Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Di Chen | -- | 2416 | 2023-07-27 08:17:15 | | | |
| 2 | Conner Chen | Meta information modification | 2416 | 2023-07-31 03:16:45 | | | | |
| 3 | Conner Chen | Meta information modification | 2416 | 2023-07-31 03:16:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Qi, M.; Li, Y.; Zhu, Z.; Du, B.; Chen, D. Sample Preparation Methods of Edible Oils. Encyclopedia. Available online: https://encyclopedia.pub/entry/47342 (accessed on 07 February 2026).
Qi M, Li Y, Zhu Z, Du B, Chen D. Sample Preparation Methods of Edible Oils. Encyclopedia. Available at: https://encyclopedia.pub/entry/47342. Accessed February 07, 2026.
Qi, Menghui, Yanyan Li, Zheng Zhu, Bin Du, Di Chen. "Sample Preparation Methods of Edible Oils" Encyclopedia, https://encyclopedia.pub/entry/47342 (accessed February 07, 2026).
Qi, M., Li, Y., Zhu, Z., Du, B., & Chen, D. (2023, July 27). Sample Preparation Methods of Edible Oils. In Encyclopedia. https://encyclopedia.pub/entry/47342
Qi, Menghui, et al. "Sample Preparation Methods of Edible Oils." Encyclopedia. Web. 27 July, 2023.
Copy Citation
The matrix of edible oils is complex, and it is necessary to perform efficient sample preparation to extract the target components before detection. Sample preparation generally includes steps such as sample collection, extraction, purification, and concentration.
edible oils
phthalic acid esters
sample preparation methods
1. Sample Preparation Methods
The matrix of edible oils is complex, and it is necessary to perform efficient sample preparation to extract the target components before detection [1][2][3]. Sample preparation generally includes steps such as sample collection, extraction, purification, and concentration. At present, the sample preparation methods for detecting PAEs in edible oils mainly include liquid-based extraction techniques [4][5][6][7], gel permeation chromatography (GPC) [8][9], sorptive-based extraction techniques [10][11][12], and QuEChERS (quick, easy, cheap, effective, rugged, and safe) [13][14][15].
2. Liquid-Based Extraction Techniques
Liquid-liquid extraction (LLE) is a traditional method for sample preparation. It transfers target analytes from the sample solution to the extractant based on the different distribution coefficients of the target analytes in two immiscible or slightly soluble solvents. For the extraction of PAEs from edible oils, the commonly used extraction solvents are mainly nonpolar solvents such as n-hexane, isooctane, and dichloromethane. Table 1 summarizes the methods for analyzing PAEs in edible oils by LLE combined with appropriate determination techniques.
Table 1. Applications of LLE-based methods for extracting PAEs in edible oil samples.
| Sample Amount | Analytes | Solvent Usage | Sample Preparation | Detection | LODs | Ref. |
|---|---|---|---|---|---|---|
| 0.1 g | 12 PAEs (DMP etc.) | Extraction solvent: 900 µL of n-hexane saturated acetonitrile | LLE | LC-HRMS | 0.02–0.35 mg/kg | [4] |
| 0.5 g | 17 PAEs (DMP etc.) | Extraction solvent: 40 mL of hexane-saturated acetonitrile | LLE | GC-MS/MS | 0.1–4.0 µg/kg | [7] |
| 2 g | 10 PAEs (DMP etc.) | Extraction solvent: 5 mL of acetonitrile | LLE | LC-MS/MS | 1–9 µg/L | [5] |
| 50 g | DEP | Extraction solvent: 75 mL of a mixture of hexane and acetone (1:1, v/v) | LLE | GNP-rt-IPCR | 1.06 pg/L | [6] |
| 1 g | PA (hydrolysate of total PAEs) | Extraction solvent: 600 µL of TBP | LPME | LC-MS/MS | 1.0 µmol/kg | [16] |
| 5 mL | o-PA, m-PA, p-PA | Extraction solvent: 34 µL of ammoniacal buffer | AA-LPME | LC-DAD | 0.11–0.29 ng/mL | [17] |
| 0.5 mL | 5 PAEs (DMP etc.) | For AALLE, extraction solvent: 1 mL of DMSO;For DLLME, dispersive solvent: DMSO (from AALLE); extraction solvent: 0.45 mL of chloroform | AALLE and DLLME | GC-FID/GC-MS | 0.007–0.023 µg/L | [18] |
AA-LPME: air-assisted liquid-phase microextraction; DAD: diode array detector; DLLME: dispersed liquid-liquid microextraction; DMP: dimethyl phthalate; DMSO: dimethyl sulfoxide; DEP: diethyl phthalate; FID: flame ionization detector; GC: gas chromatography; GNP-rt-IPCR: gold nanoparticles improved real-time immuno-Polymerase Chain Reaction; HRMS: high-resolution mass spectrometry; MS: mass spectrum; PA: phthalic acid; TBP: Tributyl phosphate.
LLE is simple to perform and does not require expensive instruments, making it widely used in the detection of PAEs [4][5][6][7]. However, traditional LLE is not environmentally friendly because it often results in the formation of emulsions and consumes large volumes of organic solvents during the extraction process. Zhou et al. used hexane-saturated acetonitrile (ACN) to extract PAEs from edible oils. The entire extraction process took more than 15 min, with a high consumption of over 40 mL of hexane-saturated acetonitrile and approximately 0.5 g of vegetable oils [7].
With the development of the new concept of green chemistry, the advantages of microextraction technology have become prominent [19]. Compared with traditional extraction methods, microextraction technology consumes lower amounts of organic solvents and is more environmentally friendly [20][21][22]. Liquid-phase microextraction (LPME) is a miniaturized extraction technology developed on the basis of LLE. Since the introduction of LPME in 1996 [23], this technology has been widely used in the analysis of various samples as a new approach for sample preparation [17][24][25]. In recent years, various modes have been derived from LPME, among which dispersed liquid–liquid microextraction (DLLME) has been widely used for the extraction of PAEs in edible oils. The DLLME system is a ternary solvent system composed of samples, dispersants, and extractants. During DLLME, a mixture of extractant and dispersant is quickly injected into the sample solution through a syringe. Under the action of the dispersant, the extractant that was originally immiscible with the sample solution forms small droplets. The droplets are dispersed in the extractant, thus increasing the contact area between the extraction phase and the sample, shortening the extraction time. Currently, these extraction technologies are often combined with auxiliary means, such as ultrasonic-assisted extraction [26] and air-assisted extraction [17][18][25][26], for the extraction of PAEs in edible oils. These auxiliary methods can further reduce the consumption of organic solvents and effectively improve the extraction efficiency. Khoshmaram et al. used air-assisted liquid–liquid extraction (AA-LLE) coupled with DLLME for the extraction and preconcentration of some PAEs from edible oils prior to their detection by gas chromatography (GC). The entire extraction process only required about 10 min, with the low consumption of 1 mL of dimethyl sulfoxide and 0.45 mL of chloroform as the extractant and 0.5 mL of edible oils [18].
Extraction technologies based on traditional LLE generally use organic solvents as extractants, which are harmful to the operator and the environment. Farajzadeh et al. used an alkaline aqueous solution instead of organic solvents and proposed an organic-solvent-free AA-LPME method to extract and preconcentrate phthalic acid residues from edible oil samples. They combined this technology with liquid chromatography (LC)-diode array detector (DAD) to detect three types of phthalic acids (o-phthalic acid, m-phthalic acid, and p-phthalic acid) in edible oil samples, and the method showed low limits of detection (LODs) of 0.11–0.29 ng/mL and high extraction recoveries of 81–97%. Moreover, they compared this method with other dispersive methods, including ultrasound-assisted dispersive liquid–liquid microextraction (USA-DLLME), manual shaking-liquid-phase microextraction (MSh-LPME), and vortex-assisted liquid–liquid microextraction (VA-LLME). Under the same conditions, the amounts of time required by USA-DLLME, MSh-LPME, and VA-LLME were 12, 5, and 4 min, respectively, with the consumption of 5 mL of oil samples and 34 µL of the ammoniacal buffer. At the same time, the LODs for all the analytes were improved when using the proposed method compared with those of other methods [17]. This demonstrates that the organic-solvent-free AA-LPME method proposed above has the advantages of being more rapid, efficient, and sensitive. Most importantly, the application of an aqueous extractive phase instead of organic extraction solvents makes traditional LLE technology more environmentally friendly. Given that phthalic acid is the main hydrolysate of PAEs, it will be a good choice to adopt the method proposed above to indirectly determine the contents of PAEs in edible oils.
In addition, there are various PAE plasticizers in edible oils, some of which are still unknown due to the lack of corresponding standard compounds. Therefore, establishing a suitable method to determine all of the PAEs in edible oils is challenging. Liu et al. [16] and Xie et al. [26] used phase transfer catalyst to accelerate the oil/water biphasic base hydrolysis of PAEs; PAEs were hydrolyzed into phthalic acid, and the total contents of PAEs were indirectly measured by determining phthalic acid (Figure 2). This method provided new ideas for measuring the total contents of PAEs in edible oils.
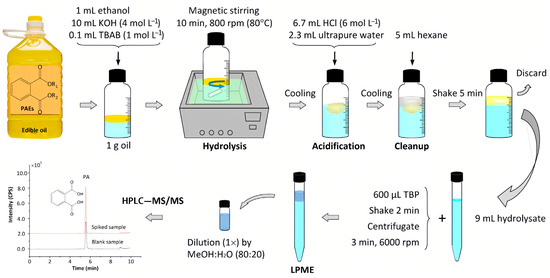
Figure 1. Schematic phase-transfer catalyst-assisted hydrolysis-LPME-LC-MS/MS procedure [16].
3. Gel Permeation Chromatography
GPC uses a porous gel as the stationary phase. Based on the spatial size effect of the gel pores, molecules of different sizes elute from large to small in order, thus achieving the goal of separating the target analyte. GPC has the characteristics of high purification efficiency, reusability, wide applicability, and a high degree of automation. GPC is suitable for separating substances with significant differences in molecular size. The matrix of edible oil samples is complex and rich in a large amount of macromolecular interfering substances. Therefore, using GPC to remove macromolecular substances to purify small-molecule PAEs is a good method. In the pretreatment process of edible oil samples, macromolecular substances such as oils and pigments are first leached out, while small-molecule substances such as PAEs are leached out later, thus achieving effective purification.
Li et al. used GPC to extract 15 kinds of PAEs from edible oils with a glass chromatography column filled with a Bio-Beads (S-X3) filler. Then, the contents of the extracted solution were detected by GC-mass spectrometry (MS). The experimental results showed that the LODs of the 15 kinds of PAEs were between 0.001 and 2.000 µg/L, the average standard addition recoveries of three concentrations were in the range of 70.50–112.00%, and the average deviations (n = 6) were in the range of 1.59–7.54%. This indicated that GPC had a good purification ability for oil samples [8]. The application of the fully automatic purification and concentration of the GPC system greatly simplified the extraction process, enabling unattended and reliable operation [27]. However, GPC also has many shortcomings. In 2018, Li et al. found that in sesame oil, PAEs had a significant overlap with the matrix, and the interference of the matrix after the GPC approach was difficult to compensate for [9]. Moreover, conventional GPC technology consumes large volumes of solvents and has high costs. The GPC system itself has a PAE background value, which makes the quantification of trace PAEs difficult to achieve [9]. Therefore, in recent years, for the determination of PAEs in edible oils, GPC technology has gradually been replaced by other technologies, such as solid-phase extraction (SPE) and LLE.
4. Sorptive-Based Extraction Techniques
SPE is by far one of the most common sample preparation methods for the determination of PAEs [28]. When a complex sample solution passes through the extraction column, the sorbent will selectively retain the target substance and some of the interferents through polar, hydrophobic, or ion exchange interactions. Next, the extraction column is washed to remove interferents adsorbed by the sorbent, followed by choosing another solvent to elute the target analyte, thus achieving the purpose of separating, purifying, and enriching the target compound [29][30]. Compared with traditional LLE method, SPE has the advantages of avoiding emulsification phenomena, being easy to automate, and achieving efficient purification [10][11][12]. There have been many new reports on the extraction of PAEs in edible oils using SPE combined with various measurement methods.
The development of SPE technology mainly depends on the innovation of the sorbents. For the extraction and purification of PAEs in edible oils, traditional sorbents for SPE mainly include C18 [31], primary secondary amine (PSA) [28][32][33], and Florisil® [34][35]. However, conventional SPE sorbents exhibit low adsorption and selectivity. Chen et al. invented novel molecularly imprinted polymers of PAEs by atom transfer radical polymerization to replace traditional SPE sorbents. They successfully detected 10 PAEs in edible oils using molecularly imprinted solid-phase extraction (MISPE) combined with GC and a flame ionization detector (FID). The LODs of this method were 0.10–0.25 μg/mL, and the recoveries of the spiked samples were 82.5–101.4%. The extraction effectiveness of MISPE for PAEs was compared with that of commercial SPE columns, and the results indicated that the performance of MISPE was better than those of C18-SPE, PSA-SPE, PAE-SPE, and silica SPE under optimized extraction conditions [36].
Solid-phase microextraction (SPME) pretreatment method was developed on the basis of SPE. It is a new sample pretreatment technology that integrates sampling, extraction, enrichment, and injection into one step [37]. In SPME, the sorbent is coated onto a matrix material such as quartz fibers to extract, enrich, and concentrate the target compounds. Once the extraction process is finished, the fibers undergo desorption using either thermal desorption or liquid desorption methods. Subsequently, the target compounds are detected [38]. The entire process is easy to perform, time-saving, and solvent-free [39]. In SPME, the performance of the coating materials is the most critical factor in improving the extraction efficiency [40]. Therefore, similar to SPE, the development of SPME technology also mainly depends on the innovation of the coating materials. To date, various coating materials have been developed for SPME to extract PAEs from edible oils, such as metal-organic framework deep eutectic solvent/molecularly imprinted polymers (MOF-DES/MIPs) [41], polydimethylsiloxane/divinylbenzene (PDMS/DVB) fiber and polyethylene glycol (PEG) fiber [42], materials institute lavoisier-88(Fe)/graphene oxide (MIL-88(Fe)/GO)-coated fibers [43], graphene/polyvinylchloride (G/PVC) nanocomposite [44], and divinylbenzene–carboxen–polydimethylsiloxane (DVB/CAR/PDMS) [45].
Magnetic solid-phase extraction (MSPE), which uses magnetic materials as sorbents and utilizes an external magnetic field to conveniently and quickly separate the adsorbent and analyte from the solution during the purification process, has also received great interest for achieving separation operation more easily [46]. Zhao et al. fabricated new magnetic covalent organic framework nanospheres named Fe3O4@covalent organic framework (1,3,5-triformylbenzene-benzidine) (Fe3O4@COF(TbBD)) as the magnetic sorbent and combined them with LC-DAD to achieve the extraction and detection of seven PAEs in edible vegetable oils (Figure 2). Under optimal conditions, the proposed method possessed a high sensitivity with LODs of 0.55–0.95 µg/kg and limits of quantification (LOQs) of 1.80–3.10 µg/kg, as well as satisfactory recoveries of 80.2–102.9% [47].
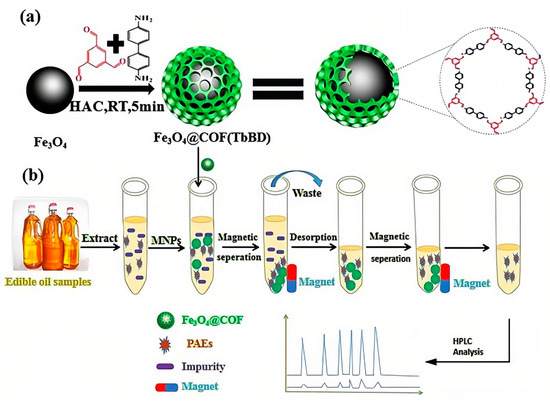
Figure 3. Schematic MSPE-LC-DAD procedure for the determination of PAEs in edible oils: (a) Fabrication process for Fe3O4@COF(TbBD) materials; (b) MSPE procedure for the detection of PAEs [47].
5. QuEChERS
QuEChERS was first proposed by Anastassuades et al. in 2003 [13] and has received significant attention for its advantages such as its simplicity, low solvent consumption, and flexibility. In recent years, QuEChERS has gradually become a new trend for the detection of trace organic matter in foods, with a wide range of applications in the detection of plasticizers. This method consists of two major steps: LLE with ACN (acetonitrile) and subsequent purification by dispersive solid-phase extraction (DSPE). As shown in Figure 3, Gan et al. successfully applied QuEChERS followed by supercritical fluid chromatography (SFC) and UV detector for the qualitative and quantitative analysis of 12 chemical additives (including three plasticizers: BBP, DEHP, and trioctyl trimellitate (TOTM)) in various edible vegetable oils [14]. In detail, they performed LLE with 0.4 g of edible vegetable oils and 4 mL of ACN, followed by salting-out with anhydrous magnesium sulfate. After DSPE with the sorbent, the supernatant was analyzed by SFC. In this process, they used ultracentrifugation to help with the purification.
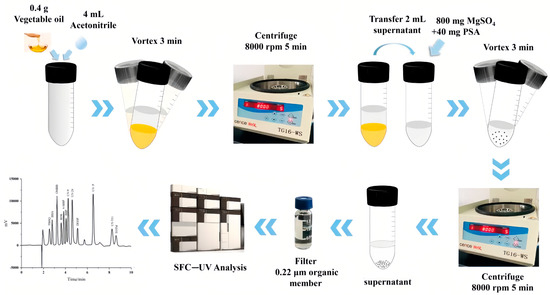
Figure 3. Schematic diagram of the QuEChERS-SFC method for the determination of PAEs in edible oils [14].
In addition to SFC, QuEChERS is usually combined with other detection technologies, such as GC-MS/MS [15][48] and LC-DAD [49], to detect plasticizers in edible oils. All these methods have achieved satisfactory results. Although QuEChERS has been able to meet the extraction requirements for most of the tested samples, further removal of lipids from edible oils for more accurate detection of the target analytes is still of great significance. Sun et al. combined QuEChERS with freezing-lipid precision to further remove the lipids in the edible oil sample during the process of extracting PAEs, thus preventing the lipids from affecting the performance of the instrument [48]. The results showed that the extraction solution without freezing-lipid precipitation was turbid due to the dispersion of lipids in it, while the extraction solution after freezing-lipid precipitation was significantly clearer, and a large amount of lipids was deposited at the bottom of the glass centrifuge bottle. The effective removal of lipids would further improve the accuracy of detection and reduce the pollution of lipids on the instrument. As an emerging technology, QuEChERS will have an increasingly wider application in the pretreatment of edible oil samples.
References
- Zhang, M.; Bu, X.; Xu, X.; Wang, B.; Yang, S.; Luo, Y.; Xu, X.; Chen, D. Miniaturized kapok fiber-supported liquid extraction for convenient extraction of pesticide residues in vegetable oils: Determination of organochlorine pesticides as a proof-of-concept study. Talanta 2023, 253, 123982.
- Wang, H.; Liu, X.; Tu, M.; Xu, X.; Yang, S.; Chen, D. Current sample preparation methods and analytical techniques for the determination of synthetic antioxidants in edible oils. J. Sep. Sci. 2022, 45, 3874–3886.
- Wang, B.; Xu, X.L.; Zhang, M.Y.; Bu, X.M.; Wang, H.L.; Shi, X.Z.; Xu, X.; Chen, D. A fully green sample preparation method for synthetic antioxidants determination in edible oils based on natural feather fiber-supported liquid extraction. J. Chromatogr. A 2023, 1698, 464004.
- Amelio, M.; Gandalinia, M. Proposal for a fast method to determine routinely 15 plasticisers in olive oil by liquid extraction and Ultra High-Performance Liquid Chromatography Heated Electro Spray Ionisation High Resolution Mass Spectrometry (Orbitrap) analysis. Riv. Ital. Sostanze Grasse 2021, 98, 169–175.
- Pardo-Mates, N.; Serrano, F.; Núñez, O. Determination of phthalic acid esters in drinking water and olive oil by ultra-high performance liquid chromatography-electrospray-tandem mass spectrometry: Study of phthalate migration from plastic bottles to drinking water at different domestic exposure conditions. Trends Chromatogr. 2017, 11, 27–48.
- Sun, R.; Zhuang, H. An ultrasensitive gold nanoparticles improved real-time immuno-PCR assay for detecting diethyl phthalate in foodstuff samples. Anal. Biochem. 2015, 480, 49–57.
- Zhou, R.-Z.; Jiang, J.; Mao, T.; Zhao, Y.-S.; Lu, Y. Multiresidue analysis of environmental pollutants in edible vegetable oils by gas chromatography-tandem mass spectrometry. Food Chem. 2016, 207, 43–50.
- Li, L.; Sun, Q.; Xin, S.; Yu, L.; Jiang, Z. Detection of Phthalate Esters from Plastic Packaging Materials into Edible Oil by Gas Chromatography-Mass. Appl. Mech. Mater. 2013, 395, 355–358.
- Li, X.; Zhang, Q.; Chen, L.; Zhao, J.; Li, H. Determination of 16 phthalate esters in sesame oil by isotope dilution liquid chromatography with tandem mass spectrometry. Anal. Methods 2018, 10, 3197–3206.
- Helle, N.; Baden, M.; Petersen, K. Automated solid phase extraction. Methods Mol. Biol. 2011, 747, 93–129.
- Buszewski, B.; Szultka, M. Past, present, and future of solid phase extraction: A review. Crit. Rev. Anal. Chem. 2012, 42, 198–213.
- Żwir-Ferenc, A.; Biziuk, M. Solid Phase Extraction Technique--Trends, Opportunities and Applications. Pol. J. Environ. Stud. 2006, 15, 677–690.
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431.
- Gan, Y.; Zhu, Y. Multi-Residue Analysis of Chemical Additives in Edible Vegetable Oils Using QuEChERS Extraction Method Followed by Supercritical Fluid Chromatography. Molecules 2022, 27, 1681.
- Wang, X.; Sun, X.; Wang, X.; Qi, X.; Wang, D.; Jiang, J.; Mao, J.; Ma, F.; Yu, L.; Zhang, L.; et al. Determination of 15 phthalic acid esters based on GC-MS/MS coupled with modified QuEChERS in edible oils. Food Chem. X 2022, 16, 100520.
- Liu, S.; Liu, L.; Han, Y.; Sun, J.; Feng, J.; Wang, J.; Zhong, C. Rapid screening of edible oils for phthalates using phase-transfer catalyst-assisted hydrolysis and liquid phase microextraction coupled to high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2015, 1420, 26–34.
- Farajzadeh, M.A.; Mogaddam, M.R.A.; Feriduni, B.; Alizadeh, A.A. Determination of migrated phthalic acid residues into edible oils using a green mode of air-assisted liquid–liquid microextraction followed by high-performance liquid chromatography–diode array detector. J. Iran. Chem. Soc. 2016, 14, 551–559.
- Khoshmaram, L.; Abdolmohammad-Zadeh, H.; Ghaffarzadeh, E. Air-assisted liquid-liquid extraction coupled with dispersive liquid-liquid microextraction and a drying step for extraction and preconcentration of some phthalate esters from edible oils prior to their determination by GC. J. Sep. Sci. 2019, 42, 736–743.
- Pereira, J.A.M.; Casado, N.; Porto-Figueira, P.; Camara, J.S. The Potential of Microextraction Techniques for the Analysis of Bioactive Compounds in Food. Front. Nutr. 2022, 9, 825519.
- Chen, D.; Zhang, M.Y.; Bu, X.M.; Wang, B.; Xu, X.L.; Yang, S.; Sun, Z.; Xu, X. In-syringe cotton fiber-supported liquid extraction coupled with gas chromatography-tandem mass spectrometry for the determination of free 3-mono-chloropropane-1,2-diol in edible oils. J. Chromatogr. A 2022, 1673, 463081.
- Chen, D.; Bu, X.; Xu, X.; Wang, B.; Zhang, M.; Gan, Y.; Yuan, H.; Xia, X. In-pipette-tip kapok fiber-supported liquid extraction/in-situ derivatization coupled with high-performance liquid chromatography for conveniently determining three furfurals. Food Chem. 2023, 415, 135788.
- Rutkowska, M.; Plotka-Wasylka, J.; Sajid, M.; Andruch, V. Liquid-phase microextraction: A review of reviews. Microchem. J. 2019, 149, 103989.
- Liu, H.; Dasgupta, P.K. Analytical chemistry in a drop. Solvent extraction in a microdrop. Anal. Chem. 1996, 68, 1817–1821.
- Mehravar, A.; Feizbakhsh, A.; Sarafi, A.H.M.; Konoz, E.; Faraji, H. Deep eutectic solvent-based headspace single-drop microextraction of polycyclic aromatic hydrocarbons in aqueous samples. J. Chromatogr. A 2020, 1632, 461618.
- Santos, A.P.; Korn, M.G.A.; Lemos, V.A. Methods of liquid phase microextraction for the determination of cadmium in environmental samples. Environ. Monit. Assess. 2017, 189, 444.
- Xie, Q.; Sun, D.; Han, Y.; Jia, L.; Hou, B.; Liu, S.; Li, D. Determination of total phthalates in edible oils by high-performance liquid chromatography coupled with photodiode array detection. J. Sep. Sci. 2016, 39, 857–863.
- Kanu, A.B. Recent developments in sample preparation techniques combined with high-performance liquid chromatography: A critical review. J. Chromatogr. A 2021, 1654, 462444.
- Wang, X.; Chen, C.; Chen, Y.; Kong, F.; Xu, Z. Detection of dibutyl phthalate in food samples by fluorescence ratio immunosensor based on dual-emission carbon quantum dot labelled aptamers. Food Agric. Immunol. 2020, 31, 813–826.
- Han, W.C.; Shi, N.; Wang, X.Y.; Wang, Z.H.; Wang, K.L.; Gao, M.; Yu, L.; Chen, D.; Xu, X. Application of natural cotton fibers as an extraction sorbent for the detection of trans-resveratrol in adulterated peanut oils. Food Chem. 2021, 339, 127885.
- Ötles, S.; Kartal, C. Solid-Phase Extraction (SPE): Principles and Applications in Food Samples. Acta Sci. Pol. Technol. Aliment. 2016, 15, 5–15.
- He, F.; Tian, Y.; Xu, Z.; Luo, L.; Yang, J.; Wang, H.; Sun, Y.; Du, Q.; Shen, Y. Development of an immunochromatographic assay as a screen for detection of total phthalate acid esters in cooking oil. J. Toxicol. Environ. Health Part A 2018, 81, 80–88.
- Wang, X.; Chen, C.; Xu, L.; Zhang, H.; Xu, Z. Development of molecularly imprinted biomimetic immunoassay method based on quantum dot marker for detection of phthalates. Food Agric. Immunol. 2019, 30, 1007–1019.
- Wu, P.; Yang, D.; Zhang, L.; Shen, X.; Pan, X.; Wang, L.; Zhang, J.; Tan, Y.; Feng, L.; Ying, Y. Simultaneous determination of 17 phthalate esters in edible vegetable oils by GC-MS with silica/PSA-mixed solid-phase extraction. J. Sep. Sci. 2012, 35, 2932–2939.
- Li, X.; Xiong, W.; Lin, H.; Zhuo, L.; Lv, S.; Tang, X.; Chen, M.; Zou, Z.; Lin, Z.; Qiu, B.; et al. Analysis of 16 phthalic acid esters in food simulants from plastic food contact materials by LC-ESI-MS/MS. J. Sep. Sci. 2013, 36, 477–484.
- Oh, M.-S.; Lee, S.-H.; Moon, M.H.; Lee, D.S.; Park, H.-M. Simultaneous analysis of phthalates, adipate and polycyclic aromatic hydrocarbons in edible oils using isotope dilution-gas chromatography-mass spectrometry. Food Addit. Contam. B 2014, 7, 168–175.
- Chen, N.; He, J.; Wu, C.; Li, Y.; Suo, A.; Wei, H.; He, L.; Zhang, S. Synthesis of molecularly imprinted polymers by atom transfer radical polymerization for the solid-phase extraction of phthalate esters in edible oil. J. Sep. Sci. 2017, 40, 1327–1333.
- Duan, C.; Shen, Z.; Wu, D.; Guan, Y. Recent developments in solid-phase microextraction for on-site sampling and sample preparation. TrAC Trends Anal. Chem. 2011, 30, 1568–1574.
- Kataoka, H. SPME techniques for biomedical analysis. Bioanalysis 2015, 7, 2135–2144.
- Risticevic, S.; Niri, V.H.; Vuckovic, D.; Pawliszyn, J. Recent developments in solid-phase microextraction. Anal. Bioanal. Chem. 2009, 393, 781–795.
- Jiang, G.; Huang, M.; Cai, Y.; Lv, J.; Zhao, Z. Progress of solid-phase microextraction coatings and coating techniques. J. Chromatogr. Sci. 2006, 44, 324–332.
- Mirzajani, R.; Kardani, F.; Ramezani, Z. Fabrication of UMCM-1 based monolithic and hollow fiber—Metal-organic framework deep eutectic solvents/molecularly imprinted polymers and their use in solid phase microextraction of phthalate esters in yogurt, water and edible oil by GC-FID. Food Chem. 2020, 314, 126179.
- Uansiri, S.; Vichapong, J.; Kanchanamayoon, W. HS-SPME for the Determination of Phthalate Esters in Vegetable Oil and Soft Drink Samples. Chiang Mai J. Sci. 2018, 45, 1052–1061.
- Zhang, S.; Yang, Q.; Li, Z.; Wang, W.; Zang, X.; Wang, C.; Wang, Z. Solid phase microextraction of phthalic acid esters from vegetable oils using iron (III)-based metal-organic framework/graphene oxide coating. Food Chem. 2018, 263, 258–264.
- Amanzadeh, H.; Yamini, Y.; Moradi, M.; Asl, Y.A. Determination of phthalate esters in drinking water and edible vegetable oil samples by headspace solid phase microextraction using graphene/polyvinylchloride nanocomposite coated fiber coupled to gas chromatography-flame ionization detector. J. Chromatogr. A 2016, 1465, 38–46.
- Rios, J.J.; Morales, A.; Marquez-Ruiz, G. Headspace solid-phase microextraction of oil matrices heated at high temperature and phthalate esters determination by gas chromatography multistage mass spectrometry. Talanta 2010, 80, 2076–2082.
- Jiang, H.; Li, N.; Cui, L.; Wang, X.; Zhao, R. Recent application of magnetic solid phase extraction for food safety analysis. TrAC Trends Anal. Chem. 2019, 120, 115632.
- Zhao, Y.; Zhu, Z.; Liu, J.; Liu, J.; Li, G. Magnetic Solid-Phase Extraction Followed by HPLC–DAD for Highly Sensitive Determination of Phthalate Esters in Edible Vegetable Oils. Food Anal. Methods 2021, 14, 2375–2385.
- Sun, L.; Tian, W.; Fang, Y.; Yang, W.; Hu, Q.; Pei, F. Rapid and simultaneous extraction of phthalates, polychlorinated biphenyls and polycyclic aromatic hydrocarbons from edible oil for GC–MS determination. J. Food Compos. Anal. 2022, 114, 104827.
- Xie, Q.; Liu, S.; Fan, Y.; Sun, J.; Zhang, X. Determination of phthalate esters in edible oils by use of QuEChERS coupled with ionic-liquid-based dispersive liquid-liquid microextraction before high-performance liquid chromatography. Anal. Bioanal. Chem. 2014, 406, 4563–4569.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
811
Revisions:
3 times
(View History)
Update Date:
31 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




