
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | M.N.A. Uda | -- | 3237 | 2023-07-24 21:36:50 | | | |
| 2 | Lindsay Dong | Meta information modification | 3237 | 2023-07-25 02:42:43 | | |
Video Upload Options
Owing to its non-toxicity, biodegradability, and biocompatibility, starch is a naturally occurring polysaccharide that scientists are looking into as a possible environmentally friendly material for sustainable water remediation. Starch could exhibit significant adsorption capabilities towards pollutants with the substitution of amide, amino, carboxyl, and other functional groups for hydroxyl groups. Starch derivatives may effectively remove contaminants such as oil, organic solvents, pesticides, heavy metals, dyes, and pharmaceutical pollutants by employing adsorption techniques at a rate greater than 90%. The maximal adsorption capacities of starch-based adsorbents for oil and organic solvents, pesticides, heavy metal ions, dyes, and pharmaceuticals are 13,000, 66, 2000, 25,000, and 782 mg/g, respectively.
1. Introduction
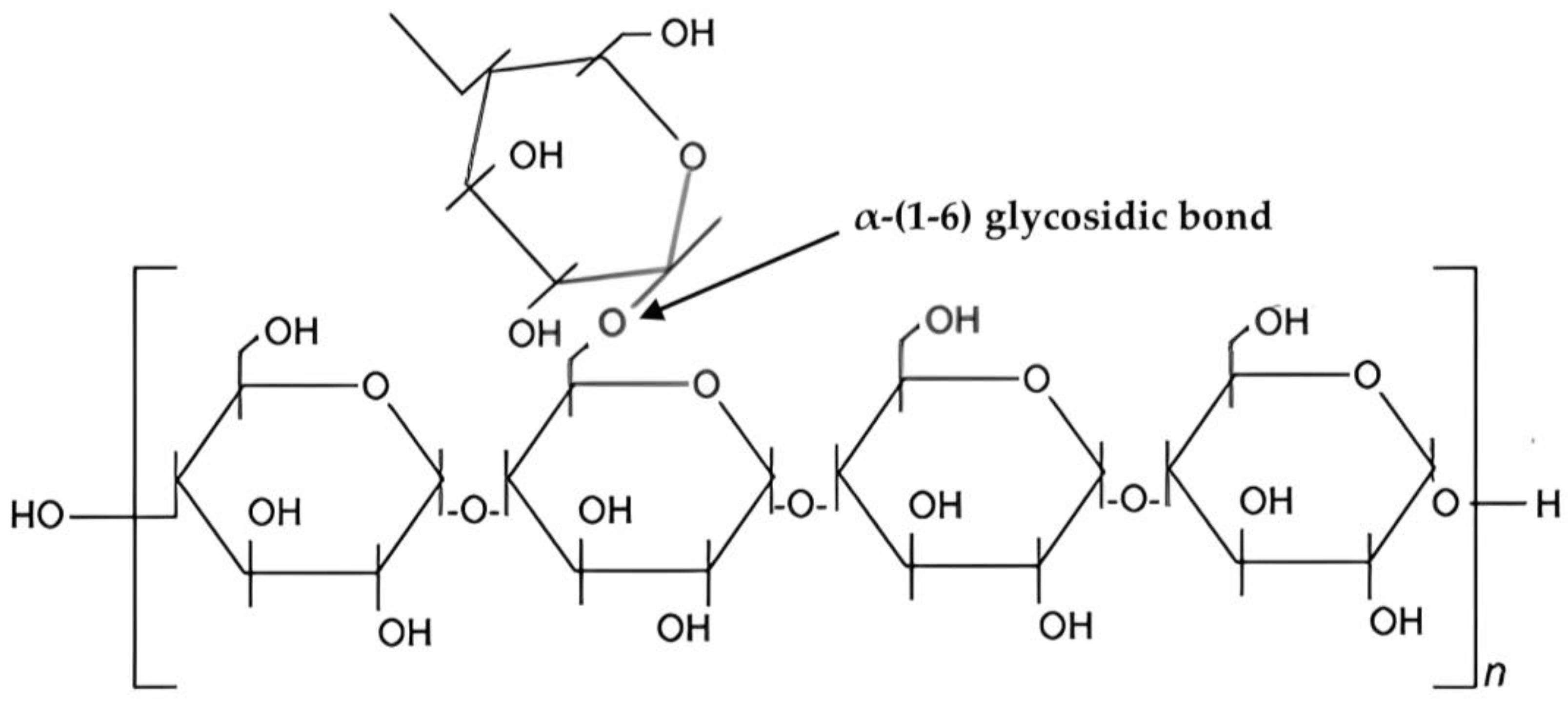
Starch is a carbohydrate and a natural component of most plants, is commercially derived from grains and serves as an important raw material in various industries such as medicine, food, chemicals, etc. Starch has been extensively studied for its potential in wastewater treatment [1][2], it is a relatively good option for wastewater treatment due to its chemical structure, biocompatibility, and biodegradability which can enhance its utilization as green adsorbent. Cassava starch, rice starch, corn starch, and potato starch are documented botanical sources of starch [3]. Starch molecules exist in two structural forms: amylose and amylopectin. Amylopectin contains a higher glucose content compared to amylose [4]. In its natural state, amylose accounts for about 20–30% of starch and amylopectin accounts for 70–80%. Starch is primarily synthesized in the chloroplast of plant leaves or the amyloplast of plant storage organs and contains lipids as well as phosphate groups [5]. However, native starch has considerable limitations when used in wastewater treatment. These limitations include low surface area, limited thermal stability, low water solubility, low molecular weight, quick degradability in water, and a lack of reactive functional groups [6][7]. To overcome these limitations and enhance its adsorption capabilities for wastewater treatment, researchers have explored various modifications of starch. Researchers discovered that incorporating a chemical functional group into the starch backbone improves the adsorption efficacy of modified starch to a variety of pollutants [6][7][8]. Several starch modifications, including starch-based grafts, polymer nanocomposites, nanofibers, nanoparticles, activated carbon, biochar, hydrogels, aerogels, and beads, have been developed to overcome these limitations [6]. Through modification and functionalization approaches, ongoing research aims to improve the adsorption capacity and selectivity of starch-based adsorbents.
2. Adsorption
2.1. Adsorbents
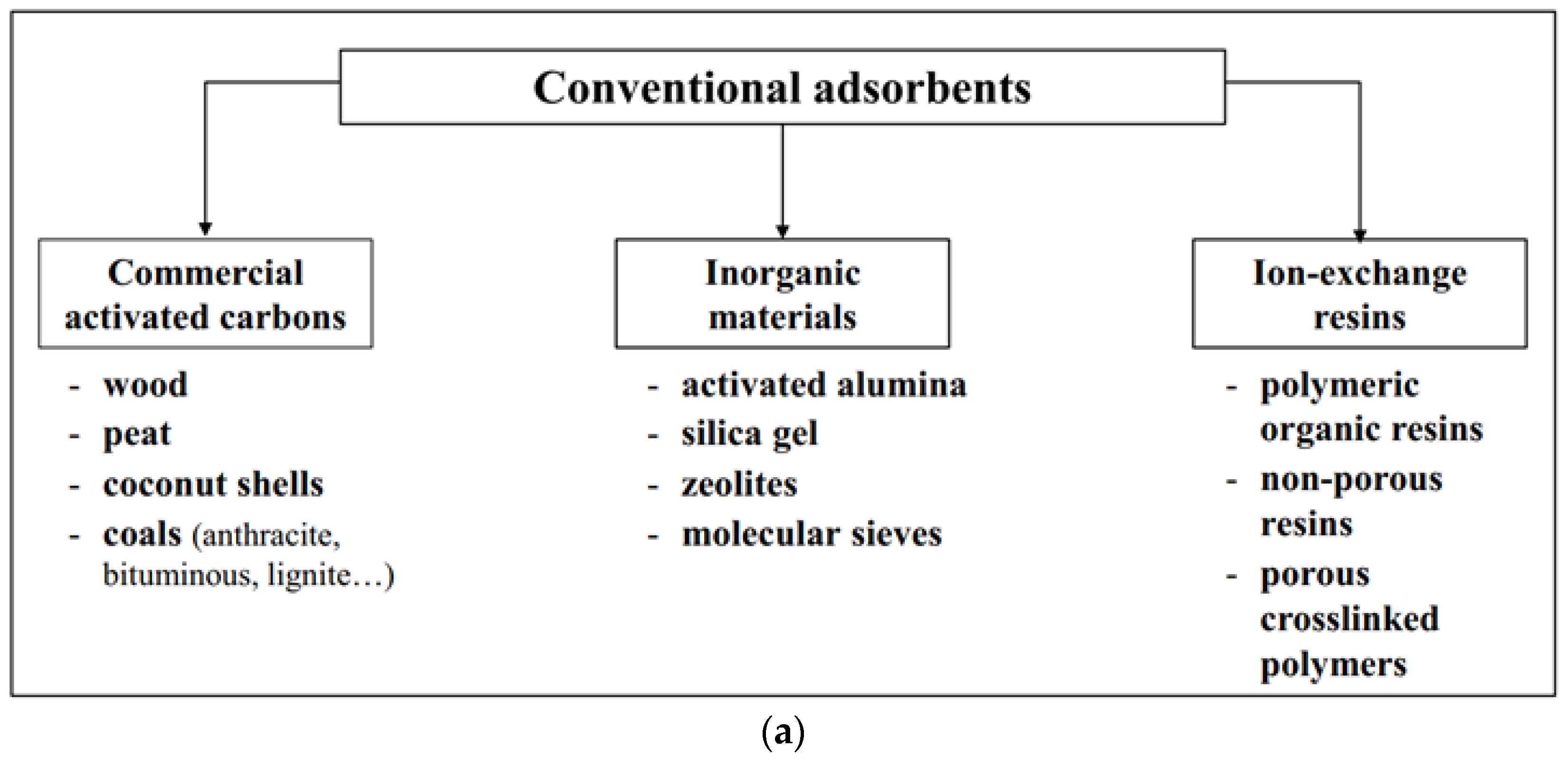
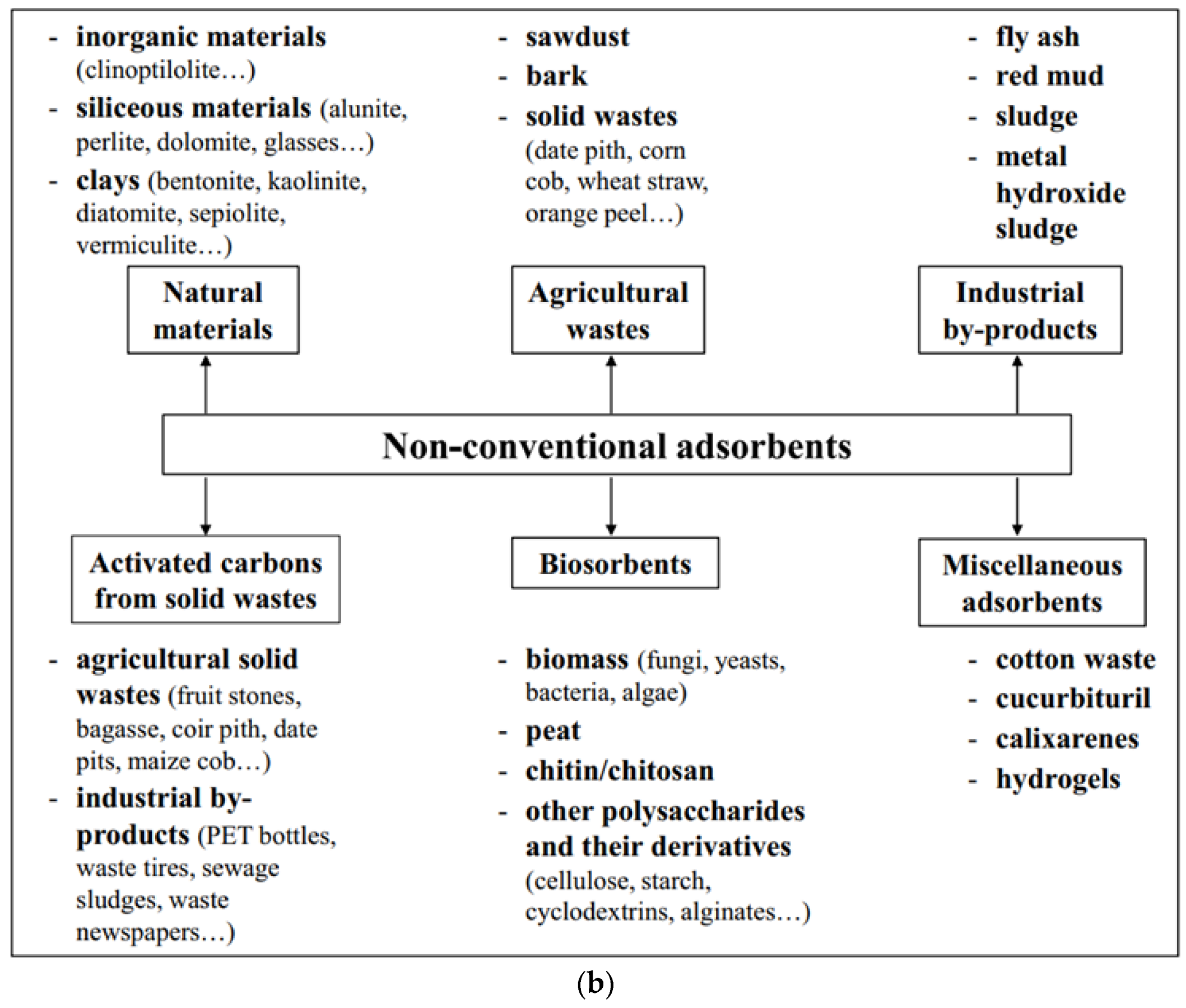
2.2. Starch-Based Adsorbents
2.3. Starch-Based Adsorbents for In situ and Ex Situ Water Remediation
3. Chemical Structure and Properties of Starch
Chemistry and Properties
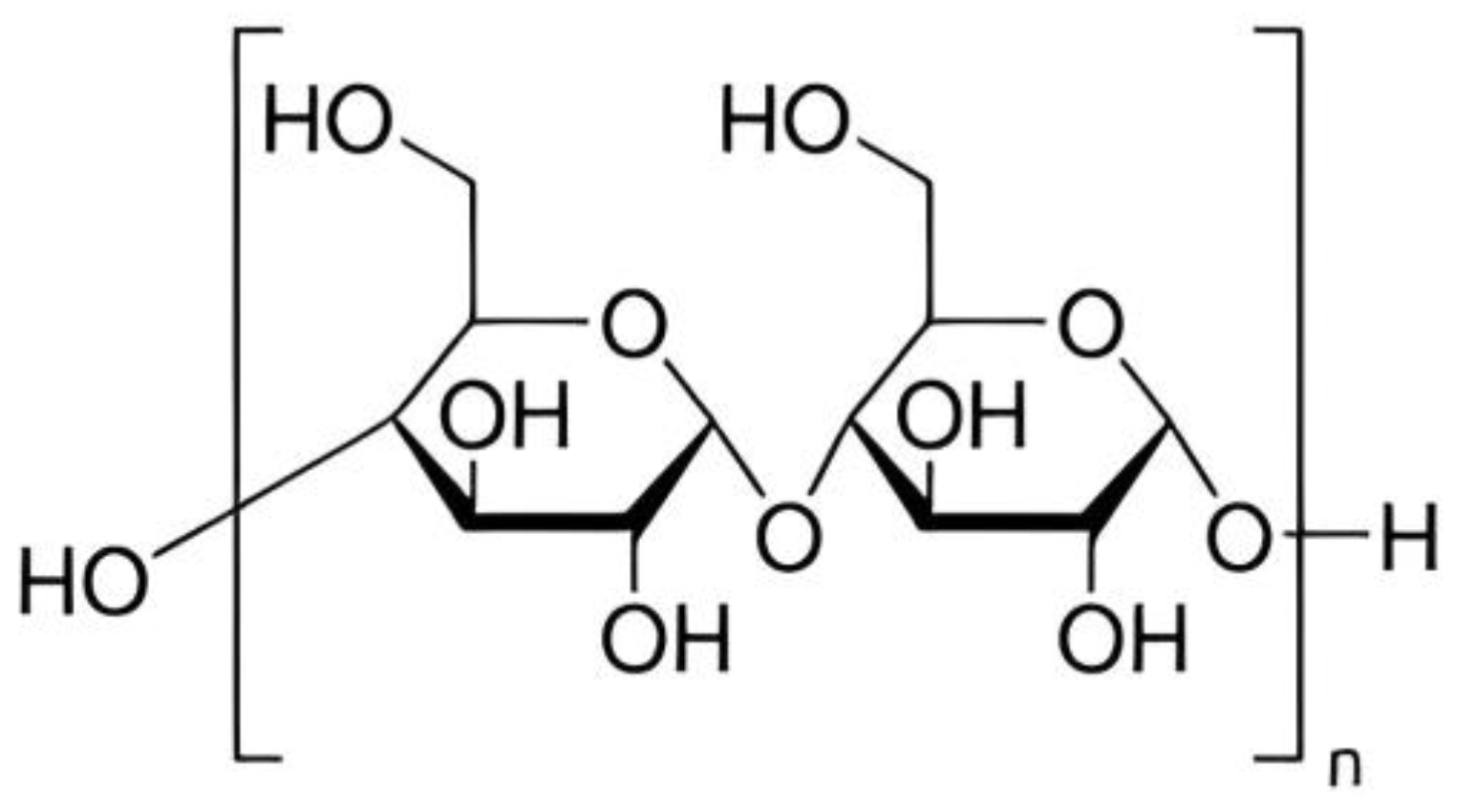
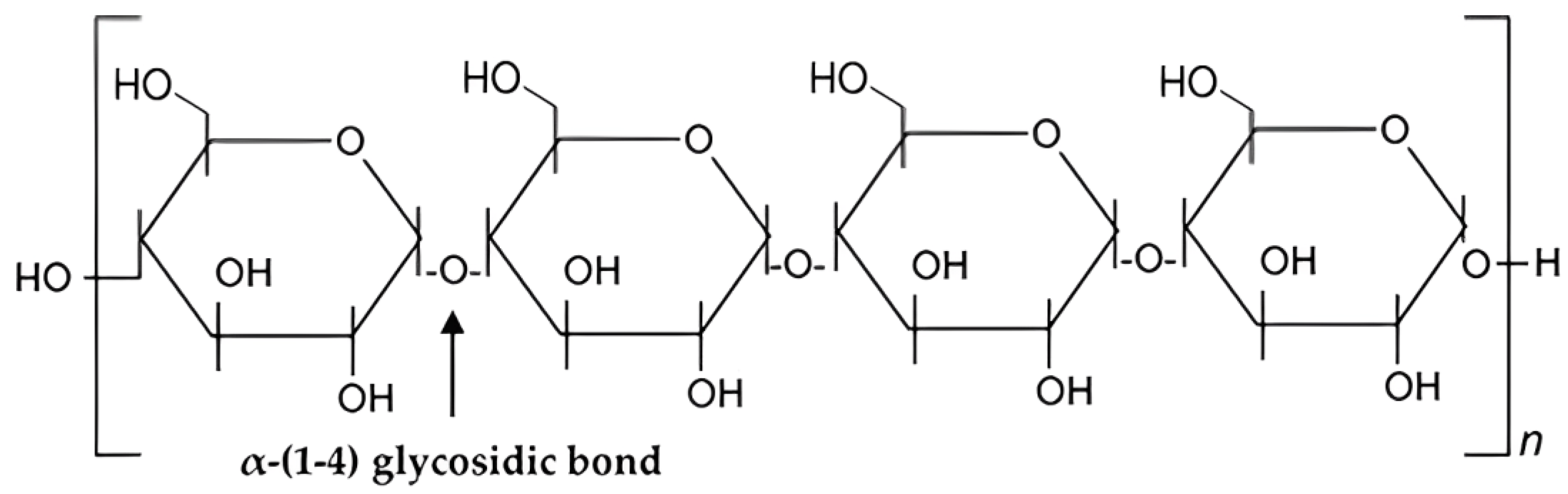
4. Applications for Water Treatment
4.1. Removal of Oil and Organic Solvent
4.2. Removal of Pesticides
4.3. Removal of Heavy Metal Ions
4.4. Removal of Dye
4.5. Removal of Pharmaceutical Pollutants
5. Conclusions
References
- Ahamad, T.; Naushad, M.; Mousa, R.H.; Alshehri, S.M. Fabrication of Starch-Salicylaldehyde Based Polymer Nanocomposite (PNC) for the Removal of Pollutants from Contaminated Water. Int. J. Biol. Macromol. 2020, 165, 2731–2738.
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.P.; Fernando, N.M.L.; Wanninayaka, D.B.; Dassanayake, R.S.; Rajapaksha, S.M.; Manamperi, A.; Fernando, C.A.N.; Kulatunga, A.K.; et al. Development of Starch-Based Materials Using Current Modification Techniques and Their Applications: A Review. Molecules 2021, 26, 6880.
- Zhang, D.; Mu, T.; Sun, H. Effects of Starch from Five Different Botanical Sources on the Rheological and Structural Properties of Starch-Gluten Model Doughs. Food Res. Int. 2018, 103, 156–162.
- Liu, S. An Overview of Biological Basics. In Bioprocess Engineering: Kinetics, Sustainability, and Reactor Design; Elsevier: Amsterdam, The Netherlands, 2017; pp. 21–80. ISBN 978-0-444-63783-3.
- Wang, S. Starch Structure, Functionality and Application in Foods; Springer: Berlin/Heidelberg, Germany, 2020.
- Abd El-Ghany, N.A.; Elella, M.H.A.; Abdallah, H.M.; Mostafa, M.S.; Samy, M. Recent Advances in Various Starch Formulation for Wastewater Purification via Adsorption Technique: A Review; Springer: Berlin/Heidelberg, Germany, 2023; ISBN 0123456789.
- Fang, K.; Deng, L.; Yin, J.; Yang, T.; Li, J.; He, W. Recent Advances in Starch-Based Magnetic Adsorbents for the Removal of Contaminants from Wastewater: A Review. Int. J. Biol. Macromol. 2022, 218, 909–929.
- Chen, Q.; Yu, H.; Wang, L.; Ul Abdin, Z.; Chen, Y.; Wang, J.; Zhou, W.; Yang, X.; Khan, R.U.; Zhang, H.; et al. Recent Progress in Chemical Modification of Starch and Its Applications. RSC Adv. 2015, 5, 67459–67474.
- McKay, G. Use of Adsorbents for the Removal of Pollutants from Wastewaters; CRC Press: Boca Raton, FL, USA, 1996.
- Park, D.; Yun, Y.S.; Park, J.M. The Past, Present, and Future Trends of Biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102.
- Crini, G. Recent Developments in Polysaccharide-Based Materials Used as Adsorbents in Wastewater Treatment. Prog. Polym. Sci. 2005, 30, 38–70.
- Crini, G. Non-Conventional Low-Cost Adsorbents for Dye Removal: A Review. Bioresour. Technol. 2006, 97, 1061–1085.
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and Non-Conventional Adsorbents for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 195–213.
- Li, W.; Wei, H.; Liu, Y.; Li, S.; Wang, G.; Guo, T.; Han, H. An in Situ Reactive Spray-Drying Strategy for Facile Preparation of Starch-Chitosan Based Hydrogel Microspheres for Water Treatment Application. Chem. Eng. Process. Process Intensif. 2021, 168, 108548.
- Zhang, B.; Li, X.; Xie, Q.; Tao, H.; Wang, W.; Chen, H.Q. Preparation and Characterization of Non-Crystalline Granular Starch and Corresponding Carboxymethyl Starch. Int. J. Biol. Macromol. 2017, 103, 656–662.
- Haq, F.; Yu, H.; Wang, L.; Teng, L.; Haroon, M.; Khan, R.U.; Mehmood, S.; Bilal-Ul-Amin; Ullah, R.S.; Khan, A.; et al. Advances in Chemical Modifications of Starches and Their Applications. Carbohydr. Res. 2019, 476, 12–35.
- Schwantes, D.; Gonçalves, A.C.; Junior Miola, A.; Coelho, G.F.; Dos Santos, M.G.; Leismann, E.A.V. Removal of Cu (II) and Zn (II) from Water with Natural Adsorbents from Cassava Agroindustry Residues. Acta Sci. Technol. 2015, 37, 409–417.
- Musarurwa, H.; Tavengwa, N.T. Application of Carboxymethyl Polysaccharides as Bio-Sorbents for the Sequestration of Heavy Metals in Aquatic Environments. Carbohydr. Polym. 2020, 237, 116142.
- Luo, H.; Dong, F.; Wang, Q.; Li, Y.; Xiong, Y. Construction of Porous Starch-Based Hydrogel via Regulating the Ratio of Amylopectin/Amylose for Enhanced Water-Retention. Molecules 2021, 26, 3999.
- Wang, Y.; Zhang, Y.; Hou, C.; Qi, Z.; He, X.; Li, Y. Facile Synthesis of Monodisperse Functional Magnetic Dialdehyde Starch Nano-Composite and Used for Highly Effective Recovery of Hg(II). Chemosphere 2015, 141, 26–33.
- Zhang, F.; Wang, C.; Wang, X.; Wang, J.; Jiang, H.; Xu, K.; Bai, Y.; Wang, P. Fabrication of Raspberry-like Starch-Based Polymer Microspheres for W/O and O/W Emulsions Separation and Purification. J. Environ. Chem. Eng. 2023, 11, 109260.
- Irani, M.; Ismail, H.; Ahmad, Z.; Fan, M. Synthesis of Linear Low-Density Polyethylene-g-Poly (Acrylic Acid)-Co-Starch/Organo-Montmorillonite Hydrogel Composite as an Adsorbent for Removal of Pb(ΙΙ) from Aqueous Solutions. J. Environ. Sci. 2015, 27, 9–20.
- Keirudin, A.A.; Zainuddin, N.; Yusof, N.A. Crosslinked Carboxymethyl Sago Starch/Citric Acid Hydrogel for Sorption of Pb2+, Cu2+, Ni2+ and Zn2+ from Aqueous Solution. Polymers 2020, 12, 2465.
- Haroon, M.; Wang, L.; Yu, H.; Abbasi, N.M.; Zain-Ul-Abdin; Saleem, M.; Khan, R.U.; Ullah, R.S.; Chen, Q.; Wu, J. Chemical Modification of Starch and Its Application as an Adsorbent Material. RSC Adv. 2016, 6, 78264–78285.
- Gunawardene, O.H.P.; Gunathilake, C.A.; Amaraweera, A.P.S.M.; Fernando, N.M.L.; Manipura, A.; Manamperi, W.A.; Kulatunga, K.M.A.K.; Rajapaksha, S.M.; Gamage, A.; Dassanayake, R.S.; et al. Removal of Pb(II) Ions from Aqueous Solution Using Modified Starch. J. Compos. Sci. 2021, 5, 46.
- Dang, X.; Yu, Z.; Yang, M.; Wai, M.; Song, Y.; Wang, X.; Zhang, H. Sustainable Electrochemical Synthesis of Natural Starch-Based Biomass Adsorbent with Ultrahigh Adsorption Capacity for Cr(VI) and Dyes Removal. Sep. Purif. Technol. 2022, 288, 120668.
- Mittal, H.; Alhassan, S.M.; Ray, S.S. Efficient Organic Dye Removal from Wastewater by Magnetic Carbonaceous Adsorbent Prepared from Corn Starch. J. Environ. Chem. Eng. 2018, 6, 7119–7131.
- Yu, M.; Zheng, Y.; Tian, J. Study on the Biodegradability of Modified Starch/Polylactic Acid (PLA) Composite Materials. RSC Adv. 2020, 10, 26298–26307.
- Ju, B.; Yan, D.; Zhang, S. Micelles Self-Assembled from Thermoresponsive 2-Hydroxy-3-Butoxypropyl Starches for Drug Delivery. Carbohydr. Polym. 2012, 87, 1404–1409.
- Ogunsona, E.; Ojogbo, E.; Mekonnen, T. Advanced Material Applications of Starch and Its Derivatives. Eur. Polym. J. 2018, 108, 570–581.
- Posada-Velez, M.C.; Pineda-Gomez, P.; Martinez-Hernandez, H.D. Acetylated Corn and Potato Starches as an Alternative to the Toxic Inorganic Coagulants/Flocculants for Wastewater Treatment. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100786.
- Sun, J.; Shi, M.; Wang, W. Ethanol-Water near-Azeotropic Mixture Dehydration by Compound Starch-Based Adsorbent. Trans. Tianjin Univ. 2015, 21, 427–432.
- Suo, F.; Liu, X.; Li, C.; Yuan, M.; Zhang, B.; Wang, J.; Ma, Y.; Lai, Z.; Ji, M. Mesoporous Activated Carbon from Starch for Superior Rapid Pesticides Removal. Int. J. Biol. Macromol. 2019, 121, 806–813.
- Tang, J.; Zhang, Q.; Zhou, J.; Fang, H.; Yang, H.; Wang, F. Investigation of Pesticide Residue Removal Effect of Gelatinized Starch Using Surface-Enhanced Raman Scattering Mapping. Food Chem. 2021, 365, 130448.
- Md. Munjur, H.; Hasan, M.N.; Awual, M.R.; Islam, M.M.; Shenashen, M.A.; Iqbal, J. Biodegradable Natural Carbohydrate Polymeric Sustainable Adsorbents for Efficient Toxic Dye Removal from Wastewater. J. Mol. Liq. 2020, 319, 114356.
- Junlapong, K.; Maijan, P.; Chaibundit, C.; Chantarak, S. Effective Adsorption of Methylene Blue by Biodegradable Superabsorbent Cassava Starch-Based Hydrogel. Int. J. Biol. Macromol. 2020, 158, 258–264.
- Bai, W.; Fan, L.; Zhou, Y.; Zhang, Y.; Shi, J.; Lv, G.; Wu, Y.; Liu, Q.; Song, J. Removal of Cd2+ Ions from Aqueous Solution Using Cassava Starch–based Superabsorbent Polymers. J. Appl. Polym. Sci. 2017, 134, 44758.
- Fang, K.; Li, K.; Yang, T.; Li, J.; He, W. Starch-Based Magnetic Nanocomposite as an Efficient Absorbent for Anticancer Drug Removal from Aqueous Solution. Int. J. Biol. Macromol. 2021, 184, 509–521.
- Sun, J.; Wang, W.; Wang, P.; Lv, H.; Luo, X.; Gao, H. Characterization of a Compound Starch-Based Adsorbent for Alcohol-Water Azeotrope Dehydration. Adsorpt. Sci. Technol. 2013, 31, 829–844.
- Wu, P.; Gao, H.; Sun, J.; Ma, T.; Liu, Y.; Wang, F. Biosorptive Dehydration of Tert-Butyl Alcohol Using a Starch-Based Adsorbent: Characterization and Thermodynamics. Bioresour. Technol. 2012, 107, 437–443.
- Mahmoud, M.E.; Nabil, G.M.; Zaki, M.M.; Saleh, M.M. Starch Functionalization of Iron Oxide By-Product from Steel Industry as a Sustainable Low Cost Nanocomposite for Removal of Divalent Toxic Metal Ions from Water. Int. J. Biol. Macromol. 2019, 137, 455–468.
- Aly, A.A.; Hebeish, A.A. Development of Starch-Based Cationic Adsorbents for Removal of Anionic Dyes from Aqueous Systems. Int. J. Sci. Res. 2015, 4, 4–12.
- Foroutan, R.; Peighambardoust, S.J.; Mohammadi, R.; Peighambardoust, S.H.; Ramavandi, B. Development of New Magnetic Adsorbent of Walnut Shell Ash/Starch/Fe3O4 for Effective Copper Ions Removal: Treatment of Groundwater Samples. Chemosphere 2022, 296, 133978.
- Jiryaei Sharahi, F.; Shahbazi, A. Melamine-Based Dendrimer Amine-Modified Magnetic Nanoparticles as an Efficient Pb(II) Adsorbent for Wastewater Treatment: Adsorption Optimization by Response Surface Methodology. Chemosphere 2017, 189, 291–300.
- Mahmoud, G.A.; Abdel-Aal, S.E.; Badway, N.A.; Abo Farha, S.A.; Alshafei, E.A. Radiation Synthesis and Characterization of Starch-Based Hydrogels for Removal of Acid Dye. Starch Staerke 2014, 66, 400–408.
- Gadd, G.M. Biosorption: Critical Review of Scientific Rationale, Environmental Importance and Significance for Pollution Treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28.
- Crini, G.; Badot, P.-M. Sorption Processes and Pollution: Conventional and Non-Conventional Sorbents for Pollutant Removal from Wastewaters; PUFC: Besançon, France, 2010.
- Hoover, R. Composition, Molecular Structure, and Physicochemical Properties of Tuber and Root Starches: A Review. Carbohydr. Polym. 2001, 45, 253–267.
- Denyer, K.A.Y.; Johnson, P.; Zeeman, S.; Smith, A.M. The Control of Amylose Synthesis. J. Plant Physiol. 2001, 158, 479–487.
- Pacsu, E.; Lejaren Arthur Hiller, J. Cellulose Studies IV. The Chemical Structure of Cellulose and Starch. Text. Res. J. 1946, 16, 243–248.
- Morrison, W.R.; Karkalas, J. Starch. In Methods in Plant Biochemistry; Academic Press: Cambridge, MA, USA, 1990; Volume 2, pp. 323–352. ISBN 0124610129.
- Liu, X.; Chao, C.; Yu, J.; Copeland, L.; Wang, S. Mechanistic Studies of Starch Retrogradation and Its Effects on Starch Gel Properties. Food Hydrocoll. 2021, 120, 106914.
- French, D. Organization of Starch Granules. In Starch: Chemistry and Technology; Whistler, R.L., Bemiller, J.N., Paschall, E.F., Eds.; Academic Press: Cambridge, MA, USA, 1984; pp. 183–247.
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch Granules: Structure and Biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112.
- Ahmed, S.; Hussain, C.M. Green and Sustainable Advanced Materials, Volume 2: Applications; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2018.
- Saurav, K.; Majhi, M.R.; Singh, V.K. Preperation of Porous Magenesia by Decomposing an Ex-Potato Known as Starch Soluble (C6H10O5)N. Am. J. Sci. Ind. Res. 2014, 5, 120–125.
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and Functionality of Starch. Food Hydrocoll. 2009, 23, 1527–1534.
- Xu, J.; Sagnelli, D.; Faisal, M.; Perzon, A.; Taresco, V.; Mais, M.; Giosafatto, C.V.L.; Hebelstrup, K.H.; Ulvskov, P.; Jørgensen, B.; et al. Amylose/Cellulose Nanofiber Composites for All-Natural, Fully Biodegradable and Flexible Bioplastics. Carbohydr. Polym. 2021, 253, 117277.
- Nakanishi, Y.; Norisuye, T.; Teramoto, A.; Kitamura, S. Conformation of Amylose in Dimethyl Sulfoxide. Macromolecules 1993, 26, 4220–4225.
- Mendes, J.F.; Paschoalin, R.T.; Carmona, V.B.; Sena Neto, A.R.; Marques, A.C.P.; Marconcini, J.M.; Mattoso, L.H.C.; Medeiros, E.S.; Oliveira, J.E. Biodegradable Polymer Blends Based on Corn Starch and Thermoplastic Chitosan Processed by Extrusion. Carbohydr. Polym. 2016, 137, 452–458.
- Obadi, M.; Qi, Y.; Xu, B. High-Amylose Maize Starch: Structure, Properties, Modifications and Industrial Applications. Carbohydr. Polym. 2023, 299, 120185.
- Chi, C.; Guo, X.; Zhou, Y.; Chen, B.; He, Y. A Facile Method for Isolating Long Branch-Chains of Amylopectin from Starch by Preheating and Pullulanase Treatment. Ind. Crops Prod. 2023, 191, 115987.
- Wang, R.; Zhang, H.; Chen, Z.; Zhong, Q. Structural Basis for the Low Digestibility of Starches Recrystallized from Side Chains of Amylopectin Modified by Amylosucrase to Different Chain Lengths. Carbohydr. Polym. 2020, 241, 116352.
- Kim, B.S.; Kim, H.S.; Yoo, S.H. Characterization of Enzymatically Modified Rice and Barley Starches with Amylosucrase at Scale-up Production. Carbohydr. Polym. 2015, 125, 61–68.
- Wang, F.; Ma, R.; Tian, Y. Superhydrophobic Starch-Based Nanocomposite Cryogel for Oil Removal Underwater and Magnetically Guided Oil Slick Cleanup. Carbohydr. Polym. 2022, 287, 119297.
- Wang, F.; Ma, R.; Zhan, J.; Shi, W.; Zhu, Y.; Tian, Y. Superhydrophobic/Superoleophilic Starch-Based Cryogels Coated by Silylated Porous Starch/Fe3O4 Hybrid Micro/Nanoparticles for Removing Discrete Oil Patches from Water. Sep. Purif. Technol. 2022, 291, 120872.
- Fallah, Z.; Zare, E.N.; Ghomi, M.; Ahmadijokani, F.; Amini, M.; Tajbakhsh, M.; Arjmand, M.; Sharma, G.; Ali, H.; Ahmad, A.; et al. Toxicity and Remediation of Pharmaceuticals and Pesticides Using Metal Oxides and Carbon Nanomaterials. Chemosphere 2021, 275, 130055.
- Yang, C.; Jiang, J.; Wu, Y.; Fu, Y.; Sun, Y.; Chen, F.; Yan, G.; Hu, J. High Removal Rate and Selectivity of Hg(II) Ions Using the Magnetic Composite Adsorbent Based on Starch/Polyethyleneimine. J. Mol. Liq. 2021, 337, 116418.
- Sekhavat Pour, Z.; Ghaemy, M. Removal of Dyes and Heavy Metal Ions from Water by Magnetic Hydrogel Beads Based on Poly(Vinyl Alcohol)/Carboxymethyl Starch-g-Poly(Vinyl Imidazole). RSC Adv. 2015, 5, 64106–64118.
- Sancey, B.; Trunfio, G.; Charles, J.; Minary, J.F.; Gavoille, S.; Badot, P.M.; Crini, G. Heavy Metal Removal from Industrial Effluents by Sorption on Cross-Linked Starch: Chemical Study and Impact on Water Toxicity. J. Environ. Manag. 2011, 92, 765–772.
- Wang, S.; Zhang, C.; Chang, Q. Synthesis of Magnetic Crosslinked Starch-Graft-Poly(Acrylamide)-Co-Sodium Xanthate and Its Application in Removing Heavy Metal Ions. J. Exp. Nanosci. 2017, 12, 270–284.
- Chen, H.; Xie, H.; Zhou, J.; Tao, Y.; Zhang, Y.; Zheng, Q.; Wang, Y. Removal Efficiency of Hexavalent Chromium from Wastewater Using Starch-Stabilized Nanoscale Zero-Valent Iron. Water Sci. Technol. 2019, 80, 1076–1084.
- Lu, Q.; Gao, P.; Zhi, H.; Zhao, H.; Yang, Y.; Sun, B. Preparation of Cu(II) Ions Adsorbent from Acrylic Acid-Grafted Corn Starch in Aqueous Solutions. Starch Staerke 2013, 65, 417–424.
- Sami, A.J.; Khalid, M.; Iqbal, S.; Afzal, M.; Shakoori, A.R. Synthesis and Application of ChitosanStarch Based Nanocomposite in Wastewater Treatment for the Removal of Anionic Commercial Dyes. Pak. J. Zool. 2017, 49, 21–26.
- Guibal, E. Interactions of Metal Ions with Chitosan-Based Sorbents: A Review. Sep. Purif. Technol. 2004, 38, 43–74.
- Chassary, P.; Vincent, T.; Guibal, E. Metal Anion Sorption on Chitosan and Derivative Materials: A Strategy for Polymer Modification and Optimum Use. React. Funct. Polym. 2004, 60, 137–149.
- Chiou, M.S.; Li, H.Y. Adsorption Behavior of Reactive Dye in Aqueous Solution on Chemical Cross-Linked Chitosan Beads. Chemosphere 2003, 50, 1095–1105.
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673.
- Shen, Q.; Xu, M.H.; Wu, T.; Pan, G.X.; Tang, P.S. Adsorption Behavior of Tetracycline on Carboxymethyl Starch Grafted Magnetic Bentonite. Chem. Pap. 2022, 76, 123–135.
- Okoli, C.P.; Ofomaja, A.E. Degree of Time Dependency of Kinetic Coefficient as a Function of Adsorbate Concentration; New Insights from Adsorption of Tetracycline onto Monodispersed Starch-Stabilized Magnetic Nanocomposite. J. Environ. Manag. 2018, 218, 139–147.
- Okoli, C.P.; Ofomaja, A.E. Development of Sustainable Magnetic Polyurethane Polymer Nanocomposite for Abatement of Tetracycline Antibiotics Aqueous Pollution: Response Surface Methodology and Adsorption Dynamics. J. Clean. Prod. 2019, 217, 42–55.
- Okoli, C.P.; Naidoo, E.B.; Ofomaja, A.E. Role of Synthesis Process Variables on Magnetic Functionality, Thermal Stability, and Tetracycline Adsorption by Magnetic Starch Nanocomposite. Environ. Nanotechnol. Monit. Manag. 2018, 9, 141–153.
- Mohamed, A.K.; Mahmoud, M.E. Encapsulation of Starch Hydrogel and Doping Nanomagnetite onto Metal-Organic Frameworks for Efficient Removal of Fluvastatin Antibiotic from Water. Carbohydr. Polym. 2020, 245, 116438.
- He, Q.; Song, P.; Zhang, Z.; You, Z.; Tu, W. Preparation of Magnetic Gelatin-Starch Microspheres and Adsorption Performance for Bovine Serum Album. J. Cent. South Univ. 2015, 22, 1220–1226.




