Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alireza Z. Moshfegh | -- | 3490 | 2023-07-25 15:10:34 | | | |
| 2 | Sirius Huang | Meta information modification | 3490 | 2023-07-26 03:33:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Goodarzi, N.; Ashrafi-Peyman, Z.; Khani, E.; Moshfegh, A.Z. Semiconductor Heterogeneous Photocatalysts. Encyclopedia. Available online: https://encyclopedia.pub/entry/47260 (accessed on 07 February 2026).
Goodarzi N, Ashrafi-Peyman Z, Khani E, Moshfegh AZ. Semiconductor Heterogeneous Photocatalysts. Encyclopedia. Available at: https://encyclopedia.pub/entry/47260. Accessed February 07, 2026.
Goodarzi, Nahal, Zahra Ashrafi-Peyman, Elahe Khani, Alireza Z. Moshfegh. "Semiconductor Heterogeneous Photocatalysts" Encyclopedia, https://encyclopedia.pub/entry/47260 (accessed February 07, 2026).
Goodarzi, N., Ashrafi-Peyman, Z., Khani, E., & Moshfegh, A.Z. (2023, July 25). Semiconductor Heterogeneous Photocatalysts. In Encyclopedia. https://encyclopedia.pub/entry/47260
Goodarzi, Nahal, et al. "Semiconductor Heterogeneous Photocatalysts." Encyclopedia. Web. 25 July, 2023.
Copy Citation
Semiconductor-based photocatalytic reactions are a practical class of advanced oxidation processes (AOPs) to address energy scarcity and environmental pollution. By utilizing solar energy as a clean, abundant, and renewable source, this process offers numerous advantages, including high efficiency, eco-friendliness, and low cost.
advanced oxidation process (AOPs)
semiconductor-based photocatalysts
H2 production
water splitting
CO2 reduction
dye and drug photodegradation
1. Introduction
With the continuous increase in global population and industrialization, the necessity for clean energy and a sustainable environment is increasing dramatically [1]. Based on U.S. Energy Information Administration (EIA) survey in 2019, energy consumption worldwide is expected to rise by approximately 50% between 2018 and 2050 [2]. About 83% of today’s energy supply comes from fossil fuels (e.g., gas, petroleum, and coal). Fossil fuel combustion is responsible for releasing large amounts of CO2, accounting for around 76% of annual greenhouse gas emissions, which creates severe problems for the environment and public health, such as global warming and climate change [3][4]. Accordingly, eco-friendly and renewable energy resources, including solar, biomass, wind, geothermal, and hydropower, have been successfully exploited as practical alternatives for fossil fuels to address these problems [5][6]. Among several energy as mentioned above, solar energy, which is emitted from the sun at a rate of 3 × 1023 kW and 1.8 × 1014 kW of which is intercepted by the earth, is the most affordable and applicable energy source [7]. Hence, designing an efficient technique to transform solar energy into hydrogen and clean fuels such as carbon-based fuels through water splitting and carbon dioxide (CO2) reduction, respectively, shows great potential to address both energy crises and environmental problems [8][9]. As a non-carbon-based energy carrier, hydrogen energy has attracted considerable attention due to its numerous advantages and may become a chemical fuel in the future. It has been proven that hydrogen has the potential to be a preferred alternative to reduce human society’s reliance on non-renewable resources and decrease environmental concerns. Water, which covers 71% of the earth’s surface, has excellent potential as a source of hydrogen energy. Using water power, we can extract H2 by using an appropriate photocatalyst. This process not only provides a means of generating energy, but also helps to reduce greenhouse gas emissions. While the high flammability of hydrogen is considered a disadvantage, it is also beneficial because it eliminates the need for additional energy to combust the fuel. Additionally, once released, hydrogen diffuses quickly and is non-toxic. These properties make hydrogen a potentially safe and efficient energy source for the future [10].
There are various approaches for solar hydrogen production, including photocatalytic (PC) [11], photothermal catalytic (PTC) [12], photovoltaic electrochemical (PV-EC) [13], photoelectrochemical (PEC) [14], and photobiological (PB) [15]. In addition, some of these methods, including PV-EC [16], PEC [17], and PC [18], are also utilized for CO2 reduction. The core of all the mentioned methods is the semiconductor photocatalysis, which is known as one of the promising approaches in carbon dioxide reduction and hydrogen production [19]. Inspiring natural photosynthesis, Figure 1 illustrates the application of solar energy by using semiconductor photocatalysts toward hydrogen generation from water splitting (Figure 1a) and CO2 reduction to valuable chemicals (Figure 1b).
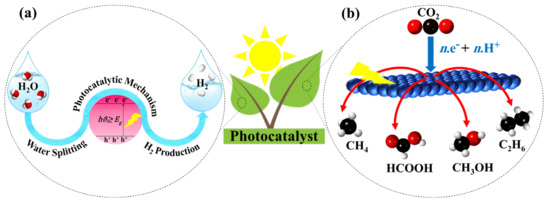
Figure 1. A schematic diagram of (a) hydrogen production from water splitting and (b) reduction of CO2 to useful chemicals using solar light.
The other most important aspect of environmental remediation in the modern world of today is water purification. Regarding the expansion of human activities, a significant number of organic contaminants, including industrial dyes, disinfectants, pesticides, agricultural fertilizers and pharmaceuticals compounds, as well as inorganic pollutants, including heavy metals, salts, solvents, and nutrients, are daily being released into the wastewater streams [20][21]. A schematic of various organic and inorganic water pollution sources is depicted in Figure 2.
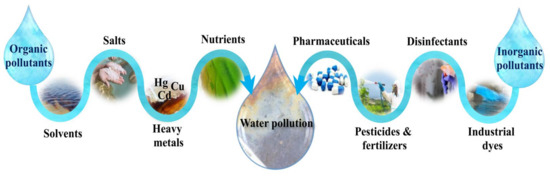
Figure 2. Various organic and inorganic sources of water pollution in the environment.
Long-term persistence in the environment and the water solubility of a wide range of compounds mentioned above have a significant potential to cause severe problems to the environment and health globally [20]. To overcome these issues, various conventional purification methods, including physical techniques (e.g., adsorption, membrane separation, flocculation, and coagulation), chemical methods (e.g., advanced oxidation process (AOPs)), and several microorganism-assistant biological treatments have been extensively used [22]. Physical methods by using adsorption techniques can remove organic and inorganic pollutants under different operating conditions [23][24]. However, the incomplete removal of organic pollutants and the generation of waste products during treatment can lead to several challenges for the desired process [23][25]. On the other hand, despite the excellent selectivity, membrane separation technology often leads to high operating costs and increased energy consumption because of membrane fouling [23][26]. Biological treatments using microbiological organisms will play an essential role in removing emergent contaminants from wastewater in the future. However, the need for physicochemical pretreatment and generation of biological sludge are the significant disadvantages of these methods [27]. Chemical methods, significantly advanced oxidation processes (AOPs), can be considered as a beneficial alternative in water purification treatments [28]. AOPs, introduced in 1981 by Glaze et al. [29], are defined as water purification processes by generating a sufficient amount of free hydroxyl radicals (•OH) as one of the most effective reactive oxygen species (ROS). In addition to hydroxyl radicals, other reactive species, including hydrogen peroxide (H2O2), anion radical (•O2−), and singlet oxygen (1O2), can be generated in AOPs [30]. Meanwhile, 1O2 is a highly selective oxidant that can quickly oxidize electron-rich moieties in organic pollutants owning to its electrophilic nature. This form of oxygen species possesses a prolonged lifetime (nearly 2–4 μs) and high concentration (e.g., 10−14–10−11 M) in water and demonstrates excellent resilience to inorganic anions and natural organic matter in wastewater [31]. These properties make this species suitable for selectively oxidizing high-priority contaminants commonly found in wastewater, such as endocrine-disrupting chemicals (EDCs), antibiotics, and pharmaceuticals [32]. Moreover, 1O2 can be utilized for the inactivation of pathogenic microorganisms and the elimination of antibiotic-resistance genes in both natural and drinking water [33]. Several AOPs systems, such as catalytic ozonation, hydrogen peroxide (H2O2), persulfate (PS) activation processes, and photocatalysis, can be applied to generate 1O2 [31]. In some ozone-based systems, O3 can be activated and produce free peroxide species (•O2) that can be directly converted to 1O2 through an electron transfer mechanism. H2O2, as a common oxidant, can generate a variety of ROS after catalytic activation, followed by 1O2 generation in the system. In persulfate (PS)-based AOPs, in the presence of carbon-based materials, the carbonyl (C=O) functional groups on the catalyst reacts with PS and generate 1O2. Among the aforementioned approaches, photocatalysis is the most effective method to produce 1O2 through either a directly energy transfer on the photocatalyst or indirectly from other ROS. Despite all the advantages of 1O2, this species has low redox potential (0.81 V/SHE). As a result, its reaction rate with most organic compounds is lower than that of observed with radicals [34]. Owing to the high oxidation ability of the •OH radical (E° (•OH/H2O) = 2.80 V/SHE), this species acts as a powerful agent to degrade a broad spectrum of toxic organic pollutants into harmless compounds and even mineralize them into CO2 and H2O [35][36]. There are six types of AOPs based on the source nature of the •OH radical generation, including (a) photocatalysis [37], (b) UV–H2O2 processes [38], (c) photo-Fenton [39], (d) ozonation [40], (e) electro-Fenton [41], and (f) Sonlysis [42]. Different types of AOPs processes are depicted in Figure 3a–f.
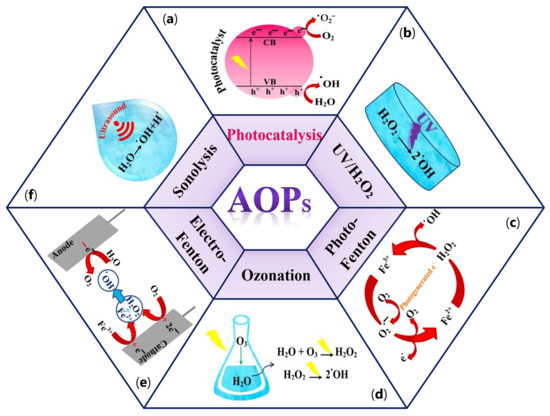
Figure 3. Different types of AOPs processes. (a) photocatalysis; (b) UV-H2O2 processes; (c) photo-Fenton; (d) ozonation; (e) electro-Fenton; (f) sonolysis.
Among various types of AOPs processes, the semiconductor-based photocatalytic process is highly expected to be the more desirable approach for wastewater treatment owing to its excellent properties, such as its environmentally friendly nature, simple manufacturing, complete mineralization capability, and reusability. Furthermore, the semiconductor photocatalyst can effectively decompose organic contaminants because of its high surface area using solar energy via a simple route and low operating cost without producing harmful byproducts [36][43][44].
2. Photoelectrochemical H2 Production from Water Splitting
It is well known that the efficient conversion of solar energy to chemical energy for producing clean energy is based on utilizing an appropriate semiconductor photocatalyst. Thus, hydrogen can be produced via photoelectrochemical (PEC) water splitting on semiconductor photocatalysts with effective nanostructures with high surface-to-volume ratio and high light absorption ability. A typical PEC cell consists of two half-reactions (a) oxygen evolution reaction (OER) usually occurs on an n-type semiconductor as a photoanode, and (b) hydrogen evolution reaction (HER) occurs on a cathode as a counter electrode, as illustrated in Figure 4.
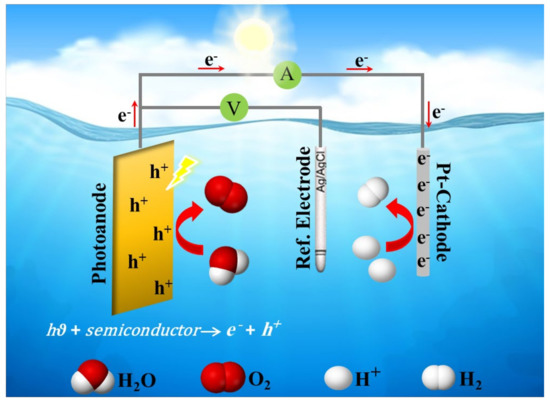
Figure 4. A schematic diagram of a typical photoelectrochemical (PEC) cell.
There are extensive efforts conducted by many researchers to maximize the efficiency of hydrogen production rate under solar irradiation by examining different nanostructured semiconductor photocatalysts. Table 1 presents some of the results obtained on different photoanode semiconductors.
Table 1. Some of the newest synthesized photocatalysts for H2 production from water splitting.
| Entry | Photocatalyst | Synthesis Method | Preparation Condition | Photocatalytic Test Condition | Photocurrent Density, Efficiency | Ref. |
|---|---|---|---|---|---|---|
| 1 | Sb-SnS | hydrothermal | 180 ◦C for 16 h. | Simulated solar, AM 1.5 G, 100 mW cm−2, 0.5 M Na2SO4 |
3.3 mA cm−2 | [45] |
| 2 | BiVO4, Bi2O3, TiO2 | spin-coating and calcination |
sol-gel-based spin coating method | Na2SO4 solution without hole scavengers | 5.1, 3.4, and 2.1 mA cm−2 | [46] |
| 3 | CN/Mn2O3 | plasma-assisted liquid-based |
melamine and C3H3N3O3 |
0.1 M Na2SO4, pH = 6.5 | 25 μA cm−2 | [47] |
| 4 | TiB2@AuNPs | spin-coating deposition |
Ultrasonic assisted liquid-phase exfoliation | sunlight simulator (1 Sun), 0.1 M KOH |
10 mA cm−2 | [48] |
| 5 | NiO/BiVO4 | dip-coating | Ink: Ni nanocrystals | 1 M KBi, hole scavenger: 0.2 M Na2SO3 |
4.41 mA cm−2 | [49] |
| 6 | β-FeOOH/CN | particle-to-substrate contacts |
precursor: DCDA and TCY |
AM 1.5G illumination, 0.1 M NaOH |
320 μA cm−2 | [50] |
| 7 | Fe2O3 (Hematite) | CVD | precursor: ferrocene organometallic compound | Simulator solar AM 1.5 G filter, 1 M NaOH |
2.5 mA cm−2 | [51] |
| 8 | Ir SAs/NiO/Ni/ ZrO2/n-Si |
ALD process |
precursor: TICP | simulated solar AM 1.5 G, 1 M NaOH |
27.7 mA cm−2 | [52] |
| 9 | Ni(OH)2/Cl-BiVO4 | impregnation method | precursor: 0.5 M NH4Cl |
AM1.5 G filter, 100 mW cm− 2, 0.5 M K3BO3, pH = 9.5 |
4.33 mA cm− 2 | [53] |
| 10 | Bi2S3-Cu3BiS3 | in-situ decoration | precursor: Cu:Bi 3:1 ink | simulated solar light (AM 1.5G), K–Pi buffer |
7.8 mA cm−2 | [54] |
| 11 | BiVO4/Vox | electrodeposition | precursor: BiOI |
simulated solar light (AM 1.5G), 1 M borate buffer |
6.29 mA cm−2 | [55] |
3. Photocatalytic CO2 Reduction
The substantial fossil fuels consumption resulted in vast carbon emissions with an atmospheric concentration of over 400 ppm, as reported in 2019. The over usage of fossil fuels ((e.g., petroleum, gas, and coal)) consumption is responsible for discharging a large amount of CO2 into the environment after their combustion. The release of CO2 accounts for around 76% of annual greenhouse gas (GHG) emissions which creates severe problems for the environment and public health, such as global warming and climate changes, seawater acidification, and the upsurge of ocean levels. To solve these severe ecological issues, the conversion of CO2 into valued small-molecule chemical products or energy fuels (such as CO, CH4, HCOOH, and other chemicals) through photocatalytic technology, can solve the energy crisis as well as environmental issues. Extensive efforts have been conducted by many researchers to convert GHG, such as CO2, into valuable products and reduce its catastrophic emission. As a result of this process, environmental problems such as climatic turbulences, which comprise ecological deterioration, seawater acidification, and the upsurge of ocean levels will be reduced [56][57][58][59][60][61][62]. Table 2 presents some results obtained on different photocatalyst semiconductors along with preparation methods, photocatalytic conditions, and product yield.
Table 2. A summary of the synthesized photocatalysts for CO2 reaction.
| Entry | photocatalyst | Synthesis Method | Preparation Condition | Photocatalytic Conditions | Photocatalytic Activity (Product Yield) |
Ref. |
|---|---|---|---|---|---|---|
| 1 | MOF [Cu3Th6(µ3-O)4(µ3-OH)4(cpb•)12] [FeIII(CN)6]6 | mixed, stirred and centrifuged | precursors: Th(NO3)4, formic acid, |
300 W Xe lamp, 420 or 800 nm filter |
CO production rate: 570.3 µmol g−1h−1 | [63] |
| 2 | Porphyrin-based MOFs | solvothermal | precursor: TPP and MnCl2 |
300 W Xe lamp, 1 sun (AM1.5 G) |
CO production rate: 46.148 µmol g−1h−1 |
[62] |
| 3 | boron-doped g-C3N4/TiO2−x | thermal reduction process | precursor: mixture of urea and NaBH4 | 300 W Xe lamp | CO production rate: 265.2 µmol g−1h−1 | [58] |
| 4 | Co-COF: | mix and sonication | precursor: TAPT, 120 °C, 96 h | 300 W Xe lamp (Microsolar 300, cut-off 420 nm), (λ ≥ 420 nm) |
CO production rate: 18,000 µmol g−1h−1 H2 production rate: 800 µmol g−1h−1 |
[59] |
| 5 | GQDs/C-BN | hydrothermal | precursors: boric acid, melamine and glucose | 300 W Xe lamp (λ = 200–420 nm or λ > 700 nm) | CO production rate: 33.47 μmol g−1h−1 | [64] |
| 6 | Ni(OH)2 | hydrolyzation of CaO | stirring for 24 h at 298.15 K. | LED lamps (5 W, λ ≥ 420 nm) |
CO production rate: 9.2 μmol g−1h−1 | [65] |
| 7 | S-ZnS/ZnIn2S4 | hydrothermal | precursor: Methylimidazole | 300 W Xe lamp cut-off filter (λ > 420 nm) |
CO production: 2075.7 ± 63.0 μmol g−1h−1 H2 production: 2912.3 ± 185.9 μmol g−1h−1 |
[66] |
| 8 | BP-CN | mix and sonication | Precursor: [NH3OH]+Cl− and melamine | 300 W Xe lamp | CO production rate: 44.6 μmol g−1h−1 | [67] |
| 9 | P-doped PCN | thermal polymerization method | precursor: Melamine and NaH2PO2⋅H2O | 300 W Xe lamp, cut-off filter 420 nm |
CH4 production rate: 1.1 μmol g−1 h−1 | [68] |
| 10 | Ni-BiOBr | hydrothermal | precursor: Bi(NO3)3⋅5H2O | 300 W xenon lamp cut-off 380 nm filter (λ ≥ 380 nm) | CO production rate: 378.7 μmol g−1h−1 |
[69] |
4. Photocatalytic Dye/Drug Degradation
Based on a World Bank report, dyeing and textile industries have contributed to about 17–20% of water pollution [70]. According to a recently published article, the textiles, leathers, food, and paper industries are primarily in charge of producing dye wastewater, with an annual global production of 8 × 105 tons of dyes, of which around 200,000 tones are textiles and dyes [71]. Numerous synthetic dyes used in textile, including both cationic dyes (e.g., safranin O (SO), rhodamine B(RhB), malachite green (MG), rhodamine 6G (Rh6G), methylene blue (MB), and crystal violet (CV)) and anionic dyes (e.g., eosin Y (EY), Eriochrome black T (EBT), phenol red (PR), methylene orange (MO), and Congo red (CR)) are toxic and harmful organic contaminants that will impede the photosynthesis process of aquatic plants and pose a threat to living creature health via the channels of food and drinking water supply [72][73][74].
Other organic wastes are produced by chemical and pharmaceutical industries that are harmful to society and the environment. During past decades, there has been a significant increase in the production and utilization of Pharmaceutical and Personal Care Products (PPCPs) to accommodate the demands of contemporary lifestyles and increased health care [75]. According to the European Union market, there are approximately 3000 frequently used drugs, and their usage is still on the rise worldwide [76]. Based on a recent report, the worldwide usage of antibiotics is believed to range from 100,000 to 200,000 metric tons. When excreted from the body, 70–90% of consumed antibiotics remain chemically unchanged or as active metabolites [77]. Moreover, the global production and consumption of PPCPs have significantly increased as a result of the COVID-19 pandemic in recent years. The findings of the National Health Commission of the People’s Republic of China indicate a dramatic increase in the consumption of antiviral and antibiotic drugs during the pandemic [78]. Pharmaceutical contaminants are found in water at concentrations as trace as ng·L−1 to μg·L−1. However, owning their chemical and physical properties, they can pose a serious threat to living organisms’ health even at these low concentration levels [79]. Given the severe problems resulting from water pollution caused by dye and pharmaceutical pollutants, as well as regarding the significant advantages of photocatalysis processes in removing harmful pollutants from water, numerous attempts have been undertaken to develop highly effective semiconductor-based photocatalysts to photodegrade of dye and pharmaceutical pollutants globally. A summary of various photocatalysts used for the degradation of dye and pharmaceutical contaminants in water, along with their synthesis method and reaction conditions, is presented in Table 3.
Table 3. A summary of various synthesized photocatalysts used for the degradation of dye and pharmaceutical contaminants in water.
| Entry | Photocatalyst | Synthesis Method | Preparation Condition | Pollutant Type | Light Condition | Efficiency (%) | Time (min) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | CN/CQD/BiOCl0.75Br0.25 | hydrothermal | pH = 5.8 [TC] = 100 mg·L−1 Catalyst dose = 0.1 g·L−1 |
Tetracycline (TC) | 500 W Xe lamp (λ > 400 nm) | 83.4 | 30 | [80] |
| 2 | α-Fe2O3@TiO2 | sonication and wet impregnation | pH = 4.76 [CFX] = 20.5 mg·L−1 Catalyst dose = 0.012 g·L−1 |
Cefixime (CFX) |
500 W halogen visible light (>400 nm) | 98.8 | 103 | [81] |
| 3 | DBS/CNNS | calcination | pH = 5.5 [MOX] = 50 mg·L−1 Catalyst dose = 1 g·L−1 |
moxifloxacin (MOX) | 300 W xenon lamp (λ > 420 nm) |
~100.0% | 30 | [82] |
| 4 | 0.1 chl/0.1 SA-TiO2 | incipient wetness impregnation | pH = 6 [CPX] = 10 mg·L−1 Catalyst dose = 0.75 g·L−1 |
Ciprofloxacin (CPX) | Blue LED light (λ = 457 nm) | ∼75% | 120 | [83] |
| 5 | ZnO/ZnIn2S4 (ZnO/ZIS) | hydrothermal | pH = 3 [CS] = 10 mg·L−1 Catalyst dose = 0.40 g·L−1 |
ceftriaxone sodium (CS) | 500 W xenon lamp. | 85.3% | 150 | [84] |
| 6 | In2S3/MQDs/SmFeO3 (IMS) | sonication | [SMX] = 10 mg·L−1, catalyst dose = 0.6 g·L−1 pH = 5.3 |
sulfamethoxazole (SMX) | 300 W Xe lamp | 98.0% and 95.4% of SMX | 120 and 90 min, | [85] |
| 7 | MgCr-LDH | formamide-assisted co-precipitation and mild hydrothermal | pH = 7 [MB] = 20 mg·L−1 Catalyst dose = 30 mg |
methylene blue (MB) | solar light | 90.6 | 120 | [86] |
| 8 | ZnO-CT | a green synthesis route using lemon leaf extract | pH = 7 [CR] = 20 mg·L−1 Catalyst dose = 0.4 g·L−1 |
Congo red (CR) | Natural sunlight, (λ = 408 nm) | 97 | 90 | [87] |
| 9 | Au/La2Ti2O7/Ag3PO4 | The in-situ precipitation | pH = 9.6 [RhB] = 10 mg·L−1 Catalyst dose = 1 g·L−1 |
Rhodamine (BRhB) | Natural sunlight | 100 | 6 | [88] |
| 10 | UiO-66-NH2/PhC2Cu | hydrothermal | pH = 9 [NOR] = 10 mg·L−1 Catalyst dose = 0.2 g·L−1 |
norfloxacin (NOR) | 9 W LED lamp (455 nm) |
97.9 | 60 | [89] |
| 11 | CuPd/ZnO | hydrothermal and chemical reduction | pH = 2 [OM] = 40 mg·L−1 Catalyst dose = 0.5 wt.% |
methyl orange(MO) | solar simulator (λ = 440 nm) |
95.3 | 45 | [90] |
| 12 | mesoporous Fe/Al/La trimetallic nano-oxide(FAL) | chemical route | pH = 7 [dyes] = 10−5 M Catalyst dose = 0.30 g/100 mL |
black 5 (RB5) methylene blue (MB) direct blue 71 (DB71) and |
Sunlight | 93.85 ± 2 90.51 ± 2 91.16 ± 2 |
90 45 60 |
[91] |
| 13 | Fe2O3/CNT/MIL | hydrothermal | pH = 7 [OFX] = 20 mg·L−1, Catalyst dose = 100 mg·L−1 |
ofloxacin (OFX) | 300 W Xe lamp (λ > 420 nm) | 99.3 | 60 | [92] |
| 14 | α-NiMoO4/ZnFe2O4/BC | Pyrolysis and hydrothermal | pH = 10 [KP] = 10 mg·L−1, Catalyst dose = 100 mg·L−1 |
ketoprofen (KP) | visible light (UV cutoff 150 W LS xenon arc lamp) | 98.65 | 180 | [93] |
| 15 | 0D/2D AgI/CAU-17 | deposition-precipitation | pH(for RhB degradation) = 3 [RhB] = 10 mg·L−1, [KP] = 10 mg·L−1, [MO] = 5 mg·L−1, Catalyst dose = 0.25 mg·L−1 |
Rhodamine B (RhB) Tetracycline (TC) methyl orange (MO) |
500 W Xe lamp | 96.7 81.3 50.3 |
90 | [94] |
5. Simultaneous Photocatalysis
In the last decade, heterogeneous photocatalytic reactions have experienced many efforts and devoted studies in developing advanced and innovative materials to confront both environmental and energy crises via the utilization of appropriate semiconducting photocatalysts in many essential chemical reactions, including wastewater treatment, H2 generation, CO2 reduction, organic transformations, N2 photofixation, and biomass conversion to valuable products. It is well known in a conventional photocatalytic investigation, and these processes are occurred in a controlled condition and are discussed in the literature separately. However, a recent innovative approach is to engage two or more functions in one photocatalytic system simultaneously. The challenging point is that combining of two functions in one photocatalytic system requires a novel design, control, and engineering of an appropriate semiconductor photocatalyst with unique characteristics for each application in a particular environment. The concept of dual-purpose photocatalysis was discussed in the pioneering work of Kim et al. [95] on simultaneous hydrogen production and phenolic compounds’ degradation by using a TiO2 surface decorated with platinum nanoparticles and fluorine atoms (F-TiO2/Pt) as a photocatalyst. They obtained complete mineralization of organic compounds and found anoxic degradation of 4-chlorophenol accompanied by H2 production. Because an appropriate photocatalyst characteristic is required for each process, thus the primary challenge is to conduct two or more kinds of applications simultaneously over one photocatalyst. Thus, it is an ideal approach to design and utilizes a special photocatalyst in at least two different concurrent applications. For example, 2D semiconductors (e.g., GO, rGO, and MXenes) and their hybrid combinations can be applied to produce hydrogen generation and pollutant degradation simultaneously. Nugmanova A. G. et al. [96] synthesized zinc porphyrin metal–organic frameworks non-covalently attached to graphene oxide (SURMOF/GO) in Pickering emulsions, and investigated its photocatalytic activity during photodegradation of rhodamine 6G (Rh6G) and 1,5-dihydroxynaphtalene (DHN). In another study, Nikoloudakis et al. [97] fabricated a covalently linked nickel (II) porphyrin–ruthenium(II) tris(bipyridyl) dyad for photocatalytic water oxidation reaction in dimethylformamide (DMF) using methyl viologen as a sacrificial electron acceptor.
In another study, Wu et al. [98] reported that the photodegradation yield of 4-chlorophenol was 99.9% after 60 min, and the H2 production rate by oxygen vacancies-enriched titanium dioxide (Ov-TiO2) photoanode was 198.2 μmol h−1 cm−2 that is significantly higher than that on the TiO2 working electrode. For example, in the case of simultaneous organic pollutant oxidation and H2 evolution, the chemical potential of pollutant oxidation can provide the chemical potential needed for hydrogen evolution (H+/H2; 0 V vs. NHE) from water splitting [99]. In another study, dual functional photocatalysis was conducted to tackle both environmental and energy challenges.
Additional examples of various photocatalysts used for simultaneous pollutant photodegradation and H2 evolution are presented in Table 4.
Table 4. A summary of various photocatalysts along with their reaction condition applied in simultaneous photodegradation of pollutant and H2 evolution from water splitting.
| Entry | Photocatalyst | Synthesis Method | Photocatalytic Test Condition | H2 Activity (μmol h−1 g−1) | Pollutant: Photodegradation Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | 0.5 wt.% Pt/Zn-V-20 | Calcination, hydrothermal |
300 W Xe lamp AM 1.5 G filter 0.5 M Na2SO4, 15% CH3OH |
5230.4 | 2,2′,4,4′- tetrahydroxybenzophenone (BP-2): (99.6), methylene blue (MB): (99.4), acetaminophen (AAP): (92.0) |
[100] |
| 2 | g-C3N4/BiOI/CdS | calcination, solvothermal, and solution chemical deposition |
300-W Xe lamp with a λ > 420 nm cutoff filter |
863.44 | bisphenol A: (98.62) | [101] |
| 3 | Mo1@CNNTs | template free polymerization | 300 W xenon lamp coupled with λ = 420 nm cutoff filter. | 4861 | tetracycline hydrochloride: (97.3) | [102] |
| 4 | [g-C3N4/polymethylmethacrylate (PMMA)]// [TiO2/polyaniline (PANI)/PMMA]// [self-assembled 3, 4, 9, 10-perylene tetraformyl diimide (PDI)/PMMA] (TMOP) | tri-axial parallel electrospinning | simulated sunlight | 536.7 | Ciprofloxacin: (88.99), tetracycline hydrochloride: (91.15), chlortetracycline hydrochloride: (77.55), levofloxacin: (69.51), and colored dye methylene blue: (92.50) |
[103] |
| 5 | SCN/NiS-1 | hydrothermal | visible light (400 nm filter) irradiation | 700.9 | Rhodamine B (RhB): (98.5) | [104] |
| 6 | ZnS@Zn0.58Cd0.42S | hydrothermal | Xe lamp (CEL-PF300-T9, CEAU) with an AM1.5G filter | 36000 | Helianthine: (94.2) |
[105] |
| 7 | NbO-BRGO | hydrothermal | 300 W Xe > 400 nm | 1742 | crystal violet (CV): (97.6) | [106] |
| 8 | ZnIn2S4@SiO2@TiO2 | sol–gel and solvothermal |
300 W xenon lamp | 618.3 | methylene blue: (99.7) |
[107] |
| 9 | Ag@TiO2-P25-5%MoS2 | Combination of photocatalysts | solar simulator composed of two white light bulbs (60 watts) | 1792 | Ciprofloxacin: (75) |
[108] |
| 10 | MoS2/ZnO | hydrothermal | 250 W metal halide lamp |
235 | Ciprofloxacin: (89) |
[109] |
References
- Brindha, K.; Mohanraj, S.; Rajaguru, P.; Pugalenthi, V. Simultaneous Production of Renewable Biohydrogen, Biobutanol and Biopolymer from Phytogenic CoNPs-Assisted Clostridial Fermentation for Sustainable Energy and Environment. Sci. Total Environ. 2023, 859, 160002.
- U.S. EIA. International Energy Outlook 2013 with Projections to 2040; U.S. EIA: Washington, DC, USA, 2019.
- Fareza, A.R.; Nugroho, F.A.A.; Abdi, F.; Fauzia, V. Nanoscale Metal Oxides–2D Materials Heterostructures for Photoelectrochemical Water Splitting—A Review. J. Mater. Chem. A 2022, 10, 8656–8686.
- Ostovan, A.; Papior, N.; Zahedi, M.; Moshfegh, A.Z. Towards Developing Efficient Metalloporphyrin-Based Hybrid Photocatalysts for CO2 Reduction; an Ab Initio Study. Phys. Chem. Chem. Phys. 2020, 22, 23128–23140.
- Tahir, M.; Tasleem, S.; Tahir, B. Recent Development in Band Engineering of Binary Semiconductor Materials for Solar Driven Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 15985–16038.
- Naseri, A.; Samadi, M.; Pourjavadi, A.; Moshfegh, A.Z.; Ramakrishna, S. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Solar Hydrogen Generation: Recent Advances and Future Development Directions. J. Mater. Chem. A 2017, 5, 23406–23433.
- Kannan, N.; Vakeesan, D. Solar Energy for Future World: A Review. Renew. Sustain. Energy Rev. 2016, 62, 1092–1105.
- Christoforidis, K.C.; Fornasiero, P. Photocatalysis for Hydrogen Production and CO2 Reduction: The Case of Copper-catalysts. ChemCatChem 2019, 11, 368–382.
- Shandilya, P.; Sambyal, S.; Sharma, R.; Mandyal, P.; Fang, B. Properties, Optimized Morphologies, and Advanced Strategies for Photocatalytic Applications of WO3 Based Photocatalysts. J. Hazard. Mater. 2022, 428, 128218.
- Kumar, P.; Devi, P. Photoelectrochemical Hydrogen Generation; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 9811672849.
- Riffat, M.; Ali, H.; Qayyum, H.A.; Bilal, M.; Hussain, T. Enhanced Solar-Driven Water Splitting by ZnO/CdTe Heterostructure Thin Films-Based Photocatalysts. Int. J. Hydrogen Energy 2023, 48, 22069–22078.
- He, J.; Yang, Z.; Wang, Z.; Fang, R.; Gu, L.; Yan, Y.; Ran, J. Systematic Study of H2 Production from Photothermal Reforming of α-Cellulose over Atomically Thin Bi2MoO6. Energy Convers. Manag. 2023, 277, 116605.
- Grimm, A.; Sainte-Marie, A.; Kramer, G.J.; Gazzani, M. Modeling Photovoltaic-Electrochemical Water Splitting Devices for the Production of Hydrogen under Real Working Conditions. Int. J. Hydrogen Energy 2022, 47, 11764–11777.
- Faraji, M.; Yousefi, M.; Yousefzadeh, S.; Zirak, M.; Naseri, N.; Jeon, T.H.; Choi, W.; Moshfegh, A.Z. Two-Dimensional Materials in Semiconductor Photoelectrocatalytic Systems for Water Splitting. Energy Environ. Sci. 2019, 12, 59–95.
- Nagy, V.; Podmaniczki, A.; Vidal-Meireles, A.; Tengölics, R.; Kovács, L.; Rákhely, G.; Scoma, A.; Tóth, S.Z. Water-Splitting-Based, Sustainable and Efficient H2 Production in Green Algae as Achieved by Substrate Limitation of the Calvin–Benson–Bassham Cycle. Biotechnol. Biofuels 2018, 11, 69.
- Lourenço, A.C.; Reis-Machado, A.S.; Fortunato, E.; Martins, R.; Mendes, M.J. Sunlight-Driven CO2-to-Fuel Conversion: Exploring Thermal and Electrical Coupling between Photovoltaic and Electrochemical Systems for Optimum Solar-Methane Production. Mater. Today Energy 2020, 17, 100425.
- Guo, S.-T.; Tang, Z.-Y.; Du, Y.-W.; Liu, T.; Ouyang, T.; Liu, Z.-Q. Chlorine Anion Stabilized Cu2O/ZnO Photocathode for Selective CO2 Reduction to CH4. Appl. Catal. B Environ. 2023, 321, 122035.
- Wu, L.; Zheng, S.; Lin, H.; Zhou, S.; Idris, A.M.; Wang, J.; Li, S.; Li, Z. In-Situ Assembling 0D/2D Z-Scheme Heterojunction of Lead-Free Cs2AgBiBr6/Bi2WO6 for Enhanced Photocatalytic CO2 Reduction. J. Colloid Interface Sci. 2023, 629, 233–242.
- Kumar, A.; Khosla, A.; Sharma, S.K.; Dhiman, P.; Sharma, G.; Gnanasekaran, L.; Naushad, M.; Stadler, F.J. A Review on S-Scheme and Dual S-Scheme Heterojunctions for Photocatalytic Hydrogen Evolution, Water Detoxification and CO2 Reduction. Fuel 2023, 333, 126267.
- Kaur, I.; Batra, V.; Bogireddy, N.K.R.; Torres, S.; Agarwal, V. Detection of Organic Pollutants, Food Additives and Antibiotics Using Sustainable Carbon Dots. Food Chem. 2022, 406, 135029.
- Sudhaik, A.; Raizada, P.; Ahamad, T.; Alshehri, S.M.; Nguyen, V.-H.; Van Le, Q.; Thakur, S.; Thakur, V.K.; Selvasembian, R.; Singh, P. Recent Advances in Cellulose Supported Photocatalysis for Pollutant Mitigation: A Review. Int. J. Biol. Macromol. 2022, 226, 1284–1308.
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066.
- AlJaberi, F.Y.; Ahmed, S.A.; Makki, H.F.; Naje, A.S.; Zwain, H.M.; Salman, A.D.; Juzsakova, T.; Viktor, S.; Van, B.; Le, P.-C. Recent Advances and Applicable Flexibility Potential of Electrochemical Processes for Wastewater Treatment. Sci. Total Environ. 2023, 867, 161361.
- Yousefi, M.; Villar-Rodil, S.; Paredes, J.I.; Moshfegh, A.Z. Oxidized Graphitic Carbon Nitride Nanosheets as an Effective Adsorbent for Organic Dyes and Tetracycline for Water Remediation. J. Alloys Compd. 2019, 809, 151783.
- Gao, Y.; Gao, W.; Zhu, H.; Chen, H.; Yan, S.; Zhao, M.; Sun, H.; Zhang, J.; Zhang, S. A Review on N-Doped Biochar for Oxidative Degradation of Organic Contaminants in Wastewater by Persulfate Activation. Int. J. Environ. Res. Public Health 2022, 19, 14805.
- Jallouli, S.; Buonerba, A.; Borea, L.; Hasan, S.W.; Belgiorno, V.; Ksibi, M.; Naddeo, V. Living Membrane Bioreactor for Highly Effective and Eco-Friendly Treatment of Textile Wastewater. Sci. Total Environ. 2023, 871, 161963.
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155.
- Boczkaj, G.; Fernandes, A. Wastewater Treatment by Means of Advanced Oxidation Processes at Basic pH Conditions: A Review. Chem. Eng. J. 2017, 320, 608–633.
- Glaze, W.H.; Kang, J.-W.; Chapin, D.H. The Chemistry of Water Treatment Processes Involving Ozone, Hydrogen Peroxide and Ultraviolet Radiation. J. Int. Ozone Assoc. 1987, 9, 335–352.
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336.
- Xie, Z.-H.; He, C.-S.; Pei, D.-N.; Dong, Y.; Yang, S.-R.; Xiong, Z.; Zhou, P.; Pan, Z.-C.; Yao, G.; Lai, B. Review of Characteristics, Generation Pathways and Detection Methods of Singlet Oxygen Generated in Advanced Oxidation Processes (AOPs). Chem. Eng. J. 2023, 468, 143778.
- Xu, L.; Li, L.; Yu, L.; Jimmy, C.Y. Efficient Generation of Singlet Oxygen on Modified g-C3N4 Photocatalyst for Preferential Oxidation of Targeted Organic Pollutants. Chem. Eng. J. 2022, 431, 134241.
- Li, Z.; Sun, Y.; Liu, D.; Yi, M.; Chang, F.; Li, H.; Du, Y. A Review of Sulfate Radical-Based and Singlet Oxygen-Based Advanced Oxidation Technologies: Recent Advances and Prospects. Catalysts 2022, 12, 1092.
- Wang, J.; Wang, S. Reactive Species in Advanced Oxidation Processes: Formation, Identification and Reaction Mechanism. Chem. Eng. J. 2020, 401, 126158.
- Kumar, V.; Shah, M.P. Advanced Oxidation Processes for Complex Wastewater Treatment. In Advanced Oxidation Processes for Effluent Treatment Plants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–31.
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering 2022, 6, 8.
- Kheirabadi, M.; Samadi, M.; Asadian, E.; Zhou, Y.; Dong, C.; Zhang, J.; Moshfegh, A.Z. Well-Designed Ag/ZnO/3D Graphene Structure for Dye Removal: Adsorption, Photocatalysis and Physical Separation Capabilities. J. Colloid Interface Sci. 2019, 537, 66–78.
- Wang, W.; Sha, J.; Lu, Z.; Shao, S.; Sun, P.; Hu, Q.; Zhang, X. Implementation of UV-Based Advanced Oxidation Processes in Algal Medium Recycling. Sci. Total Environ. 2018, 634, 243–250.
- Wang, T.; Bai, Y.; Si, W.; Mao, W.; Gao, Y.; Liu, S. Heterogeneous Photo-Fenton System of Novel Ternary Bi2WO6/BiFeO3/g-C3N4 Heterojunctions for Highly Efficient Degrading Persistent Organic Pollutants in Wastewater. J. Photochem. Photobiol. A Chem. 2021, 404, 112856.
- Yang, Y.; Jiang, J.; Lu, X.; Ma, J.; Liu, Y. Production of Sulfate Radical and Hydroxyl Radical by Reaction of Ozone with Peroxymonosulfate: A Novel Advanced Oxidation Process. Environ. Sci. Technol. 2015, 49, 7330–7339.
- Hassan, M.; Olvera-Vargas, H.; Zhu, X.; Zhang, B.; He, Y. Microbial Electro-Fenton: An Emerging and Energy-Efficient Platform for Environmental Remediation. J. Power Sources 2019, 424, 220–244.
- Matafonova, G.; Batoev, V. Review on Low-and High-Frequency Sonolytic, Sonophotolytic and Sonophotochemical Processes for Inactivating Pathogenic Microorganisms in Aqueous Media. Water Res. 2019, 166, 115085.
- Baaloudj, O.; Kenfoud, H.; Badawi, A.K.; Assadi, A.A.; El Jery, A.; Assadi, A.A.; Amrane, A. Bismuth Sillenite Crystals as Recent Photocatalysts for Water Treatment and Energy Generation: A Critical Review. Catalysts 2022, 12, 500.
- Khan, I.; Saeed, K.; Ali, N.; Khan, I.; Zhang, B.; Sadiq, M. Heterogeneous Photodegradation of Industrial Dyes: An Insight to Different Mechanisms and Rate Affecting Parameters. J. Environ. Chem. Eng. 2020, 8, 104364.
- Wang, X.; Liu, X.; Wu, Y.; Fu, Y.; Zhang, H.; Zhou, M.; Wang, Y. Thinnest Npn Homojunction for Inspired Photoelectrochemical Water Splitting. Appl. Catal. B Environ. 2023, 323, 122182.
- Wang, J.; Ni, G.; Liao, W.; Liu, K.; Chen, J.; Liu, F.; Zhang, Z.; Jia, M.; Li, J.; Fu, J. Subsurface Engineering Induced Fermi Level De-pinning in Metal Oxide Semiconductors for Photoelectrochemical Water Splitting. Angew. Chem. Int. Ed. 2023, 62, e202217026.
- Zheng, Y.; Ruan, Q.; Ren, J.; Guo, X.; Zhou, Y.; Zhou, B.; Xu, Q.; Fu, Q.; Wang, S.; Huang, Y. Plasma-Assisted Liquid-Based Growth of g-C3N4/Mn2O3 Pn Heterojunction with Tunable Valence Band for Photoelectrochemical Application. Appl. Catal. B Environ. 2023, 323, 122170.
- Zabelina, A.; Miliutina, E.; Zabelin, D.; Burtsev, V.; Buravets, V.; Elashnikov, R.; Neubertova, V.; Šťastný, M.; Popelková, D.; Lancok, J. Plasmon Coupling inside 2D-like TiB2 Flakes for Water Splitting Half Reactions Enhancement in Acidic and Alkaline Conditions. Chem. Eng. J. 2023, 454, 140441.
- Wen, P.; Lei, R.; Cao, X.; Ma, Q.; Zhang, G.; Guo, C.; Wang, X.; Qiu, Y. Anchored Ni Nanocrystals Boosting BiVO4 Photoanode for Highly Efficient Water Oxidation via In-Situ Generation of Co-Catalyst. Chem. Eng. J. 2023, 454, 139983.
- Lei, Y.; Si, W.; Wang, Y.; Tan, H.; Di, L.; Wang, L.; Liang, J.; Hou, F. Robust Carbon Nitride Homojunction Photoelectrode for Solar-Driven Water Splitting. ACS Appl. Mater. Interfaces 2023, 15, 6726–6734.
- Fernandez-Izquierdo, L.; Spera, E.L.; Durán, B.; Marotti, R.E.; Dalchiele, E.A.; Del Rio, R.; Hevia, S.A. CVD Growth of Hematite Thin Films for Photoelectrochemical Water Splitting: Effect of Precursor-Substrate Distance on Their Final Properties. Molecules 2023, 28, 1954.
- Jun, S.E.; Kim, Y.-H.; Kim, J.; Cheon, W.S.; Choi, S.; Yang, J.; Park, H.; Lee, H.; Park, S.H.; Kwon, K.C. Atomically Dispersed Iridium Catalysts on Silicon Photoanode for Efficient Photoelectrochemical Water Splitting. Nat. Commun. 2023, 14, 609.
- Zhang, J.; Wei, X.; Zhao, J.; Zhang, Y.; Wang, L.; Huang, J.; She, H.; Wang, Q. Electronegative Cl− Modified BiVO4 Photoanode Synergized with Nickel Hydroxide Cocatalyst for High-Performance Photoelectrochemical Water Splitting. Chem. Eng. J. 2023, 454, 140081.
- Moon, S.; Park, J.; Lee, H.; Yang, J.W.; Yun, J.; Park, Y.S.; Lee, J.; Im, H.; Jang, H.W.; Yang, W. Bi2S3-Cu3BiS3 Mixed Phase Interlayer for High-Performance Cu3BiS3-Photocathode for 2.33% Unassisted Solar Water Splitting Efficiency. Adv. Sci. 2023, 10, 2206286.
- Liu, B.; Wang, X.; Zhang, Y.; Xu, L.; Wang, T.; Xiao, X.; Wang, S.; Wang, L.; Huang, W. A BiVO4 Photoanode with a VOx Layer Bearing Oxygen Vacancies Offers Improved Charge Transfer and Oxygen Evolution Kinetics in Photoelectrochemical Water Splitting. Angew. Chem. Int. Ed. 2023, 62, e202217346.
- Gulati, S.; Vijayan, S.; Kumar, S.; Harikumar, B.; Trivedi, M.; Varma, R.S. Recent Advances in the Application of Metal-Organic Frameworks (MOFs)-Based Nanocatalysts for Direct Conversion of Carbon Dioxide (CO2) to Value-Added Chemicals. Coord. Chem. Rev. 2023, 474, 214853.
- Tafreshi, S.S.; Moshfegh, A.Z.; de Leeuw, N.H. Mechanism of Photocatalytic Reduction of CO2 by Ag3PO4 (111)/g-C3N4 Nanocomposite: A First-Principles Study. J. Phys. Chem. C 2019, 123, 22191–22201.
- Bao, X.; Lu, D.; Wang, Z.; Yin, H.; Zhu, B.; Chen, B.; Shi, M.; Zhang, Y.; Xu, Q.; Qin, Y. Significantly Enhanced Photothermal Catalytic CO2 Reduction over TiO2/g-C3N4 Composite with Full Spectrum Solar Light. J. Colloid Interface Sci. 2023, 638, 63–75.
- Zhang, Q.; Gao, S.; Guo, Y.; Wang, H.; Wei, J.; Su, X.; Zhang, H.; Liu, Z.; Wang, J. Designing Covalent Organic Frameworks with Co-O4 Atomic Sites for Efficient CO2 Photoreduction. Nat. Commun. 2023, 14, 1147.
- Shindell, D.; Smith, C.J. Climate and Air-Quality Benefits of a Realistic Phase-out of Fossil Fuels. Nature 2019, 573, 408–411.
- Bian, H.; Liu, T.; Li, D.; Xu, Z.; Lian, J.; Chen, M.; Yan, J.; Liu, S.F. Unveiling the Effect of Interstitial Dopants on CO2 Activation over CsPbBr3 Catalyst for Efficient Photothermal CO2 Reduction. Chem. Eng. J. 2022, 435, 135071.
- Liang, J.; Yu, H.; Shi, J.; Li, B.; Wu, L.; Wang, M. Dislocated Bilayer MOF Enables High-selectivity Photocatalytic Reduction of CO2 to CO. Adv. Mater. 2023, 35, 2209814.
- Hu, K.; Huang, Z.; Li, X.; Cheng, Y.; Kong, X.; Mei, L.; Zeng, L.; Zhang, Z.; Yu, J.; Gibson, J.K. Tailored Persistent Radical-containing Heterotrimetal-Organic Framework for Boosting Efficiency of Visible/NIR Light-driven Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2023, 33, 2213039.
- Du, Z.; Cai, H.; Zhao, Z.; Guo, Z.; Lin, J.; Huang, Y.; Tang, C.; Chen, G.; Fang, Y. Facile Synthesis of Graphene Quantum Dots and C-Doping Porous BN Nanoribbon Heterojunctions for Boosting CO2 Photoreduction. Sep. Purif. Technol. 2023, 311, 123321.
- Han, B.; Lin, Y.; Luo, Y.; Liu, W.; Liu, X.; Weng, Z.; Ou, X.; Lin, Z. Low-Crystalline Ni(OH)2 Ultrathin Nanosheets Upcycled from Solid Wastes for Efficient Photoconversion of Low-Concentration CO2. Chem. Eng. J. 2023, 460, 140507.
- Liu, J.; Liu, M.; Zheng, S.; Liu, X.; Yao, S.; Jing, F.; Chen, G. Interfacial Intimacy and Internal Electric Field Modulated S-Scheme Sv-ZnS/ZnIn2S4 Photocatalyst for Efficient H2 Evolution and CO2 Reduction. J. Colloid Interface Sci. 2023, 635, 284–294.
- Liu, Z.; Liang, J.; Song, Q.; Li, Y.; Zhang, Z.; Zhou, M.; Wei, W.; Xu, H.; Lee, C.-S.; Li, H. Construction Atomic-Level NP Charge Transfer Channel for Boosted CO2 Photoreduction. Appl. Catal. B Environ. 2023, 328, 122472.
- Tang, J.; Li, X.; Ma, Y.; Wang, K.; Liu, Z.; Zhang, Q. Boosting Exciton Dissociation and Charge Transfer by Regulating Dielectric Constant in Polymer Carbon Nitride for CO2 Photoreduction. Appl. Catal. B Environ. 2023, 327, 122417.
- Wang, Y.; Xie, Y.; Yu, S.; Yang, K.; Shao, Y.; Zou, L.; Zhao, B.; Wang, Z.; Ling, Y.; Chen, Y. Ni Doping in Unit Cell of BiOBr to Increase Dipole Moment and Induce Spin Polarization for Promoting CO2 Photoreduction via Enhanced Build-in Electric Field. Appl. Catal. B Environ. 2023, 327, 122420.
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic Degradation of Dyes Using Semiconductor Photocatalysts to Clean Industrial Water Pollution. J. Ind. Eng. Chem. 2021, 97, 111–128.
- Solayman, H.M.; Hossen, M.A.; Abd Aziz, A.; Yahya, N.Y.; Hon, L.K.; Ching, S.L.; Monir, M.U.; Zoh, K.-D. Performance Evaluation Of Dye Wastewater Treatment Technologies: A Review. J. Environ. Chem. Eng. 2023, 11, 109610.
- Zhang, L.-L.; Zaoui, A.; Sekkal, W.; Zheng, Y.-Y. Interlayer Adsorption of Cationic Dye on Cationic Surfactant-Modified and Unmodified Montmorillonite. J. Hazard. Mater. 2023, 442, 130107.
- Lin, Z.; Wang, R.; Tan, S.; Zhang, K.; Yin, Q.; Zhao, Z.; Gao, P. Nitrogen-Doped Hydrochar Prepared by Biomass and Nitrogen-Containing Wastewater for Dye Adsorption: Effect of Nitrogen Source in Wastewater on the Adsorption Performance of Hydrochar. J. Environ. Manag. 2023, 334, 117503.
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430.
- Silori, R.; Shrivastava, V.; Singh, A.; Sharma, P.; Aouad, M.; Mahlknecht, J.; Kumar, M. Global Groundwater Vulnerability for Pharmaceutical and Personal Care Products (PPCPs): The Scenario of Second Decade of 21st Century. J. Environ. Manag. 2022, 320, 115703.
- Kumar, M.; Sridharan, S.; Sawarkar, A.D.; Shakeel, A.; Anerao, P.; Mannina, G.; Sharma, P.; Pandey, A. Current Research Trends on Emerging Contaminants Pharmaceutical and Personal Care Products (PPCPs): A Comprehensive Review. Sci. Total Environ. 2023, 859, 160031.
- Zhang, C.; Chen, Y.; Chen, S.; Guan, X.; Zhong, Y.; Yang, Q. Occurrence, Risk Assessment, and in Vitro and in Vivo Toxicity of Antibiotics in Surface Water in China. Ecotoxicol. Environ. Saf. 2023, 255, 114817.
- Chen, X.; Lei, L.; Liu, S.; Han, J.; Li, R.; Men, J.; Li, L.; Wei, L.; Sheng, Y.; Yang, L. Occurrence and Risk Assessment of Pharmaceuticals and Personal Care Products (PPCPs) against COVID-19 in Lakes and WWTP-River-Estuary System in Wuhan, China. Sci. Total Environ. 2021, 792, 148352.
- Rapti, I.; Boti, V.; Albanis, T.; Konstantinou, I. Photocatalytic Degradation of Psychiatric Pharmaceuticals in Hospital WWTP Secondary Effluents Using G-C3N4 and g-C3N4/MoS2 Catalysts in Laboratory-Scale Pilot. Catalysts 2023, 13, 252.
- Abdurahman, M.H.; Abdullah, A.Z.; Da Oh, W.; Shopware, N.F.; Gasim, M.F.; Okoye, P.; Ul-Hamid, A.; Mohamed, A.R. Tunable Band Structure of Synthesized Carbon Dots Modified Graphitic Carbon Nitride/Bismuth Oxychlorobromide Heterojunction for Photocatalytic Degradation of Tetracycline in Water. J. Colloid Interface Sci. 2023, 629, 189–205.
- Rasouli, K.; Alamdari, A.; Sabbaghi, S. Ultrasonic-Assisted Synthesis of α-Fe2O3@TiO2 Photocatalyst: Optimization of Effective Factors in the Fabrication of Photocatalyst and Removal of Non-Biodegradable Cefixime via Response Surface Methodology-Central Composite Design. Sep. Purif. Technol. 2023, 307, 122799.
- Sun, P.; Liu, Y.; Mo, F.; Wu, M.; Xiao, Y.; Xiao, X.; Wang, W.; Dong, X. Efficient Photocatalytic Degradation of High-Concentration Moxifloxacin over Dodecyl Benzene Sulfonate Modified Graphitic Carbon Nitride: Enhanced Photogenerated Charge Separation and Pollutant Enrichment. J. Clean. Prod. 2023, 393, 136320.
- Krishnan, S.; Shriwastav, A. Chlorophyll Sensitized and Salicylic Acid Functionalized TiO2 Nanoparticles as a Stable and Efficient Catalyst for the Photocatalytic Degradation of Ciprofloxacin with Visible Light. Environ. Res. 2023, 216, 114568.
- Yang, J.; Fang, L.; Li, Z.; Meng, G.; Jia, Y.; Jiang, Y.; Lian, J.; Gan, X. Insights into the Formation of Environmentally Persistent Free Radicals during Photocatalytic Degradation Processes of Ceftriaxone Sodium by ZnO/ZnIn2S4. Chemosphere 2023, 314, 137618.
- Saravanakumar, K.; Yun, K.; Maheskumar, V.; Yea, Y.; Jagan, G.; Park, C.M. Construction of Novel In2S3/Ti3C2 MXene Quantum Dots/SmFeO3 Z-Scheme Heterojunctions for Efficient Photocatalytic Removal of Sulfamethoxazole and 4-Chlorophenol: Degradation Pathways and Mechanism Insights. Chem. Eng. J. 2023, 451, 138933.
- Nayak, S.; Das, K.K.; Parida, K. Indulgent of the Physiochemical Features of MgCr-LDH Nanosheets towards Photodegradation Process of Methylene Blue. J. Colloid Interface Sci. 2023, 634, 121–137.
- Fatima, B.; Siddiqui, S.I.; Rajor, H.K.; Malik, M.A.; Narasimharao, K.; Ahmad, R.; Vikrant, K.; Kim, T.; Kim, K.-H. Photocatalytic Removal of Organic Dye Using Green Synthesized Zinc Oxide Coupled Cadmium Tungstate Nanocomposite under Natural Solar Light Irradiation. Environ. Res. 2023, 216, 114534.
- Chen, X.; Xu, Z.; Chen, J.; Yao, L.; Xie, W.; He, J.; Li, N.; Li, J.; Xu, S.; Zhu, Y. Continuous Surface Z-Scheme and Schottky Heterojunction Au/La2Ti2O7/Ag3PO4 Catalyst with Boosted Charge Separation through Dual Channels for Excellent Photocatalysis: Highlight Influence Factors Regulation and Catalytic System Applicability. Sep. Purif. Technol. 2023, 312, 123414.
- Lin, Z.; Wu, Y.; Jin, X.; Liang, D.; Jin, Y.; Huang, S.; Wang, Z.; Liu, H.; Chen, P.; Lv, W. Facile Synthesis of Direct Z-Scheme UiO-66-NH2/PhC2Cu Heterojunction with Ultrahigh Redox Potential for Enhanced Photocatalytic Cr (VI) Reduction and NOR Degradation. J. Hazard. Mater. 2023, 443, 130195.
- Sun, H.; Lee, S.-Y.; Park, S.-J. Bimetallic CuPd Alloy Nanoparticles Decorated ZnO Nanosheets with Enhanced Photocatalytic Degradation of Methyl Orange Dye. J. Colloid Interface Sci. 2023, 629, 87–96.
- Mukherjee, A.; Dhak, P.; Hazra, V.; Goswami, N.; Dhak, D. Synthesis of Mesoporous Fe/Al/La Trimetallic Oxide for Photodegradation of Various Water-Soluble Dyes: Kinetic, Mechanistic, and PH Studies. Environ. Res. 2023, 217, 114862.
- Alamgholiloo, H.; Pesyan, N.N.; Marjani, A.P. Visible-Light-Responsive Z-Scheme α-Fe2O3/SWCNT/NH2-MIL-125 Heterojunction for Boosted Photodegradation of Ofloxacin. Sep. Purif. Technol. 2023, 305, 122442.
- Ray, S.K.; Reddy, P.A.K.; Yoon, S.; Shin, J.; Chon, K.; Bae, S. A Magnetically Separable α-NiMoO4/ZnFe2O4/Coffee Biochar Heterojunction Photocatalyst for Efficient Ketoprofen Degradation. Chem. Eng. J. 2023, 452, 139546.
- Zhang, F.; Xiao, X.; Xiao, Y.; Cheng, X. Construction of Novel 0D/2D AgI/CAU-17 Heterojunction with Excellent Photocatalytic Performance by in Situ Deposition-Precipitation. J. Environ. Chem. Eng. 2023, 11, 109641.
- Kim, J.; Choi, W. Hydrogen Producing Water Treatment through Solar Photocatalysis. Energy Environ. Sci. 2010, 3, 1042–1045.
- Nugmanova, A.G.; Safonova, E.A.; Baranchikov, A.E.; Tameev, A.R.; Shkolin, A.V.; Mitrofanov, A.A.; Eliseev, A.A.; Meshkov, I.N.; Kalinina, M.A. Interfacial Self-Assembly of Porphyrin-Based SURMOF/Graphene Oxide Hybrids with Tunable Pore Size: An Approach toward Size-Selective Ambivalent Heterogeneous Photocatalysts. Appl. Surf. Sci. 2022, 579, 152080.
- Nikoloudakis, E.; Alsaleh, A.Z.; Charalambidis, G.; Coutsolelos, A.G.; D’Souza, F. A Covalently Linked Nickel (Ii) Porphyrin–Ruthenium (Ii) Tris (Bipyridyl) Dyad for Efficient Photocatalytic Water Oxidation. Chem. Commun. 2022, 58, 12078–12081.
- Wu, J.; Tao, Y.; Zhang, C.; Zhu, Q.; Zhang, D.; Li, G. Activation of Chloride by Oxygen Vacancies-Enriched TiO2 Photoanode for Efficient Photoelectrochemical Treatment of Persistent Organic Pollutants and Simultaneous H2 Generation. J. Hazard. Mater. 2023, 443, 130363.
- Naseri, A.; Asghari Sarabi, G.; Samadi, M.; Yousefi, M.; Ebrahimi, M.; Moshfegh, A.Z. Recent Advances on Dual-Functional Photocatalytic Systems for Combined Removal of Hazardous Water Pollutants and Energy Generation. Res. Chem. Intermed. 2022, 48, 911–933.
- Zhu, C.; Lu, L.; Xu, J.; Song, S.; Fang, Q.; Liu, R.; Shen, Y.; Zhao, J.; Dong, W.; Shen, Y. Metal Monovacancy-Induced Spin Polarization for Simultaneous Energy Recovery and Wastewater Purification. Chem. Eng. J. 2023, 451, 138537.
- Zhang, Y.; Wu, Y.; Wan, L.; Yang, W.; Ding, H.; Lu, C.; Zhang, W.; Xing, Z. Double Z-Scheme g-C3N4/BiOI/CdS Heterojunction with I3−/I− Pairs for Enhanced Visible Light Photocatalytic Performance. Green Energy Environ. 2022, 7, 1377–1389.
- Mateen, M.; Cheong, W.-C.; Zheng, C.; Talib, S.H.; Zhang, J.; Zhang, X.; Liu, S.; Chen, C.; Li, Y. Molybdenum Atomic Sites Embedded 1D Carbon Nitride Nanotubes as Highly Efficient Bifunctional Photocatalyst for Tetracycline Degradation and Hydrogen Evolution. Chem. Eng. J. 2023, 451, 138305.
- Sun, F.; Xie, Y.; Xu, D.; Liu, F.; Qi, H.; Ma, Q.; Yang, Y.; Yu, H.; Yu, W.; Dong, X. Electrospun Self-Supporting Double Z-Scheme Tricolor-Typed Microfiber Oriented-Heterostructure Photocatalyst with Highly Effective Hydrogen Evolution and Organic Pollutants Degradation. J. Environ. Chem. Eng. 2023, 11, 109169.
- Zhou, G.; Zhou, Z.; Xia, Y.; Yin, W.; Hou, J.; Zhu, X.; Yi, J.; Wang, S.; Ning, X.; Wang, X. Synchronous Synthesis of S-Doped Carbon Nitride/Nickel Sulfide Photocatalysts for Efficient Dye Degradation and Hydrogen Evolution. Appl. Surf. Sci. 2023, 608, 154974.
- Bi, L.; Liu, J.; Du, M.; Huang, B.; Song, M.; Jiang, G. In-Situ Upcycling of Cadmium from Wastewater into Core–Shell Zn0. 58Cd0. 42S Heterojunction Photocatalyst for Environmental Purification and H2 Evolution. Chem. Eng. J. 2023, 454, 140258.
- Hamzad, S.; Kumar, K.-Y.; Prashanth, M.K.; Radhika, D.; Parashuram, L.; Alharti, F.-A.; Jeon, B.-H.; Raghu, M.S. Boron Doped RGO from Discharged Dry Cells Decorated Niobium Pentoxide for Enhanced Visible Light-Induced Hydrogen Evolution and Water Decontamination. Surf. Interfaces 2023, 36, 102544.
- Wang, L.; Zhou, H.; Zhang, H.; Song, Y.; Zhang, H.; Qian, X. SiO2@TiO2 Nanoparticles Deposited on 2D-Layered ZnIn2S4 to Form a Ternary Heterostructure for Simultaneous Photocatalytic Hydrogen Production and Organic Pollutant Degradation. Inorg. Chem. 2020, 59, 2278–2287.
- Machín, A.; Fontánez, K.; García, D.; Sampayo, P.; Colón-Cruz, C.; Claudio-Serrano, G.J.; Soto-Vázquez, L.; Resto, E.; Petrescu, F.I.; Morant, C. Hydrogen Production and Degradation of Ciprofloxacin by 2-MoS2 Photocatalysts. Catalysts 2022, 12, 267.
- Hunge, Y.M.; Yadav, A.A.; Kang, S.-W.; Lim, S.J.; Kim, H. Visible Light Activated MoS2/ZnO Composites for Photocatalytic Degradation of Ciprofloxacin Antibiotic and Hydrogen Production. J. Photochem. Photobiol. A Chem. 2023, 434, 114250.
More
Information
Subjects:
Chemistry, Applied
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
699
Revisions:
2 times
(View History)
Update Date:
26 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




