999, 10, 3645–3648.
- Baum, L.; Chan, I.H.S.; Cheung, S.K.-K.; Goggins, W.B.; Mok, V.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; Woo, J.; et al. Serum Zinc Is Decreased in Alzheimer’s Disease and Serum Arsenic Correlates Positively with Cognitive Ability. BioMetals 2010, 23, 173–179.
- Bhattacharjee, S.; Zhao, Y.; Hill, J.M.; Culicchia, F.; Kruck, T.P.A.; Percy, M.E.; Pogue, A.I.; Walton, J.R.; Lukiw, W.J. Selective Accumulation of Aluminum in Cerebral Arteries in Alzheimer’s Disease (AD). J. Inorg. Biochem. 2013, 126, 35–37.
- González-Domínguez, R.; García-Barrera, T.; Gómez-Ariza, J.L. Characterization of Metal Profiles in Serum during the Progression of Alzheimer’s Disease. Metallomics 2014, 6, 292–300.
- Smorgon, C.; Mari, E.; Atti, A.R.; Dalla Nora, E.; Zamboni, P.F.; Calzoni, F.; Passaro, A.; Fellin, R. Trace Elements and Cognitive Impairment: An Elderly Cohort Study. Arch. Gerontol. Geriatr. 2004, 38, 393–402.
- Du, K.; Liu, M.; Pan, Y.; Zhong, X.; Wei, M. Association of Serum Manganese Levels with Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 231.
- Koseoglu, E.; Koseoglu, R.; Kendirci, M.; Saraymen, R.; Saraymen, B. Trace Metal Concentrations in Hair and Nails from Alzheimer’s Disease Patients: Relations with Clinical Severity. J. Trace Elem. Med. Biol. 2017, 39, 124–128.
- Szabo, S.T.; Harry, G.J.; Hayden, K.M.; Szabo, D.T.; Birnbaum, L. Comparison of Metal Levels between Postmortem Brain and Ventricular Fluid in Alzheimer’s Disease and Nondemented Elderly Controls. Toxicol. Sci. 2016, 150, 292–300.
- Gorantla, N.V.; Das, R.; Balaraman, E.; Chinnathambi, S. Transition Metal Nickel Prevents Tau Aggregation in Alzheimer’s Disease. Int. J. Biol. Macromol. 2020, 156, 1359–1365.
- Tong, Y.; Yang, H.; Tian, X.; Wang, H.; Zhou, T.; Zhang, S.; Yu, J.; Zhang, T.; Fan, D.; Guo, X.; et al. High Manganese, A Risk for Alzheimer’s Disease: High Manganese Induces Amyloid-β Related Cognitive Impairment. J. Alzheimers Dis. 2014, 42, 865–878.
- Walton, J.R.; Wang, M.-X. APP Expression, Distribution and Accumulation Are Altered by Aluminum in a Rodent Model for Alzheimer’s Disease. J. Inorg. Biochem. 2009, 103, 1548–1554.
- Bouras, C.; Giannakopoulos, P.; Good, P.F.; Hsu, A.; Hof, P.R.; Perl, D.P. A Laser Microprobe Mass Analysis of Brain Aluminum and Iron in Dementia Pugilistica: Comparison with Alzheimer’s Disease. Eur. Neurol. 2007, 38, 53–58.
- Rondeau, V.; Jacqmin-Gadda, H.; Commenges, D.; Helmer, C.; Dartigues, J.-F. Aluminum and Silica in Drinking Water and the Risk of Alzheimer’s Disease or Cognitive Decline: Findings from 15-Year Follow-up of the PAQUID Cohort. Am. J. Epidemiol. 2009, 169, 489–496.
- Zhang, Q.L.; Jia, L.; Jiao, X.; Guo, W.L.; Ji, J.W.; Yang, H.L.; Niu, Q. APP/PS1 Transgenic Mice Treated with Aluminum: An Update of Alzheimer’s Disease Model. Int. J. Immunopathol. Pharmacol. 2012, 25, 49–58.
