
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joseph Mercola | -- | 3292 | 2023-07-24 11:50:48 | | | |
| 2 | Lindsay Dong | Meta information modification | 3292 | 2023-07-25 02:34:28 | | |
Video Upload Options
The intake of linoleic acid (LA) has increased dramatically in the standard American diet. LA is generally promoted as supporting human health, but there exists controversy regarding whether the amount of LA currently consumed in the standard American diet supports human health. While LA is considered to be an essential fatty acid and support health when consumed in modest amounts, an excessive intake of LA leads to the formation of oxidized linoleic acid metabolites (OXLAMs), impairments in mitochondrial function through suboptimal cardiolipin composition, and likely contributes to many chronic diseases that became an epidemic in the 20th century, and whose prevalence continues to increase. As LA consumption increases, the potential for OXLAM formation also increases. OXLAMs have been associated with various illnesses, including cardiovascular disease, cancer, and Alzheimer’s disease, among others. Lowering dietary LA intake can help reduce the production and accumulation of OXLAMs implicated in chronic diseases.
1. Introduction
2. How Much LA Is Required in the Human Diet?
3. The Omega 3:6 Ratio
4. Pathophysiological Mechanism of Elevated LA Levels
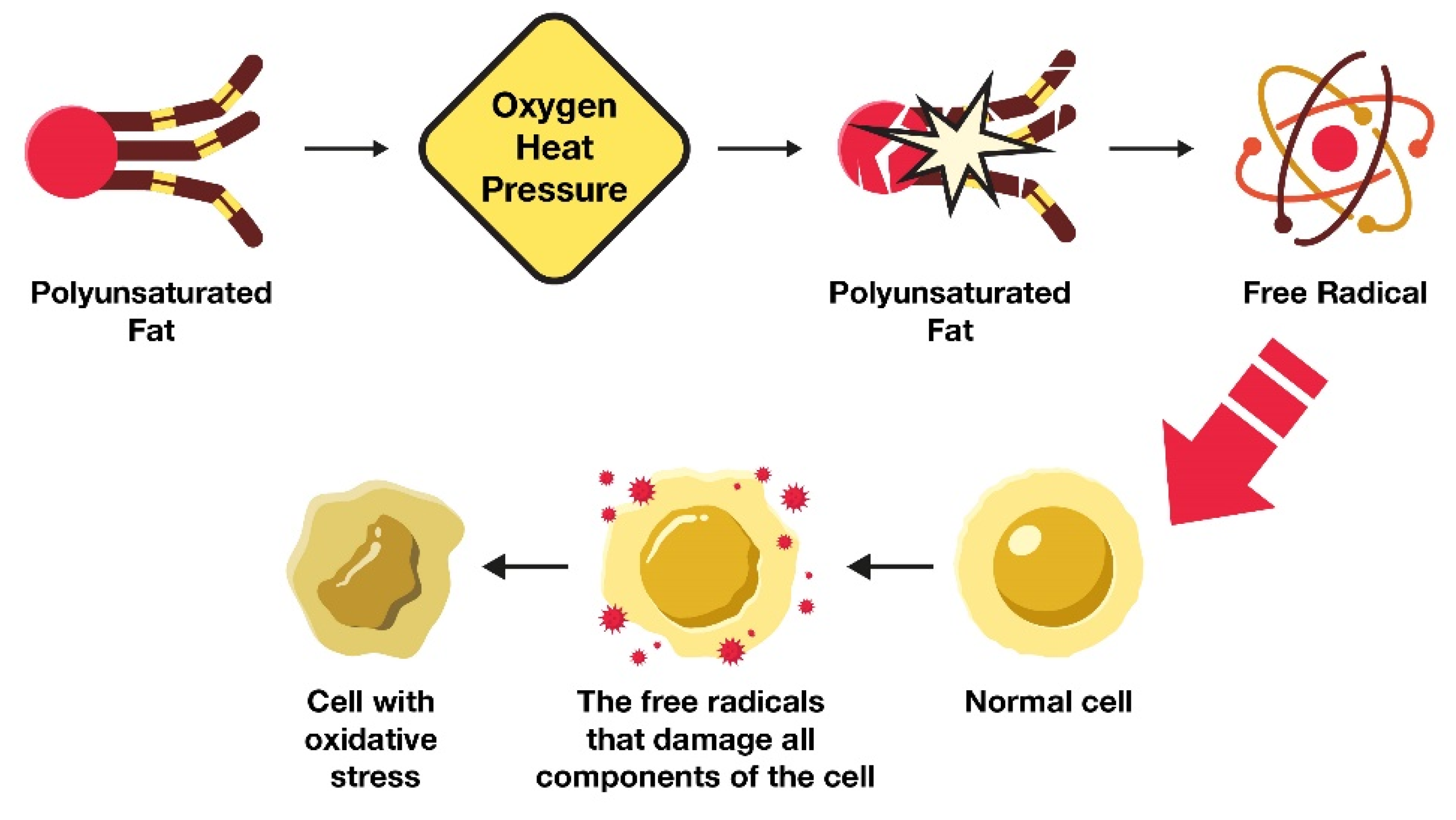
4.1. How Excess LA Consumption Alters Health
4.2. LA Remains in Tissues for Extended Time Periods
4.3. Cardiolipin: Stealth Fat in Mitochondria
5. Associations between LA Intake and Chronic Disease
| Proposed Neutral or Health Benefits | Health Risks |
|---|---|
| Reduces cardiovascular disease risk by decreasing total cholesterol levels [42] | Increases risk of cardiovascular disease by increasing oxidized LDL [2][43] |
| Unrecognized as having an impact on cancer [44] | Increases risk of cancer by impairing mitochondrial function and increasing systemic oxidative stress [45] that adversely impacts cardiolipin in the inner mitochondrial membrane [46][47][48] |
| Reduces the risk of type 2 diabetes [49] | Increases risk of diabetes [45] |
| Role in obesity is contentious [50] | Increases risk of obesity [51] |
| Unrecognized as having an impact on dementia | Increases risk of dementia [52] |
6. Dietary Sources of LA and Mitigation Strategies
In general, the lowest LA-containing source of fats would be the preferred fats of choice for lowering the LA burden in the diet. Olive oil is a popular cooking oil that is prominently featured in Mediterranean diets, which generally contain far fewer seed oils considering the abundant use of olive oil.
6.1. Sources of Animal Protein and Varying LA Contents
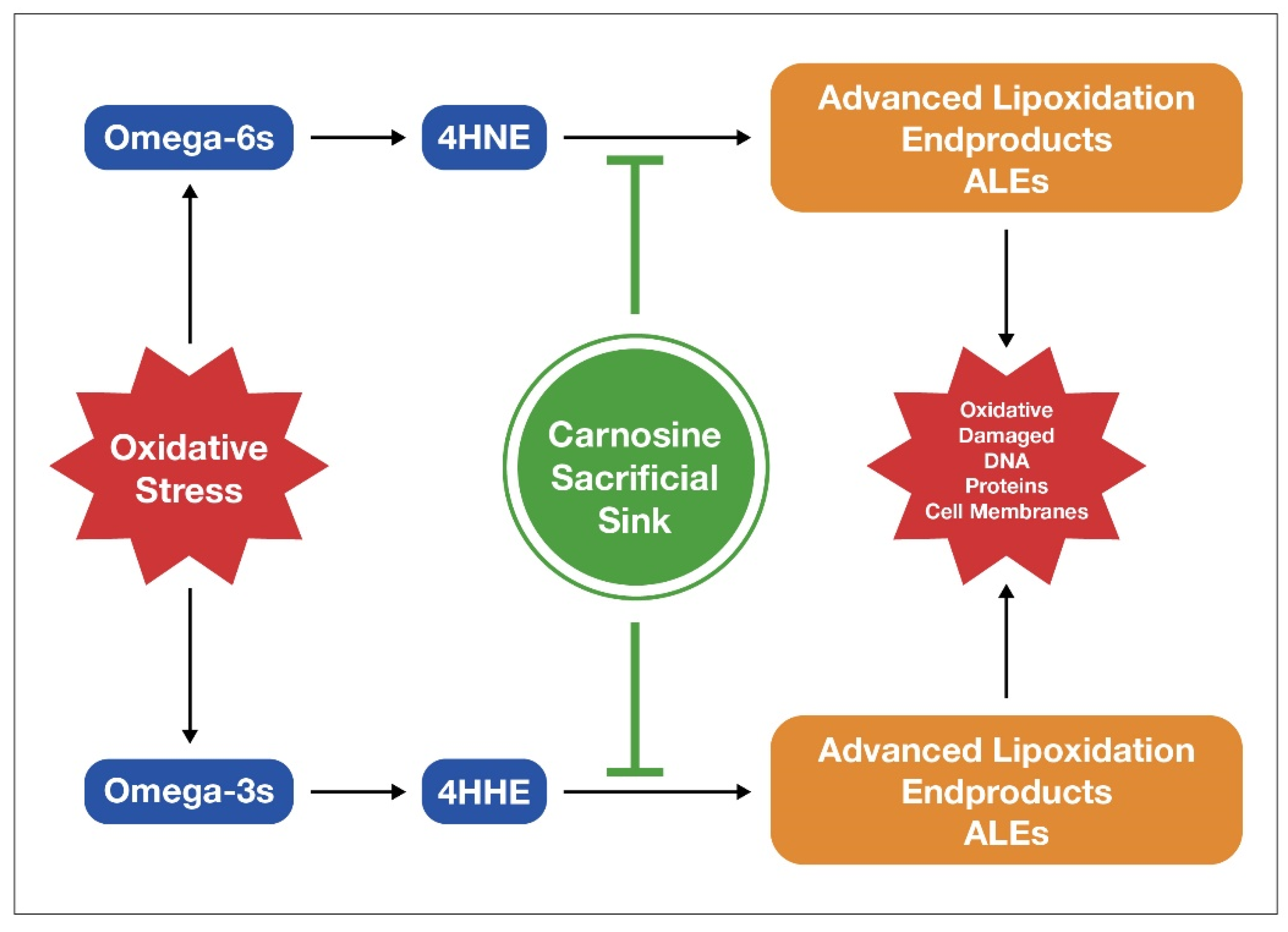
6.2. Carnosine Helps Lower Oxidative Damage from LA
References
- Chapkin, R.S.; McMurray, D.N.; Davidson, L.A.; Patil, B.S.; Fan, Y.Y.; Lupton, J.R. Bioactive dietary long-chain fatty acids: Emerging mechanisms of action. Br. J. Nutr. 2008, 100, 1152–1157.
- Ramsden, C.E.; Ringel, A.; Feldstein, A.E.; Taha, A.Y.; MacIntosh, B.A.; Hibbeln, J.R.; Majchrzak-Hong, S.F.; Faurot, K.R.; Rapoport, S.I.; Cheon, Y.; et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 135–141.
- Holman, R.T.; Johnson, S.B.; Kokmen, E. Deficiency of essential fatty acids and membrane fluidity during pregnancy and lactation. Proc. Natl. Acad. Sci. USA 1968, 61, 1053–1059.
- Behrman, R.E.; Reller, L.B.; Carey, H.V. Essential fatty acid deficiency in a patient receiving prolonged intra-venous alimentation. N. Engl. J. Med. 1971, 285, 19–21.
- Klein, P.D.; Johnson, R.M. Phosphorous metabolism in unsaturated fatty acid-deficient rats. J. Biol. Chem. 1954, 211, 103–110.
- Hayashida, T.; Portman, O.W. Swelling of liver mitochondria from rats fed diets deficient in essential fatty acids. Proc. Soc. Exp. Biol. Med. 1960, 103, 656–659.
- Cornwell, D.G.; Panganamala, R.V. Atherosclerosis an intracellular deficiency in essential fatty acids. Prog. Lipid Res. 1981, 20, 365–376.
- Smith, E.B. The effects of age and of early atheromata on the intimal lipids in men. Biochem. J. 1962, 84, 49.
- Smith, E.B. Lipids carried by S1 0–12 lipoprotein in normal and hypercholesterolaemic serum. Lancet 1962, 2, 530–534.
- Das, U.N. Bioactive lipids and vascular disease. Eur. J. Clin. Nutr. 2021, 75, 1528–1531.
- Salem, N.; Wegher, B.; Mena, P.; Uauy, R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc. Natl. Acad. Sci. USA 1996, 93, 49–54.
- Ramsden, C.E.; Zamora, D.; Faurot, K.R.; MacIntosh, B.; Horowitz, M.; Keyes, G.S.; Yuan, Z.X.; Miller, V.; Lynch, C.; Honvoh, G.; et al. Dietary alteration of n-3 and n-6 fatty acids for headache reduction in adults with migraine: Randomized controlled trial. BMJ 2021, 374, n1448.
- Burr, G.O.; Burr, M.M. A new deficiency disease produced by the rigid exclusion of fat from the diet. J. Biol. Chem. 1929, 82, 345–367.
- Suzuki, N.; Sawada, K.; Takahashi, I.; Matsuda, M.; Fukui, D.; Tokuyasu, H.; Shimizu, H.; Yokoyama, J.; Akaike, A. Association between polyunsaturated fatty acid and reactive oxygen species production of neutrophils in the general population. Nutrients 2020, 12, 3222.
- Santoro, N.; Caprio, S.; Giannini, C.; Kim, G.; Kursawe, R.; Pierpont, B.; Shaw, M.M.; Feldstein, A.E. Oxidized fatty acids: A potential pathogenic link between fatty liver and type-2 diabetes in obese adolescents. Antioxid. Redox Signal. 2014, 20, 383–389.
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763.
- Osborne, T.; Mendel, L.B. Growth on diets poor in true fats. JBC 1920, 45, 145–152.
- Spector, A.A.; Kim, H.Y. Discovery of fatty acids. J. Lipid. Res. 2015, 56, 11–21.
- Hansen, A.E. Essential fatty acids and infant nutrition; Borden award address. Pediatrics 1958, 21, 494–501.
- Hansen, A.E.; Wiese, H.F.; Boelsche, A.N. Role of linoleic acid in infant nutrition study Clinical and Chemical Study of 428 Infants Fed on Milk Mixtures Varying in Kind and Amount of Fat. Pediatrics 1963, 31, 171–192.
- Cunnane, S.C.; Guesnet, P. Linoleic acid recommendations—A house of cards. Prostaglandins Leukot. Essent Fat. Acids 2011, 85, 399–402.
- Igarashi, M.; Gao, F.; Kim, H.W.; Ma, K.; Bell, J.M.; Rapoport., S.I. Dietary n-6 PUFA deprivation for 15 weeks reduces arachidonic acid concentrations while increasing n-3 PUFA concentrations in organs of post-weaning male rats. Biochim. Biophys. Acta 2009, 1791, 132–139.
- Guesnet, P.; Lallemand, S.M.; Alessandri, J.M.; Jouin, M.; Cunnane, S.C. α-Linolenate reduces the dietary requirement for linoleate in the growing rat. Prostaglandins Leukot. Essent Fat. Acids 2011, 85, 353–360.
- Stark, A.H.; Crawford, M.A.; Reifen, R. Update on alpha-linolenic acid. Nutr. Rev. 2008, 66, 326–333.
- DiNicolantonio, J.J.; O’Keefe, J.H. Monosaturated fat vs. saturated fat: Effects on cardio-metabolic health and obesity. Mo. Med. 2022, 119, 69–73.
- Vannice, G.; Rasmussen, H. Position of the Academy of Nutrition and Dietetics: Dietary fatty acids for healthy adults. J. Acad. Nutr. Diet. 2014, 114, 136–153.
- Kris-Etherton, P.M. Fish consumption, fish oil, omega-3 Fatty Acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757.
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438.
- Hoxha, M.; Spahiu, E.; Prendi, E.; Zappacosta, B. A Systematic Review on the Role of Arachidonic Acid Pathway in Multiple Sclerosis. CNS Neurol. Disord. Drug Targets 2022, 21, 160–187.
- Birkic, N.; Azar, T.; Maddipati, K.R.; Minic, Z.; Reynolds, C.A. Excessive dietary linoleic acid promotes plasma accumulation of pronociceptive fatty acyl lipid mediators. Sci. Rep. 2022, 12, 17832.
- Singh, P.N.; Arthur, K.N.; Orlich, M.J.; James, W.; Purty, A.; Job, J.S.; Rajaram, S.; Sabaté, J. Global epidemiology of obesity, vegetarian dietary patterns, and noncommunicable disease in Asian Indians. Am. J. Clin. Nutr. 2014, 100, 359S–364S.
- Rett, B.S.; Whelan, J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: A systematic review. Nutr. Metab. 2011, 8, 36.
- Yoshida, Y.; Yoshikawa, A.; Kinumi, T.; Ogawa, Y.; Saito, Y.; Ohara, K.; Yamamoto, H.; Imai, Y.; Niki, E. Hydroxyoctadecadienoic acid and oxidatively modified peroxiredoxins in the blood of Alzheimer’s disease patients and their potential as biomarkers. Neurobiol. Aging 2009, 30, 174–185.
- Poirier, B.; Michel, O.; Bazin, R.; Bariéty, J.; Chevalier, J.; Myara, I. Conjugated dienes: A critical trait of lipoprotein oxidizability in renal fibrosis. Nephrol. Dial. Transplant. 2001, 16, 1598–1606.
- Esterbauer, H.; Gebicki, J.; Puhl, H.; Jürgens, G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radical Biol. Med. 1992, 13, 341–390.
- Dayton, S.; Hashimoto, S.; Dixon, W.; Pearce, M.L. Composition of lipids in human serum and adipose tissue during prolonged feeding of a diet high in unsaturated fat. J. Lipid. Res. 1966, 7, 103–111.
- Umhau, J.C.; Zhou, W.; Carson, R.E.; Rapoport, S.I.; Polozova, A.; Demar, J.; Hussein, N.; Bhattacharjee, A.K.; Ma, K.; Esposito, G.; et al. Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J. Lipid. Res. 2009, 50, 1259–1268.
- Braeckman, R.A.; Stirtan, W.; Soni, P.N. Pharmacokinetics of eicosapentaenoic acid in plasma and red blood cells after multiple oral dosing with icosapent ethyl in healthy subjects. Clin. Pharmacol. Drug Dev. 2014, 3, 101–108.
- Bertram, R.; Pedersen, M.G.; Luciani, D.S.; Sherman, A. A simplified model for mitochondrial ATP production. J. Theor. Biol. 2006, 243, 575–586.
- Ahmadpour, S.T.; Maheo, K.; Servais, S.; Brisson, L.; Dumas, J.F. Cardiolipin, the mitochondrial signature lipid: Implication in cancer. Int. J. Mol. Sci. 2020, 21, 8031.
- Dudek, J. Role of cardiolipin in mitochondrial signaling pathways. Front. Cell. Dev. Biol. 2017, 5, 90.
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98.
- Bu, T.; Tang, D.; Liu, Y.; Chen, D. Trends in Dietary Patterns and Diet-related Behaviors in China. Am. J. Health Behav. 2021, 45, 371–383.
- Zock, P.L.; Katan, M.B. Linoleic acid intake and cancer risk: A review and meta-analysis. Am. J. Clin. Nutr. 1998, 68, 142–153.
- Jaganjac, M.; Zarkovic, N. Lipid Peroxidation Linking Diabetes and Cancer: The Importance of 4-Hydroxynonenal. Antioxid. Redox Signal. 2022, 37, 1222–1233.
- Panov, A.V.; Dikalov, S.I. Cardiolipin, Perhydroxyl Radicals, and Lipid Peroxidation in Mitochondrial Dysfunctions and Aging. Oxidative Med. Cell. Longev. 2020, 2020, 1323028.
- Kim, J.; Minkler, P.E.; Salomon, R.G.; Anderson, V.E.; Hoppel, C.L. Cardiolipin: Characterization of distinct oxidized molecular species. J. Lipid Res. 2011, 52, 125–135.
- Kuschner, C.E.; Choi, J.; Yin, T.; Shinozaki, K.; Becker, L.B.; Lampe, J.W.; Kim, J. Comparing phospholipid profiles of mitochondria and whole tissue: Higher PUFA content in mitochondria is driven by increased phosphatidylcholine unsaturation. J. Chromatogr. B 2018, 1093–1094, 147–157.
- Belury, M.A.; Cole, R.M.; Snoke, D.B.; Banh, T.; Angelotti, A. Linoleic acid, glycemic control and Type 2 diabetes. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 30–33.
- Naughton, S.S.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. 2016, 125, 90–99.
- DiNicolantonio, J.J.; O’Keefe, J.H. Good Fats versus Bad Fats: A Comparison of Fatty Acids in the Promotion of Insulin Resistance, Inflammation, and Obesity. Mo. Med. 2017, 114, 303–307.
- Currenti, W.; Godos, J.; Alanazi, A.M.; Lanza, G.; Ferri, R.; Caraci, F.; Grosso, G.; Galvano, F.; Castellano, S. Dietary Fats and Cognitive Status in Italian Middle-Old Adults. Nutrients 2023, 15, 1429.
- Kim, H.J.; Zhao, J.; Walewski, J.L.; Sparrow, J.R. A High Fat Diet Fosters Elevated Bisretinoids. J. Biol. Chem. 2023, 299, 104784.
- Deol, P.; Kozlova, E.; Valdez, M.; Ho, C.; Yang, E.W.; Richardson, H.; Gonzalez, G.; Truong, E.; Reid, J.; Valdez, J.; et al. Dysregulation of Hypothalamic Gene Expression and the Oxytocinergic System by Soybean Oil Diets in Male Mice. Endocrinology 2020, 161, bqz044.
- Deol, P.; Evans, J.R.; Dhahbi, J.; Chellappa, K.; Han, D.S.; Spindler, S.; Sladek, F.M. Soybean oil is more obesogenic and diabetogenic than coconut oil and fructose in mouse: Potential role for the liver. PLoS ONE 2015, 10, e0132672.
- Deol, P.; Fahrmann, J.; Yang, J.; Evans, J.R.; Rizo, A.; Grapov, D.; Salemi, M.; Wanichthanarak, K.; Fiehn, O.; Phinney, B.; et al. Omega-6 and omega-3 oxylipins are implicated in soybean oil-induced obesity in mice. Sci. Rep. 2017, 7, 12488.
- Piñeros, A.R.; Kulkarni, A.; Gao, H.; Orr, K.S.; Glenn, L.; Huang, F.; Liu, Y.; Gannon, M.; Syed, F.; Wu, W.; et al. Proinflammatory signaling in islet β cells propagates invasion of pathogenic immune cells in autoimmune diabetes. Cell Rep. 2022, 39, 111011.
- Hernandez, M.L.; Sicardo, M.D.; Belaj, A.; Martínez-Rivas, J.M. The oleic/linoleic acid ratio in olive (Olea europaea Lfruit mesocarp is mainly controlled by OeFAD2-2 and OeFAD2-5 genes together with the different specificity of extraplastidial acyltransferase enzymes. Front. Plant Sci. 2021, 12, 653997.
- CBS News. 60 Minutes Overtime: Don’t Fall Victim to Olive Oil Fraud. Available online: https://www.cbsnews.com/news/60-minutes-overtime-how-to-buy-olive-oil/ (accessed on 13 April 2023).
- Shah, A.K.; Dhalla, N.S. Effectiveness of some vitamins in the prevention of cardiovascular disease: A narrative review. Front. Physiol. 2021, 12, 729255.
- Ong, D.E.; Chytil, F. Vitamin A and cancer. Vitam. Horm. 1983, 40, 105–144.
- Sommer, A. Vitamin A deficiency and clinical disease: An historical overview. J. Nutr. 2008, 128, 1835–1839.
- Cabiddu, A.; Delgadilla-Puga, C.; Decandia, M.; Molle, G. Extensive ruminant production systems and milk quality with emphasis on unsaturated fatty acids, volatile compounds, antioxidant protection degree and phenol content. Animals 2019, 9, 771.
- Nürnberg, K.; Wegner, J.; Ender, K. Factors influencing fat composition in muscle and adipose tissue of farm animals. Livest. Prod. Sci. 1998, 56, 145–156.
- Klebanov, G.I.; YuO, T.; Babenkova, I.V.; Lyubitsky, O.B.; OYu, R.; Boldyrev, A.A.; YuA, V. Effect of carnosine and its components on free-radical reactions. Membr. Cell Biol. 1998, 12, 89–99.
- Boldyrev, A.; Bulygina, E.; Leinsoo, T.; Carpenter, D.O. Protection of neuronal cells against reactive oxygen species by carnosine and related compounds. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 137, 81–88.
- Chan, K.M.; Decker, E.A. Endogenous skeletal muscle antioxidants. Crit. Rev. Food Sci. Nutr. 1994, 34, 403–426.




