
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giuseppe Murdaca | -- | 2100 | 2023-07-26 13:50:00 | | | |
| 2 | Peter Tang | Meta information modification | 2100 | 2023-07-27 04:04:45 | | |
Video Upload Options
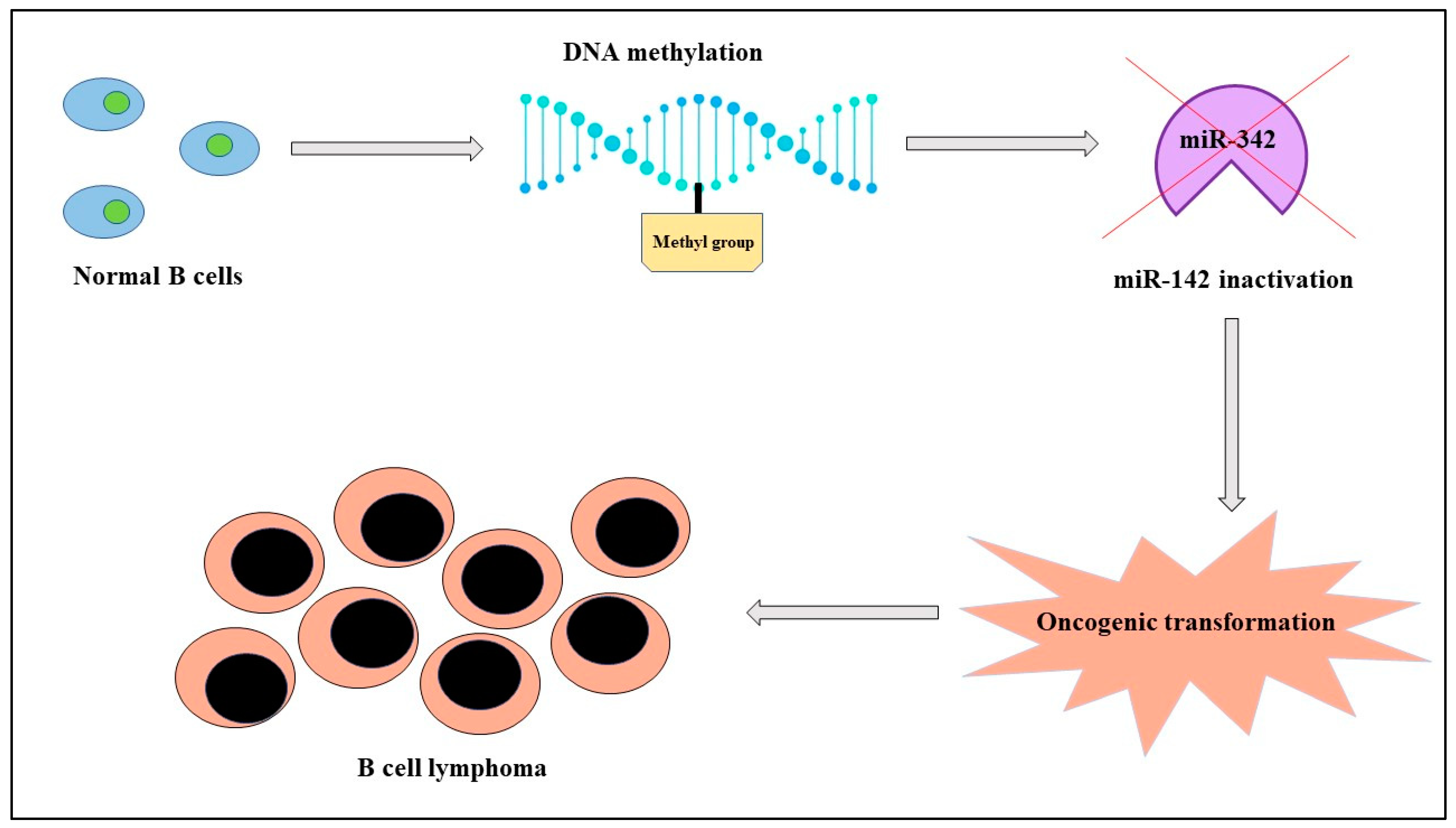
MicroRNAs are small, noncoding molecules of about twenty-two nucleotides with crucial roles in both healthy and pathological cells. Their expression depends not only on genetic factors, but also on epigenetic mechanisms like genomic imprinting and inactivation of X chromosome in females that influence in a sex-dependent manner onset, progression, and response to therapy of different diseases like cancer. There is evidence of a correlation between miRNAs, sex, and cancer both in solid tumors and in hematological malignancies; as an example, in lymphomas, with a prevalence rate higher in men than women, miR-142 is “silenced” because of its hypermethylation by DNA methyltransferase-1 and it is blocked in its normal activity of regulating the migration of the cell. This condition corresponds in clinical practice with a more aggressive tumor. In addition, cancer treatment can have advantages from the evaluation of miRNAs expression; in fact, therapy with estrogens in hepatocellular carcinoma determines an upregulation of the oncosuppressors miR-26a, miR-92, and miR-122 and, consequently, apoptosis.
1. Introduction
1.1. General Considerations on miRNAs
1.2. Sex Differences, miRNAs, and Cancer
2. Hematological Malignancies
|
Cancer |
miRNAs |
UP/DOWN Regulated |
Mechanism of Action |
Onset/Prognosis/Response to Therapy Involvement |
References |
|---|---|---|---|---|---|
|
B-cell lymphoma |
miR-142 |
DOWN |
Altered migration of cells |
Poor prognosis |
[22] |
|
Papillary thyroid cancer |
miR-21 miR-26a miR-181a miR-181b miR-219 miR-221 miR-222 miR-245 |
UP |
Modulation in protein p27Kip1 expression |
Onset |
[24] |
|
Hepatocellular carcinoma |
miR-371a-5p |
UP |
Transition from G to S phase of cell cycle |
Onset |
[25] |
|
Colorectal cancer |
miR-16 miR-22 miR-142-3p |
miR-22 UP; miR-16 and miR-142-3p DOWN |
Inhibition of autophagy (miR-16, miR-142-3p) and inhibition of estrogen activity |
Onset (miR-16, miR-142-3p) and better response to therapy (miR-22) |
[8] |
|
Gastric cancer |
miR-125 |
UP |
Block of apoptosis |
Onset |
[26] |
|
Lung cancer |
miR-143 miR-145 miR-153-3p |
UP |
Apoptosis induction |
Good prognosis |
[22] |
|
Melanoma |
miR-23a miR-221 miR-222 |
UP |
Block of cell proliferation |
Onset |
[8] |
|
Breast cancer |
miR-17 miR-21 miR-124 |
UP |
BRCA1 inactivation |
Onset |
|
|
Glioblastoma |
hsa-miR-1909-5p hsa-let-7c-5p miR-206-5p |
hsa-miR-1909-5p and hsa-let-7c-5p UP; miR-206-5p DOWN |
Promotion of cell migration and invasion (hsa-miR-1909-5p, hsa-let-7c-5p) and modulation of apoptosis (miR-206-5p) |
Onset |
[29] |
References
- Allegra, A.; Murdaca, G.; Gammeri, L.; Ettari, R.; Gangemi, S. Alarmins and MicroRNAs, a New Axis in the Genesis of Respiratory Diseases: Possible Therapeutic Implications. Int. J. Mol. Sci. 2023, 24, 1783.
- Amato, G.; Vita, F.; Quattrocchi, P.; Minciullo, P.L.; Pioggia, G.; Gangemi, S. Involvement of miR-142 and miR-155 in Non-Infectious Complications of CVID. Molecules 2020, 25, 4760.
- Kraczkowska, W.; Stachowiak, L.; Pławski, A.; Jagodziński, P.P. Circulating miRNA as potential biomarkers for diabetes mellitus type 2: Should we focus on searching for sex differences? J. Appl. Genet. 2022, 63, 293–303.
- Hasakova, K.; Bezakova, J.; Vician, M.; Reis, R.; Zeman, M.; Herichova, I. Gender-Dependent Expression of Leading and Passenger Strand of miR-21 and miR-16 in Human Colorectal Cancer and Adjacent Colonic Tissues. Physiol. Res. 2017, 66, S575–S582.
- Umansky, S. Aging and aging-associated diseases: A microRNA-based endocrine regulation hypothesis. Aging 2018, 10, 2557–2569.
- Warnefors, M.; Mössinger, K.; Halbert, J.; Studer, T.; VandeBerg, J.L.; Lindgren, I.; Fallahshahroudi, A.; Jensen, P.; Kaessmann, H. Sex-biased microRNA expression in mammals and birds reveals underlying regulatory mechanisms and a role in dosage compensation. Genome Res. 2017, 27, 1961–1973.
- Bezler, A.; Braukmann, F.; West, S.M.; Duplan, A.; Conconi, R.; Schütz, F.; Gönczy, P.; Piano, F.; Gunsalus, K.; Miska, E.A.; et al. Tissue- and sex-specific small RNAomes reveal sex differences in response to the environment. PLOS Genet. 2019, 15, e1007905.
- Bellenghi, M.; Puglisi, R.; Pontecorvi, G.; De Feo, A.; Carè, A.; Mattia, G. Sex and Gender Disparities in Melanoma. Cancers 2020, 12, 1819.
- Otmani, K.; Rouas, R.; Lewalle, P. OncomiRs as noncoding RNAs having functions in cancer: Their role in immune suppression and clinical implications. Front. Immunol. 2022, 13, 913951.
- Caserta, S.; Innao, V.; Musolino, C.; Allegra, A. Immune checkpoint inhibitors in multiple myeloma: A review of the literature. Pathol. Res. Pract. 2020, 216, 153114.
- Carè, A.; Bellenghi, M.; Matarrese, P.; Gabriele, L.; Salvioli, S.; Malorni, W. Sex disparity in cancer: Roles of microRNAs and related functional players. Cell Death Differ. 2018, 25, 477–485.
- Fernández-Torrón, R.; García-Puga, M.; Emparanza, J.-I.; Maneiro, M.; Cobo, A.-M.; Poza, J.-J.; Espinal, J.-B.; Zulaica, M.; Ruiz, I.; Martorell, L.; et al. Cancer risk in DM1 is sex-related and linked to miRNA-200/141 downregulation. Neurology 2016, 87, 1250–1257.
- Tomeva, E.; Krammer, U.D.B.; Switzeny, O.J.; Haslberger, A.G.; Hippe, B. Sex-Specific miRNA Differences in Liquid Biopsies from Subjects with Solid Tumors and Healthy Controls. Epigenomes 2023, 7, 2.
- Javed, Z.; Khan, K.; Herrera-Bravo, J.; Naeem, S.; Iqbal, M.J.; Sadia, H.; Qadri, Q.R.; Raza, S.; Irshad, A.; Akbar, A.; et al. Correction: Genistein as a regulator of signaling pathways and microRNAs in different types of cancers. Cancer Cell Int. 2022, 22, 256.
- Shiau, J.-P.; Chuang, Y.-T.; Yen, C.-Y.; Chang, F.-R.; Yang, K.-H.; Hou, M.-F.; Tang, J.-Y.; Chang, H.-W. Modulation of AKT Pathway-Targeting miRNAs for Cancer Cell Treatment with Natural Products. Int. J. Mol. Sci. 2023, 24, 3688.
- Caserta, S.; Zaccuri, A.M.; Innao, V.; Musolino, C.; Allegra, A. Immune thrombocytopenia: Options and new perspectives. Blood Coagul. Fibrinolysis 2021, 32, 427–433.
- De Luca, F.; Allegra, A.; Di Chio, C.; Previti, S.; Zappalà, M.; Ettari, R. Monoclonal Antibodies: The Greatest Resource to Treat Multiple Myeloma. Int. J. Mol. Sci. 2023, 24, 3136.
- Musolino, C.; Oteri, G.; Allegra, A.; Mania, M.; D’ascola, A.; Avenoso, A.; Innao, V.; Allegra, A.G.; Campo, S. Altered microRNA expression profile in the peripheral lymphoid compartment of multiple myeloma patients with bisphosphonate-induced osteonecrosis of the jaw. Ann. Hematol. 2018, 97, 1259–1269.
- Allegra, A.; Tonacci, A.; Giordano, L.; Musolino, C.; Gangemi, S. Targeting Redox Regulation as a Therapeutic Opportunity against Acute Leukemia: Pro-Oxidant Strategy or Antioxidant Approach? Antioxidants 2022, 11, 1696.
- Taverna, S.; Tonacci, A.; Ferraro, M.; Cammarata, G.; Cuttitta, G.; Bucchieri, S.; Pace, E.; Gangemi, S. High Mobility Group Box 1: Biological Functions and Relevance in Oxidative Stress Related Chronic Diseases. Cells 2022, 11, 849.
- Cui, X.; Zhao, X.; Liang, Y. Sex differences in normal and malignant hematopoiesis. Blood Sci. 2022, 4, 185–191.
- Matarrese, P.; Mattia, G.; Pagano, M.T.; Pontecorvi, G.; Ortona, E.; Malorni, W.; Carè, A. The Sex-Related Interplay between TME and Cancer: On the Critical Role of Estrogen, MicroRNAs and Autophagy. Cancers 2021, 13, 3287.
- Anonymous. Correction to: Hormonally Regulated Myogenic miR-486 Influences Sex-specific Differences in Cancer-induced Skeletal Muscle Defects. Endocrinology 2022, 163, bqac120, Erratum in: Endocrinology 2021, 162, 35939403.
- Huang, C.; Cai, Z.; Huang, M.; Mao, C.; Zhang, Q.; Lin, Y.; Zhang, X.; Tang, B.; Chen, Y.; Wang, X.; et al. miR-219–5p Modulates Cell Growth of Papillary Thyroid Carcinoma by Targeting Estrogen Receptor α. J. Clin. Endocrinol. Metab. 2015, 100, E204–E213.
- Bai, P.-S.; Hou, P.; Kong, Y. Hepatitis B virus promotes proliferation and metastasis in male Chinese hepatocellular carcinoma patients through the LEF-1/miR-371a-5p/SRCIN1/pleiotrophin/Slug pathway. Exp. Cell Res. 2018, 370, 174–188.
- Liu, B.; Zhou, M.; Li, X.; Zhang, X.; Wang, Q.; Liu, L.; Yang, M.; Yang, D.; Guo, Y.; Zhang, Q.; et al. Interrogation of gender disparity uncovers androgen receptor as the transcriptional activator for oncogenic miR-125b in gastric cancer. Cell Death Dis. 2021, 12, 441.
- Li, E.W.; Bai, Y. Computational Identification of Sex-Biased Biomarker MicroRNAs and Genes Associated with Immune Infiltration in Breast Cancer. Genes 2021, 12, 570.
- Singh, H. Role of Molecular Targeted Therapeutic Drugs in Treatment of Glioblastoma: A Review Article. Glob. Med. Genet. 2023, 10, 42–47.
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma. JAMA 2015, 314, 2535–2543.




