Owing to its non-toxicity, biodegradability, and biocompatibility, starch is a naturally occurring polysaccharide that scientists are looking into as a possible environmentally friendly material for sustainable water remediation. Starch could exhibit significant adsorption capabilities towards pollutants with the substitution of amide, amino, carboxyl, and other functional groups for hydroxyl groups. Starch derivatives may effectively remove contaminants such as oil, organic solvents, pesticides, heavy metals, dyes, and pharmaceutical pollutants by employing adsorption techniques at a rate greater than 90%. The maximal adsorption capacities of starch-based adsorbents for oil and organic solvents, pesticides, heavy metal ions, dyes, and pharmaceuticals are 13,000, 66, 2000, 25,000, and 782 mg/g, respectively.
- starch
- adsorbent
- wastewater treatment
- heavy metals
- dye
- oil
- organic solvents
- pesticides
- pharmaceutical pollutants
1. Introduction
Starch is a carbohydrate and a natural component of most plants, is commercially derived from grains and serves as an important raw material in various industries such as medicine, food, chemicals, etc. Starch has been extensively studied for its potential in wastewater treatment [1][2][26,27], it is a relatively good option for wastewater treatment due to its chemical structure, biocompatibility, and biodegradability which can enhance its utilization as green adsorbent. Cassava starch, rice starch, corn starch, and potato starch are documented botanical sources of starch [3][28]. Starch molecules exist in two structural forms: amylose and amylopectin. Amylopectin contains a higher glucose content compared to amylose [4][29]. In its natural state, amylose accounts for about 20–30% of starch and amylopectin accounts for 70–80%. Starch is primarily synthesized in the chloroplast of plant leaves or the amyloplast of plant storage organs and contains lipids as well as phosphate groups [5][30]. However, native starch has considerable limitations when used in wastewater treatment. These limitations include low surface area, limited thermal stability, low water solubility, low molecular weight, quick degradability in water, and a lack of reactive functional groups [6][7][31,32]. To overcome these limitations and enhance its adsorption capabilities for wastewater treatment, researchers have explored various modifications of starch. Researchers discovered that incorporating a chemical functional group into the starch backbone improves the adsorption efficacy of modified starch to a variety of pollutants [6][7][8][31,32,33]. Several starch modifications, including starch-based grafts, polymer nanocomposites, nanofibers, nanoparticles, activated carbon, biochar, hydrogels, aerogels, and beads, have been developed to overcome these limitations [6][31]. Through modification and functionalization approaches, ongoing research aims to improve the adsorption capacity and selectivity of starch-based adsorbents.
2. Adsorption
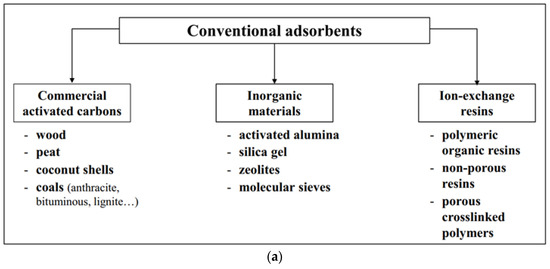
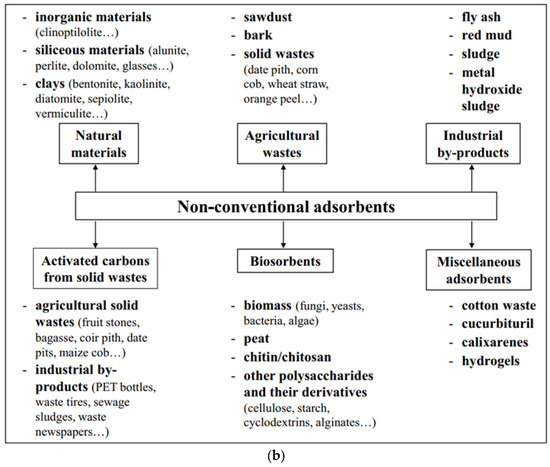
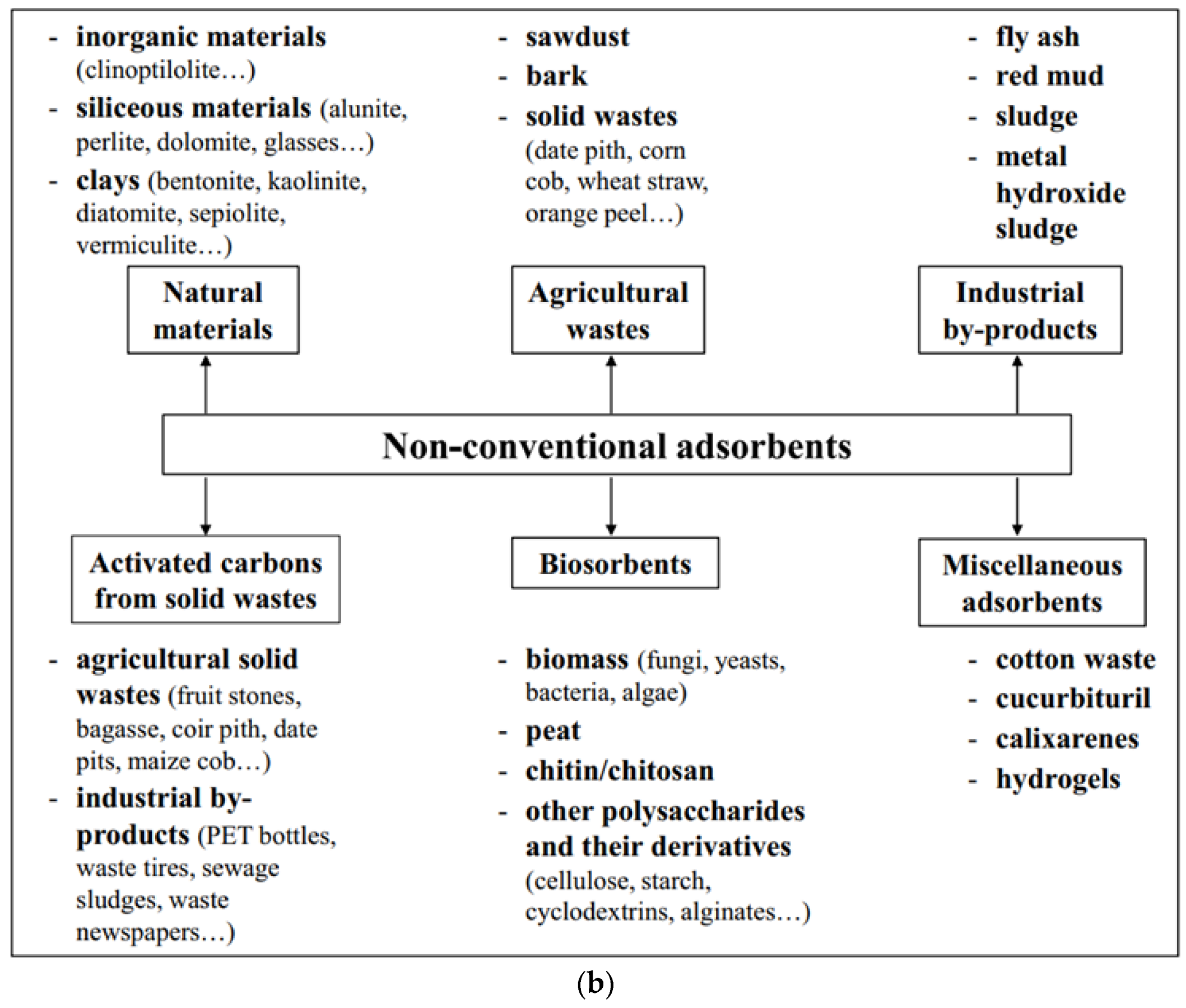
2.1. Adsorbents
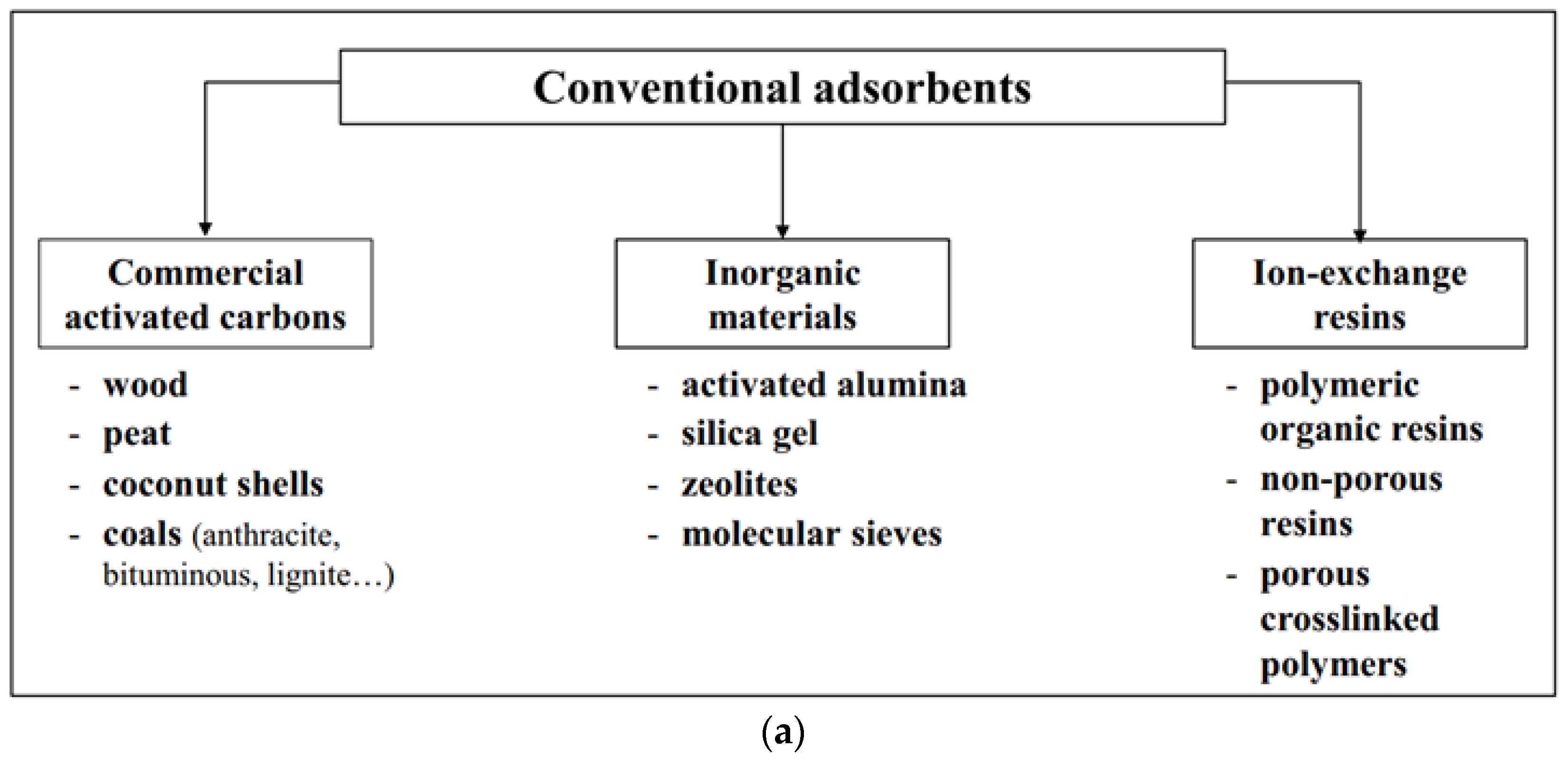
2.2. Starch-Based Adsorbents
2.3. Starch-Based Adsorbents for In situ and Ex Situ Water Remediation
Starch-based adsorbents can be used in the adsorption stage of water treatment. Various adsorbents will be applied at this stage to remove pollutants from the wastewater via adsorption mechanism. Starch-based adsorbents derived from cornstarch [31][32][33][34][84,85,86,87], rice flour [35][88], cassava starch [36][37][38][83,89,90], graham flour [35][88], and potato starch [31][32][39][40][84,85,91,92] are safe compounds with the potential to be used for in situ water remediation. Starch-functionalized iron oxide nanocomposite, for example, has been utilized to adsorb heavy metal ions from wastewater produced from different industries [41][64]. The adsorption efficiency of Cd(II) from tap, marine, and industrial wastewater samples ranged from 87 to 93%, 84 to 91%, and 76 to 90%, respectively, whereas the adsorption efficiency of Hg(II) ranged from 69 to 93%, 40 to 89%, and 70 to 94%, respectively. Pb(II) had the maximum adsorption efficiency of 97 to 98%, 92 to 97%, and 93 to 98% for tap, marine, and industrial wastewater samples. Additionally, these starch-based nanocomposites have the potential to be a sustainable adsorbent for metal ions removal from industrial wastewater, marine water, and tap water with 76 to 93%, 70 to 94%, and 93 to 97% of percentage recovery, respectively [41][64]. The optimum conditions for ex situ water remediation using starch-based adsorbents will vary depending on the type of pollutant, structure (functional group, chain length, etc.) of the starch-based adsorbent, adsorbent dose, pollutant concentration, pH of the treating bath, treatment duration, and agitation speed [42][35]. Starch-based adsorbents have been found to be effective at removing pollutants at concentrations ranging from a few milligrams per liter to several hundred milligrams per liter [22][61], as reported in Table 1. The initial pollutant concentration is a driving force in overcoming the mass transfer resistance of pollutants between water and adsorbent [43][95]. Nevertheless, the effectiveness of starch-based adsorbents may decrease at higher pollutant concentrations due to adsorbent surface saturation, increasing the electrostatic repulsion force between the saturated adsorbent and adsorbate in the aqueous solution [44][96]. Other factors, such as reaction time, pH, temperature, and competing ions in the water, may also have an impact on the performance of starch-based adsorbents [43][45][95,97]. In this case, the adsorbent of interest is starch, one of the non-conventional “green” adsorbents that are highly effective at removing a wide variety of pollutants from wastewater. They have higher adsorption capacities and longer lifetimes than conventional adsorbents. Additionally, they are highly advantageous compared to the conventional adsorbents due to their physiochemical nature, abundance, and relatively inexpensive pricing, showing their effectiveness in pollutant adsorption in water treatment processes [46][47][98,99].3. Chemical Structure and Properties of Starch
Chemistry and Properties
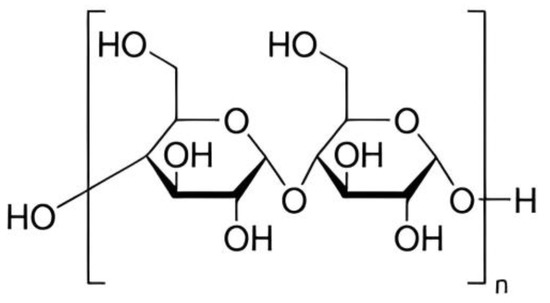
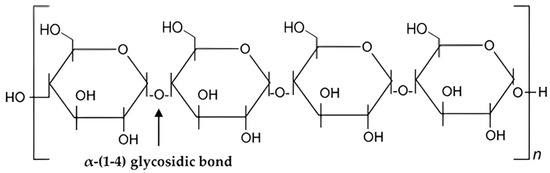
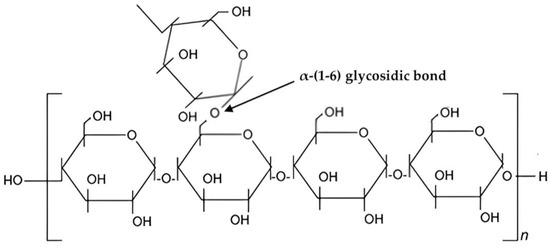
Starches with basic chemical formula, (𝐶6𝐻10𝑂5)𝑛 is a carbohydrate naturally found in many grains and vegetables, such as wheat, maize, and potatoes [48][49][100,101]. Starch is a part of polysaccharide which exists in two structural forms: amylose and amylopectin [50][51][102,103]. One of the most abundant polysaccharides in nature, starch provides us with a good supply of additional carbs [52][104]. The size of starch granules varies, ranging from the sub-micron elongated chloroplast granules to the comparatively enormous oval granules of potato and canna [53][54][105,106]. Biosynthesis of starch is a complex process [49][54][101,106]. It is also composed of glucose molecules [55][107] as shown in Figure 2.