
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Helena Korpelainen | -- | 1889 | 2023-07-25 21:18:14 | | | |
| 2 | Catherine Yang | Meta information modification | 1889 | 2023-07-26 03:54:39 | | | | |
| 3 | Helena Korpelainen | + 114 word(s) | 2075 | 2023-07-27 09:04:23 | | |
Video Upload Options
Non-indigenous species are species distributed outside their historic and native range. It has been proposed that a non-indigenous species must go through three stages to become invasive. Firstly, individuals of the species must disperse from their native range to a new area. Secondly, after the introduction, the individuals must survive and reproduce in the new area and become established. Thirdly, once established, the non-indigenous species will increase in number, expand its geographic range, and become a threat to the ecosystem, i.e., become invasive. Thus, the success of invasion depends on the combination of dispersal and demography in a non-native region, and these are affected by the processes of post-dispersal adaptation, genetic diversity, and phenotypic plasticity. However, these processes are not well understood. However, it is well known that invasive species threaten native biodiversity. Based on evidence from metacommunity models, it has been shown that species introductions could disrupt species coexistence, generating extinction debts, especially when combined with other forms of anthropogenic environmental changes. Therefore, the control and eradication of invasive species are essential for the conservation of native species, biodiversity, and ecosystem function, e.g., plant–pollinator networks.
1. Traits Important for Successful Plant Invasion
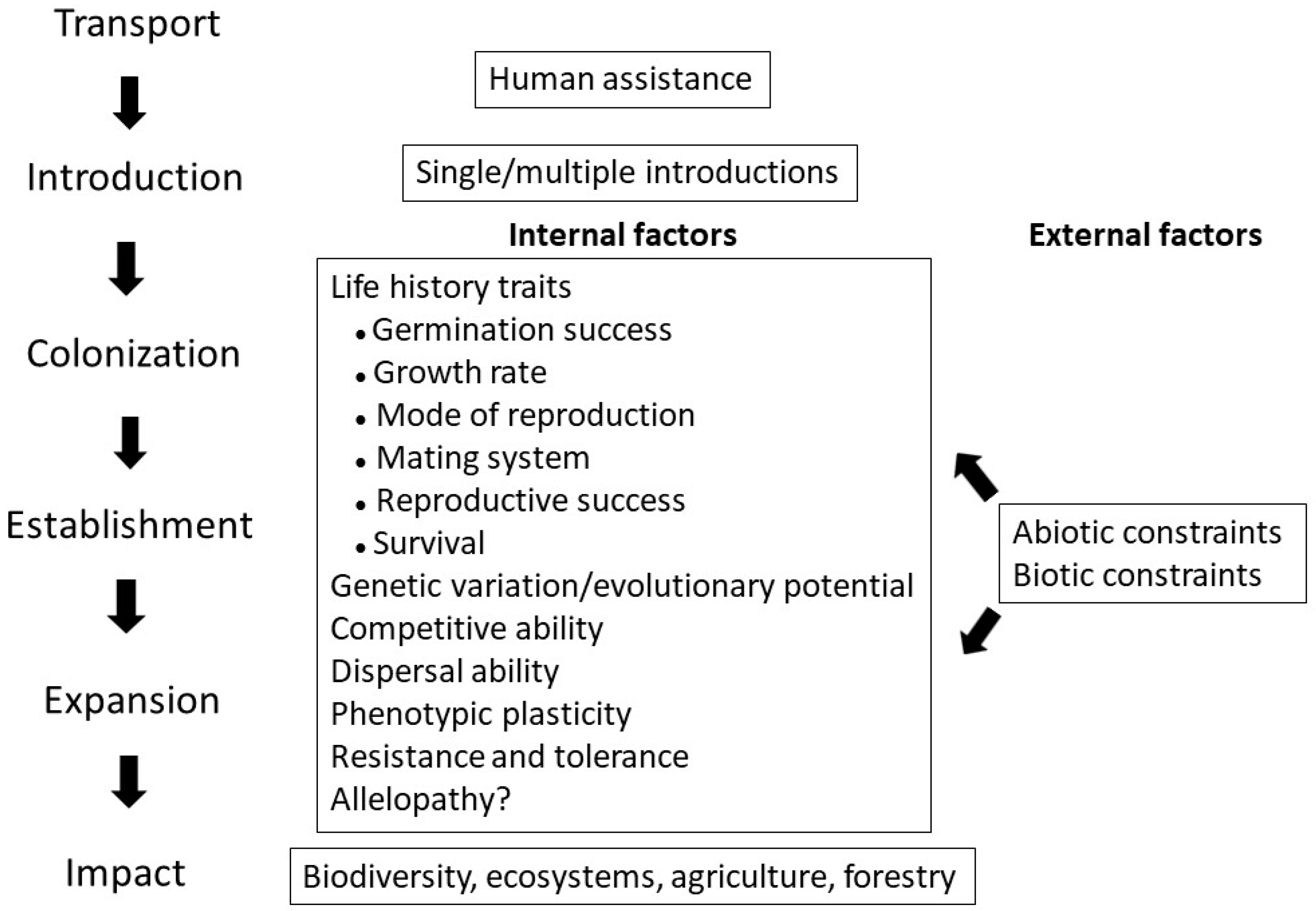
2. Interactions between Genetic Traits and Environmental Conditions Promoting Plant Invasion
3. Pathways to Successful Plant Invasion
The genetic traits of introduced populations affect their capacity to expand in non-native regions. At introduction, they may contain just a portion of the species’ gene pool and experience founder effects. Such bottlenecks could inhibit the population growth and further expansion, as decreased genetic diversity is likely to result in inbreeding depression and reduced evolutionary potential, thus lowering the ability to respond to novel selection pressures. On the other hand, these populations may benefit from being freed from the biotic pressures of the former habitat. However, the genetic diversity of invasive populations can increase through multiple introductions and/or sexual reproduction along with recombination. Invasive populations becoming established in new regions can be a source of additional introductions, leading to a self-accelerating process.
Overall, it has become clear that the processes of plant invasion vary, and there is no single universal mechanism. It is known that various inherent and external factors affect the invasion process. External factors consist of a range of abiotic and biotic constraints. How well a plant species performs under those depends on a wide range of inherent characteristics, such as multiple life history traits, the pattern of genetic variation, competitive and dispersal abilities, phenotypic plasticity, resistance and tolerance, herbivory, and allelopathic interactions. In addition, patterns of reproduction and sexual functions are important. In the case of sexual reproduction, hermaphroditism combined with self-compatibility may enhance invasiveness, since selfing allows fertilization and recombination even under low population densities. Overall, the ability for asexual propagation and, in the case of sexuality, hermaphroditism, is an asset in the invasion process.
References
- van Kleunen, M.; Weber, E.; Fischer, M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010, 13, 235–245.
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431.
- Tabassum, S.; Leishman, M.R. Have your cake and eat it too: Greater dispersal ability and faster germination towards range edges of an invasive plant species in eastern Australia. Biol. Invasions 2018, 20, 1199–1210.
- Baker, H.G. Characteristics and Modes of Origin of Weeds. In The Genetics of Colonizing Species; Baker, H.G., Stebbins, G.L., Eds.; Academic Press: New York, NY, USA, 1965; pp. 147–169.
- Hiatt, D.; Flory, S.L. Populations of a widespread invader and co-occurring native species vary in phenotypic plasticity. New Phytol. 2020, 225, 584–594.
- Rathee, S.; Ahmad, M.; Sharma, P.; Singh, H.P.; Batish, D.R.; Kaur, S.; Kaur, A.; Yadav, S.S.; Kohli, R.K. Biomass allocation and phenotypic plasticity are key elements of successful invasion of Parthenium hysterophorus at high elevation. Environ. Exp. Bot. 2021, 184, 104392.
- Zhang, J.; Huang, W.; Ding, J. Phenotypic plasticity in resource allocation to sexual trait of alligatorweed in wetland and terrestrial habitats. Sci. Total Environ. 2021, 757, 143819.
- Rodríguez, J.; Lorenzo, P.; González, L. Phenotypic plasticity of invasive Carpobrotus edulis modulates tolerance against herbivores. Biol. Invasions 2021, 23, 1859–1875.
- Xu, X.; Zhou, C.; He, Q.; Qiu, S.; Zhang, Y.; Yang, J.; Li, B.; Nie, M. Phenotypic plasticity of light use favors a plant invader in nitrogen-enriched ecosystems. Ecology 2022, 103, e3665.
- Luo, X.; Xu, X.; Zheng, Y.; Guo, H.; Hu, S. The role of phenotypic plasticity and rapid adaptation in determining invasion success of Plantago virginica. Biol. Invasions 2019, 21, 2679–2692.
- Wan, J.S.H.; Fazlioglu, F.; Bonser, S.P. Loss of plasticity in life-history strategy associated with secondary invasion into stressful environments in invasive narrowleaf plantain (Plantago lanceolata L.). Austral Ecol. 2018, 43, 752–762.
- Phillips, B.L.; Brown, G.P.; Shine, R. Life-history evolution in range-shifting populations. Ecology 2010, 91, 1617–1627.
- van Boheemen, L.A.; Atwater, D.Z.; Hodgins, K.A. Rapid and repeated local adaptation to climate in an invasive plant. New Phytol. 2019, 222, 614–627.
- Burton, O.J.; Phillips, B.L.; Travis, M.J. Trade-offs and the evolution of life histories during range expansion. Ecol. Lett. 2010, 13, 1210–1220.
- Kilkenny, F.F.; Galloway, L.F. Adaptive divergence at the margin of an invaded range. Evolution 2013, 67, 722–731.
- Ni, M.; Deane, D.C.; Li, S.; Wu, Y.; Sui, X.; Xu, H.; Chu, C.; He, F.; Fang, S. Invasion success and impacts depend on different characteristics in non-native plants. Div. Distrib. 2021, 27, 1194–1207.
- Liu, W.; Chen, X.; Wang, J.; Zhang, Y. Does the effect of flowering time on biomass allocation across latitudes differ between invasive and native salt marsh grass Spartina alterniflora? Ecol. Evol. 2022, 12, e8681.
- Broadbent, A.; Stevens, C.J.; Peltzer, D.A.; Ostle, N.J.; Orwin, K.H. Belowground competition drives invasive plant impact on native species regardless of nitrogen availability. Oecologia 2018, 186, 577–587.
- Lockwood, J.L.; Hoopes, M.F.; Marchetti, M.P. Invasion Ecology; Blackwell Publishing: Oxford, UK, 2008.
- Byun, C.; de Blois, S.; Brisson, J. Management of invasive plants through ecological resistance. Biol. Invasions 2018, 20, 13–27.
- Lustenhouwer, N.; Williams, J.L.; Levine, J.M. Evolution during population spread affects plant performance in stressful environments. J. Ecol. 2019, 107, 396–406.
- Smith, A.L.; Hodkinson, T.R.; Villellas, J.; Catford, J.A.; Csergő, A.M.; Blomberg, S.P.; Crone, E.E.; Ehrlén, J.; Garcia, M.B.; Laine, A.-L. Global gene flow releases invasive plants from environmental constraints on genetic diversity. Proc. Nat. Acad. Sci. USA 2020, 117, 4218–4227.
- Dlugosch, K.M.; Parker, I.M. Founding events in species invasions: Genetic variation, adaptive evolution, and the role of multiple introductions. Mol. Ecol. 2008, 17, 431–449.
- Schrieber, K.; Lachmuth, S. The genetic paradox of invasions revisited: The potential role of inbreeding × environment interactions in invasion success. Biol. Rev. 2017, 92, 939–952.
- Jelbert, K.; Buss, D.; McDonald, J.; Townley, S.; Franco, M.; Stott, I.; Jones, O.; Salguero-Gómez, R.; Buckley, Y.; Knight, T.; et al. Demographic amplification is a predictor of invasiveness among plants. Nat. Commun. 2019, 10, 5602.
- Keller, J.A.; Shea, K. Warming and shifting phenology accelerate an invasive plant life cycle. Ecology 2021, 102, e03219.
- Campoy, J.G.; Lema, M.; Fenollosa, E.; Munné-Bosch, S.; Retuerto, R. Functional responses to climate change may increase invasive potential of Carpobrotus edulis. Am. J. Bot. 2021, 108, 1902–1916.
- Mao, R.; Bajwa, A.A.; Adkins, S. A superweed in the making: Adaptations of Parthenium hysterophorus to a changing climate. A review. Agron. Sustain. Dev. 2021, 41, 47.
- Lopez, B.E.; Allen, J.M.; Dukes, J.S.; Lenoir, J.; Vilà, M.; Blumenthal, D.M.; Beaury, E.M.; Fusco, E.J.; Laginhas, B.B.; Morelli, T.L.; et al. Global environmental changes more frequently offset than intensify detrimental effects of biological invasions. Proc. Natl. Acad. Sci. USA 2022, 119, e2117389119.
- Hierro, J.L.; Callaway, R.M. The ecological importance of allelopathy. Ann. Rev. Ecol. Evol. Syst. 2021, 52, 25–45.
- Schandry, N.; Becker, C. Allelopathic plants: Models for studying plant-interkingdom interactions. Trends Plant Sci. 2020, 25, 176–185.
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an invasive plant. Plants 2021, 10, 1028.
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and allelochemicals of Leucaena leucocephala as an invasive plant species. Plants 2022, 11, 1672.
- Kato-Noguchi, H. Involvement of allelopathy in the invasive potential of Tithonia diversifolia. Plants 2020, 9, 766.
- Kalisz, S.; Kivlin, S.N.; Bialic-Murphy, L. Allelopathy is pervasive in invasive plants. Biol. Invasions 2021, 23, 367–371.
- Uesugi, A.; Kessler, A. Herbivore release drives parallel patterns of evolutionary divergence in invasive plant phenotypes. J. Ecol. 2016, 104, 876–886.
- Wan, J.; Huang, B.; Yu, H.; Peng, S. Reassociation of an invasive plant with its specialist herbivore provides a test of the shifting defence hypothesis. J. Ecol. 2019, 107, 361–371.
- Colomer-Ventura, F.; Martínez-Vilalta, J.; Zuccarini, P.; Escolà, A.; Armengot, L.; Castells, E. Contemporary evolution of an invasive plant is associated with climate but not with herbivory. Funct. Ecol. 2015, 29, 1475–1485.
- Joshi, J.; Vrieling, K. The enemy release and EICA hypothesis revisited: Incorporating the fundamental difference between specialist and generalist herbivores. Ecol. Lett. 2005, 8, 704–714.
- Zhang, Z.; Pan, X.; Blumenthal, D.; van Kleunen, M.; Liu, M.; Li, B. Contrasting effects of specialist and generalist herbivores on resistance evolution in invasive plants. Ecology 2018, 99, 866–875.
- Lucero, J.E.; Callaway, R.M. Native granivores reduce the establishment of native grasses but not invasive Bromus tectorum. Biol. Invasions 2018, 20, 3491–3497.




