
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ali Mirzaei | -- | 2824 | 2023-07-20 16:12:05 | | | |
| 2 | Peter Tang | Meta information modification | 2824 | 2023-07-21 09:10:53 | | | | |
| 3 | Peter Tang | Meta information modification | 2824 | 2023-07-21 09:11:20 | | |
Video Upload Options
Vanadium pentoxide (V2O5) is a transition metal oxide with features such as high availability, good catalytic activity, unique electrical properties and high conductivity which are appropriate for gas sensing applications.
1. Introduction

2. Pristine Nanostructured V2O5 Gas Sensors

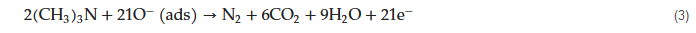
|
V2O5 Morphology |
Synthesis Method |
Target Gas |
Conc. (ppm) |
Response (Ra/Rg) or (Rg/Ra) |
T (°C) |
Response time/Recovery Time(s) |
Ref. |
|---|---|---|---|---|---|---|---|
|
Hollow spheres Solid spheres |
Solvothermal |
C3H9N |
500 500 |
9.7 3.08 |
370 |
20/83 45/150 |
[36] |
|
Hollow spheres |
Chemical synthesis |
H2 |
200 |
2.8 |
RT |
50/10 |
[37] |
|
Nanoneedles |
PVD |
C3H6O |
140 |
2.35 |
RT |
67/- |
[38] |
|
Hierarchical nanostructures |
Hydrothermal |
C3H9N |
10–200 |
1.13-3.57 |
240 |
5/28 |
[41] |
|
Flower-like |
Hydrothermal |
5 |
2.25 |
200 |
13/13 |
[42] |
|
|
Nanorods |
CVD |
NH3 |
100 |
235 * |
400 |
-/- |
[43] |
|
Nanorods |
Solvothermal |
C2H5OH NH3 |
500 |
1.04 1.02 |
RT |
-/- |
[44] |
|
Spherical |
Precipitation |
C2H5OH NH3 |
1.04 1.06 |
-/- |
[45] |
||
|
Flower-like |
Hydrothermal |
1-butylamine |
100 |
2.6 |
140 |
9/49 |
[46] |
|
Nanofibers |
Electrospinning |
9.5 |
500 * |
RT |
-/- |
[47] |
|
|
Flower-like Sheet-like |
Hydrothermal |
100 |
3.6 2.8 |
300 |
25/14 17/14 |
[48] |
|
|
Nanofibers |
Sol–gel |
NH3 |
2.1 |
11 * |
200 |
50/350 |
[49] |
|
Flower-like |
DC sputtering |
CH4 |
500 |
100 |
206/247 |
[50] |
|
|
Hydrothermal |
C8H10 |
3 |
300 |
44/74 |
[51] |
||
|
Nano stars |
Hydrothermal |
He |
300 |
53 * |
RT |
9/10 |
[52] |
|
Nanorods |
Chemical spray pyrolysis |
NO2 |
100 |
24.2 * |
200 |
13/140 |
[53] |
|
Nanofibers |
Chemical spray pyrolysis |
C8H10 |
27 |
RT |
80/50 |
[54] |
|
|
Nanowires |
Melt quenching |
C2H5OH |
1000 |
9.09 |
330 |
-/- |
[55] |
|
Thin film |
Plasma focus method |
H2 |
50 * |
275 |
-/- |
[56] |
|
|
Chemical spray pyrolysis |
NO2 |
100 |
41 * |
200 |
20/150 |
[57] |
* Response = Δ𝑅/𝑅𝑎×100; RT: Room temperature; PVD: Physical vapor deposition; CVD: Chemical vapor deposition.
3. Decorated/Doped V2O5 Gas Sensors
|
Sensor |
Synthesis Method |
Target Gas |
Conc. (ppm) |
Response (Ra/Rg) or (Rg/Ra) |
Working Temp. (°C) |
Response Time/Recovery Time(s) |
Ref. |
|---|---|---|---|---|---|---|---|
|
Pd decorated porous Si/V2O5 nanopillars |
DC sputtering |
NO2 |
2 |
4.5 |
RT |
-/- |
[59] |
|
Ru-decorated layer structure V2O5 |
Hydrothermal |
NH3 |
130 |
4 * |
RT |
~2/~12 |
[62] |
|
V2O5-decorated α-Fe2O3 nanorods |
Electrospinning |
C4H11N |
300 |
9 |
350 |
2/40 |
[63] |
|
V2O5 decorated SnO2 NWs |
VLS/ALD |
NO2 |
200 ppb |
3.6 |
250 |
-/- |
[75] |
|
Porous Si/V2O5 NR composite |
Galvanostatic electrochemical etching |
NO2 |
2 |
7.4 |
RT |
-/- |
[65] |
|
rGO/Mn3O4/V2O5 nanocomposite |
Hydrothermal |
H2 |
50 |
175 |
RT |
82/92 |
[71] |
|
Pd-decorated CuO NWs |
UV irradiation |
H2S |
100 |
1.962 |
100 |
-/- |
[72] |
|
V2O5/CuO nano-string of pearls |
Electrospinning |
C3H6O |
500 |
9 |
440 |
~40/~100 |
[74] |
|
CuO-decorated V2O5 NWs |
Hydrothermal and wet-deposition |
H2S |
23 |
31.86 |
220 |
130/218 |
[76] |
|
SnO2 NP-decorated V2O5 NWs |
Hydrothermal |
C2H5OH |
1000 |
1.3 |
RT |
-/- |
[77] |
|
Fe2O3 activated V2O5 nanotubes |
Hydrolysis |
C2H5OH |
1000 |
2.2 |
270 |
-/- |
[78] |
|
TiO2-decorated V2O5 NWs |
Hydrothermal |
O3 |
1.25 |
2.6 * |
300 |
~180/~180 |
[79] |
|
RGO-decorated V2O5 thin film |
Reactive sputtering and drop casting |
NO2 |
100 |
50.7 |
150 |
-/- |
[72] |
|
Au NP-decorated V2O5 |
Two-step in-situ reduction of Au and thermal oxidization as V2O5 |
Amines |
100 |
7.5 |
240 |
90/35 |
[80] |
|
Pd-decorated V2O5 thin film |
DC magnetron reactive sputtering |
H2 |
100 |
5.7 |
100 |
~6/14.8 |
[81] |
|
V2O5- doped SnO2 NFs |
Electrospinning |
C6H6 |
25 |
6.32 |
325 |
3/47 |
[82] |
* Response = Δ𝑅/𝑅𝑎×100; RT: Room temperature; VLS: Vapor-liquid-solid; NR; Nanorod; NP; Nanoparticle; NF; Nanofiber.
4. Nanocomposites/Nanohybrids of V2O5 Gas Sensors
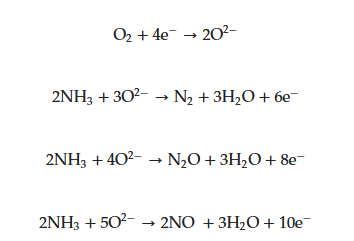
|
Sensing Material |
Synthesis Method |
Target Gas |
Conc. (Ppm) |
Response (Ra/Rg) Or (Rg/Ra) |
T (°C) |
Response Time/Recovery Time(S) |
Ref. |
|---|---|---|---|---|---|---|---|
|
V2O5/In2O3 core–shells |
Hydrothermal |
n-propylamine |
200 |
4 |
190 |
48/121 |
[86] |
|
MoO3-V2O5 thin films |
Chemical spray pyrolysis |
NO2 |
120 |
80 * |
200 |
118/1182 |
[87] |
|
(MoO3)0.4(V2O5)0.6 sheet composite |
Chemical spray pyrolysis |
100 |
115 |
39/453 |
[88] |
||
|
Au/V2O5/CuWO4 composite |
Hydrothermal |
NH3 |
5 |
2.7 |
150 |
35/33 |
[89] |
|
SnO2/V2O5 composite |
Sol-gel |
C6H6 |
200 |
10.5 |
275 |
-/- |
[90] |
|
V2O5/polyvinyl acetate NF composite |
Electrospinning |
NH3 |
0.8 |
6 * |
260 |
-/- |
[91] |
|
V2O5/ZnV2O6 nanobelt composite |
Chemical route |
C2H5OH |
2000 |
16.5 |
240 |
-/- |
[92] |
|
TiO2/V2O5 NF composite |
Electrospinning |
100 |
24.6 |
350 |
6/7 |
[93] |
|
|
ZnO/V2O5 thin films |
Spray pyrolysis |
C7H8 |
400 |
2.3 |
27 |
23/28 |
[94] |
* Response = Δ𝑅/𝑅𝑎×100.
References
- Le, T.K.; Kang, M.; Kim, S.W. A review on the optical characterization of v2o5 micro-nanostructures. Ceram. Int. 2019, 45, 15781–15798.
- Mane, A.A.; Maldar, P.S.; Dabhole, S.H.; Nikam, S.A.; Moholkar, A.V. Effect of substrate temperature on physicochemical and gas sensing properties of sprayed orthorhombic v2o5 thin films. Measurement 2019, 131, 223–234.
- Bouzidi, A.; Benramdane, N.; Bresson, S.; Mathieu, C.; Desfeux, R.; Marssi, M.E. X-ray and raman study of spray pyrolysed vanadium oxide thin films. Vib. Spectrosc. 2011, 57, 182–186.
- Su, Q.; Lan, W.; Wang, Y.Y.; Liu, X.Q. Structural characterization of β-v2o5 films prepared by dc reactive magnetron sputtering. Appl. Surf. Sci. 2009, 255, 4177–4179.
- Ramana, C.; Hussain, O.; Naidu, B.S.; Reddy, P. Spectroscopic characterization of electron-beam evaporated v2o5 thin films. Thin Solid Film 1997, 305, 219–226.
- Laubach, S.; Schmidt, P.C.; Thißen, A.; Fernandez-Madrigal, F.J.; Wu, Q.-H.; Jaegermann, W.; Klemm, M.; Horn, S. Theoretical and experimental determination of the electronic structure of v2o5, reduced v2o5− x and sodium intercalated nav2o5. Phys. Chem. Chem. Phys. 2007, 9, 2564–2576.
- Aawani, E.; Memarian, N.; Dizaji, H.R. Synthesis and characterization of reduced graphene oxide–v2o5 nanocomposite for enhanced photocatalytic activity under different types of irradiation. J. Phys. Chem. Solids 2019, 125, 8–15.
- Rathika, R.; Kovendhan, M.; Joseph, D.P.; Pachaiappan, R.; Kumar, A.S.; Vijayarangamuthu, K.; Venkateswaran, C.; Asokan, K.; Jeyakumar, S.J. Tailoring the properties of spray deposited v2o5 thin films using swift heavy ion beam irradiation. Nucl. Eng. Technol. 2020.
- Hou, T.-F.; Johar, M.A.; Boppella, R.; Hassan, M.A.; Patil, S.J.; Ryu, S.-W.; Lee, D.-W. Vertically aligned one-dimensional zno/v2o5 core–shell hetero-nanostructure for photoelectrochemical water splitting. J. Energy Chem. 2020, 49, 262–274.
- Slewa, L.H.; Abbas, T.A.; Ahmed, N.M. Effect of sn doping and annealing on the morphology, structural, optical, and electrical properties of 3d (micro/nano) v2o5 sphere for high sensitivity ph-egfet sensor. Sens. Actuators B Chem. 2020, 305, 127515.
- Abd-Alghafour, N.M.; Ahmed, N.M.; Hassan, Z.; Almessiere, M.A.; Bououdina, M.; Al-Hardan, N.H. High sensitivity extended gate effect transistor based on v2o5 nanorods. J. Mater. Sci. Mater. Electron. 2017, 28, 1364–1369.
- Yin, Z.; Xu, J.; Ge, Y.; Jiang, Q.; Zhang, Y.; Yang, Y.; Sun, Y.; Hou, S.; Shang, Y.; Zhang, Y. Synthesis of v2o5 microspheres by spray pyrolysis as cathode material for supercapacitors. Mater. Res. Express 2018, 5, 036306.
- Deepak Raj, P.; Gupta, S.; Sridharan, M. Studies on nanostructured v2o5/v/v2o5 films for un-cooled ir detector application. J. Mater. Sci. Mater. Electron. 2016, 27, 7494–7500.
- Abd-Alghafour, N.M.; Ahmed, N.M.; Hassan, Z. Fabrication and characterization of v2o5 nanorods based metal–semiconductor–metal photodetector. Sens. Actuators A Phys. 2016, 250, 250–257.
- Kim, D.; Yun, J.; Lee, G.; Ha, J.S. Fabrication of high performance flexible micro-supercapacitor arrays with hybrid electrodes of mwnt/v2o5 nanowires integrated with a sno2 nanowire uv sensor. Nanoscale 2014, 6, 12034–12041.
- Singh, N.; Umar, A.; Singh, N.; Fouad, H.; Alothman, O.Y.; Haque, F.Z. Highly sensitive optical ammonia gas sensor based on sn doped v2o5 nanoparticles. Mater. Res. Bull. 2018, 108, 266–274.
- Santos, M.C.; Hamdan, O.H.C.; Valverde, S.A.; Guerra, E.M.; Bianchi, R.F. Synthesis and characterization of v2o5/pani thin films for application in amperometric ammonia gas sensors. Org. Electron. 2019, 65, 116–120.
- Wang, C.; Li, X.; Yuan, Y.; Wang, B.; Huang, J.; Xia, F.; Zhang, H.; Xiao, J. Effects of sintering temperature on sensing properties of v2o5-wo3-tio2 electrode for potentiometric ammonia sensor. Sens. Actuators B Chem. 2017, 241, 268–275.
- Alam, M.M.; Uddin, M.T.; Asiri, A.M.; Rahman, M.M.; Islam, M.A. Development of reproducible thiourea sensor with binary sno2/v2o5 nanomaterials by electrochemical method. Arab. J. Chem. 2020, 13, 5406–5416.
- Rajesh, K.; Santhanalakshmi, J. Design and development of graphene intercalated v2o5 nanosheets based electrochemical sensors for effective determination of potentially hazardous 3,5–dichlorophenol. Mater. Chem. Phys. 2017, 199, 497–507.
- Zhang, H.; Zhang, L.; Hu, J.; Cai, P.; Lv, Y. A cataluminescence gas sensor based on nanosized v2o5 for tert-butyl mercaptan. Talanta 2010, 82, 733–738.
- Karthikeyan, P.S.; Dhivya, P.; Deepak Raj, P.; Sridharan, M. V2o5 thin film for 2-propanol vapor sensing. Mater. Today Proc. 2016, 3, 1510–1516.
- Imawan, C.; Steffes, H.; Solzbacher, F.; Obermeier, E. Structural and gas-sensing properties of v2o5–moo3 thin films for h2 detection. Sens. Actuators B Chem. 2001, 77, 346–351.
- Chen, C.L.; Dong, C.L.; Ho, Y.K.; Chang, C.C.; Wei, D.H.; Chan, T.C.; Chen, J.L.; Jang, W.L.; Hsu, C.C.; Kumar, K.; et al. Electronic and atomic structures of gasochromic v2o5 films. EPL (Europhys. Lett.) 2013, 101, 17006.
- Tadeo, I.J.; Parasuraman, R.; Krupanidhi, S.B.; Umarji, A.M. Enhanced humidity responsive ultrasonically nebulised v2o5 thin films. Nano Express 2020, 1, 010005.
- Schneider, K.; Lubecka, M.; Czapla, A. V2o5 thin films for gas sensor applications. Sens. Actuators B Chem. 2016, 236, 970–977.
- Vasanth Raj, D.; Ponpandian, N.; Mangalaraj, D.; Viswanathan, C. Effect of annealing and electrochemical properties of sol–gel dip coated nanocrystalline v2o5 thin films. Mater. Sci. Semicond. Process. 2013, 16, 256–262.
- Gandasiri, R.; Sreelatha, C.J.; Nagaraju, P.; Vijayakumar, Y. Effect of annealing temperature on micro-structural, optical and electrical characterization of nanostructured v2o5 thin films prepared by spray pyrolysis technique. Phys. B Condens. Matter 2019, 572, 220–224.
- Thangarasu, R.; Thangavel, E.; Chandrasekaran, J.; Balasundaram, O.N. Synthesis, characterization and gas sensing performance of v2o5 nano-structure on pet substrate. J. Mater. Sci. Mater. Electron. 2019, 30, 4238–4249.
- Yıldırım, M.A.; Tuna Yıldırım, S.; Çağirtekin, A.O.; Karademir, M.; Karaduman Er, I.; Coşkun, A.; Ateş, A.; Acar, S. The effect of deposition time on the structural, morphological and h2s gas sensing properties of the v2o5 nanostructures deposited by hydrothermal method. J. Mater. Sci. Mater. Electron. 2019, 30, 12215–12223.
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (vocs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141.
- Mirzaei, A.; Neri, G. Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: A review. Sens. Actuators B Chem. 2016, 237, 749–775.
- Amiri, V.; Roshan, H.; Mirzaei, A.; Neri, G.; Ayesh, A.I. Nanostructured metal oxide-based acetone gas sensors: A review. Sensors 2020, 20, 3096.
- Mirzaei, A.; Lee, J.-H.; Majhi, S.M.; Weber, M.; Bechelany, M.; Kim, H.W.; Kim, S.S. Resistive gas sensors based on metal-oxide nanowires. J. Appl. Phys. 2019, 126, 241102.
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. A novel gas sensor based on ag/fe2o3 core-shell nanocomposites. Ceram. Int. 2016, 42, 18974–18982.
- Wu, M.; Zhang, X.; Gao, S.; Cheng, X.; Rong, Z.; Xu, Y.; Zhao, H.; Huo, L. Construction of monodisperse vanadium pentoxide hollow spheres via a facile route and triethylamine sensing property. CrystEngComm 2013, 15, 10123–10131.
- Wang, Y.-T.; Whang, W.-T.; Chen, C.-H. Hollow v2o5 nanoassemblies for high-performance room-temperature hydrogen sensors. ACS Appl. Mater. Interfaces 2015, 7, 8480–8487.
- Hakim, S.A.; Liu, Y.; Zakharova, G.S.; Chen, W. Synthesis of vanadium pentoxide nanoneedles by physical vapour deposition and their highly sensitive behavior towards acetone at room temperature. RSC Adv. 2015, 5, 23489–23497.
- Li, Y.; Liu, J.; Zhang, J.; Liang, X.; Zhang, X.; Qi, Q. Deposition of in2o3 nanofibers on polyimide substrates to construct high-performance and flexible trimethylamine sensor. Chin. Chem. Lett. 2019.
- Zhang, J.; Song, P.; Li, Z.; Zhang, S.; Yang, Z.; Wang, Q. Enhanced trimethylamine sensing performance of single-crystal moo3 nanobelts decorated with au nanoparticles. J. Alloys Compd. 2016, 685, 1024–1033.
- Wang, D.; Gu, K.; Zhao, Q.; Zhai, C.; Yang, T.; Lu, Q.; Zhang, J.; Zhang, M. Synthesis and trimethylamine sensing properties of spherical v2o5 hierarchical structures. New J. Chem. 2018, 42, 14188–14193.
- Meng, D.; Si, J.; Wang, M.; Wang, G.; Shen, Y.; San, X.; Meng, F. In-situ growth of v2o5 flower-like structures on ceramic tubes and their trimethylamine sensing properties. Chin. Chem. Lett. 2019.
- Akande, A.A.; Mosuang, T.; Ouma, C.N.M.; Benecha, E.M.; Tesfamichael, T.; Roro, K.; Machatine, A.G.J.; Mwakikunga, B.W. Ammonia gas sensing characteristics of v2o5 nanostructures: A combined experimental and ab initio density functional theory approach. J. Alloys Compd. 2020, 821, 153565.
- Dhayal Raj, A.; Pazhanivel, T.; Suresh Kumar, P.; Mangalaraj, D.; Nataraj, D.; Ponpandian, N. Self assembled v2o5 nanorods for gas sensors. Curr. Appl. Phys. 2010, 10, 531–537.
- Dhayal Raj, A.; Suresh Kumar, P.; Yang, Q.; Mangalaraj, D. Synthesis and gas sensors behavior of surfactants free v2o5 nanostructure by using a simple precipitation method. Phys. E Low-Dimens. Syst. Nanostructures 2012, 44, 1490–1494.
- Yang, T.; Yu, H.; Xiao, B.; Li, Z.; Zhang, M. Enhanced 1-butylamine gas sensing characteristics of flower-like v2o5 hierarchical architectures. J. Alloys Compd. 2017, 699, 921–927.
- Raible, I.; Burghard, M.; Schlecht, U.; Yasuda, A.; Vossmeyer, T. V2o5 nanofibres: Novel gas sensors with extremely high sensitivity and selectivity to amines. Sens. Actuators B Chem. 2005, 106, 730–735.
- Yang, X.H.; Xie, H.; Fu, H.T.; An, X.Z.; Jiang, X.C.; Yu, A.B. Synthesis of hierarchical nanosheet-assembled v2o5 microflowers with high sensing properties towards amines. RSC Adv. 2016, 6, 87649–87655.
- Modafferi, V.; Panzera, G.; Donato, A.; Antonucci, P.L.; Cannilla, C.; Donato, N.; Spadaro, D.; Neri, G. Highly sensitive ammonia resistive sensor based on electrospun v2o5 fibers. Sens. Actuators B Chem. 2012, 163, 61–68.
- Mounasamy, V.; Mani, G.K.; Ponnusamy, D.; Tsuchiya, K.; Reshma, P.R.; Prasad, A.K.; Madanagurusamy, S. Investigation on ch4 sensing characteristics of hierarchical v2o5 nanoflowers operated at relatively low temperature using chemiresistive approach. Anal. Chim. Acta 2020, 1106, 148–160.
- Cao, P.; Gui, X.; Navale, S.T.; Han, S.; Xu, W.; Fang, M.; Liu, X.; Zeng, Y.; Liu, W.; Zhu, D.; et al. Design of flower-like v2o5 hierarchical nanostructures by hydrothermal strategy for the selective and sensitive detection of xylene. J. Alloys Compd. 2020, 815, 152378.
- Chauhan, P.S.; Bhattacharya, S. Highly sensitive v2o5·1.6h2o nanostructures for sensing of helium gas at room temperature. Mater. Lett. 2018, 217, 83–87.
- Mane, A.A.; Suryawanshi, M.P.; Kim, J.H.; Moholkar, A.V. Fast response of sprayed vanadium pentoxide (v2o5) nanorods towards nitrogen dioxide (no2) gas detection. Appl. Surf. Sci. 2017, 403, 540–550.
- Vijayakumar, Y.; Mani, G.K.; Ponnusamy, D.; Shankar, P.; Kulandaisamy, A.J.; Tsuchiya, K.; Rayappan, J.B.B.; Ramana Reddy, M.V. V2o5 nanofibers: Potential contestant for high performance xylene sensor. J. Alloys Compd. 2018, 731, 805–812.
- Jin, W.; Yan, S.; An, L.; Chen, W.; Yang, S.; Zhao, C.; Dai, Y. Enhancement of ethanol gas sensing response based on ordered v2o5 nanowire microyarns. Sens. Actuators B Chem. 2015, 206, 284–290.
- Panahi, N.; Shirazi, M.; Hosseinnejad, M.T. Fabrication, characterization and hydrogen gas sensing performance of nanostructured v2o5 thin films prepared by plasma focus method. J. Mater. Sci. Mater. Electron. 2018, 29, 13345–13353.
- Mane, A.A.; Moholkar, A.V. Effect of film thickness on no2 gas sensing properties of sprayed orthorhombic nanocrystalline v2o5 thin films. Appl. Surf. Sci. 2017, 416, 511–520.
- Fu, H.; Jiang, X.; Yang, X.; Yu, A.; Su, D.; Wang, G. Glycothermal synthesis of assembled vanadium oxide nanostructures for gas sensing. J. Nanopart. Res. 2012, 14, 871.
- Qiang, X.; Hu, M.; Zhou, L.; Liang, J. Pd nanoparticles incorporated porous silicon/v2o5 nanopillars and their enhanced p-type no2-sensing properties at room temperature. Mater. Lett. 2018, 231, 194–197.
- Bolokang, A.S.; Motaung, D.E. Reduction-oxidation of v2o5-wo3 nanostructured by ball milling and annealing: Their improved h2s gas sensing performance. Appl. Surf. Sci. 2019, 473, 164–173.
- Liang, Y.-C.; Cheng, Y.-R. Combinational physical synthesis methodology and crystal features correlated with oxidizing gas detection ability of one-dimensional zno–vox crystalline hybrids. CrystEngComm 2015, 17, 5801–5807.
- Birajdar, S.N.; Hebalkar, N.Y.; Pardeshi, S.K.; Kulkarni, S.K.; Adhyapak, P.V. Ruthenium-decorated vanadium pentoxide for room temperature ammonia sensing. RSC Adv. 2019, 9, 28735–28745.
- Zhang, H.; Luo, Y.; Zhuo, M.; Yang, T.; Liang, J.; Zhang, M.; Ma, J.; Duan, H.; Li, Q. Diethylamine gas sensor using v2o5-decorated α-fe2o3 nanorods as a sensing material. RSC Adv. 2016, 6, 6511–6515.
- Ozdemir, S.; Gole, J.L. The potential of porous silicon gas sensors. Curr. Opin. Solid State Mater. Sci. Semicond. Process. 2007, 11, 92–100.
- Yan, W.; Hu, M.; Wang, D.; Li, C. Room temperature gas sensing properties of porous silicon/v2o5 nanorods composite. Appl. Surf. Sci. 2015, 346, 216–222.
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B Chem. 2015, 221, 1170–1181.
- Meng, F.-L.; Guo, Z.; Huang, X.-J. Graphene-based hybrids for chemiresistive gas sensors. TrAC Trends Anal. Chem. 2015, 68, 37–47.
- Toda, K.; Furue, R.; Hayami, S. Recent progress in applications of graphene oxide for gas sensing: A review. Anal. Chim. Acta 2015, 878, 43–53.
- Wang, T.; Huang, D.; Yang, Z.; Xu, S.; He, G.; Li, X.; Hu, N.; Yin, G.; He, D.; Zhang, L. A review on graphene-based gas/vapor sensors with unique properties and potential applications. Nano-Micro Lett. 2016, 8, 95–119.
- Lu, G.; Ocola, L.E.; Chen, J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 445502.
- Amarnath, M.; Heiner, A.; Gurunathan, K. Surface bound nanostructures of ternary r-go/ mn3o4/v2o5 system for room temperature selectivity of hydrogen gas. Ceram. Int. 2020, 46, 7336–7345.
- Bhati, V.S.; Sheela, D.; Roul, B.; Raliya, R.; Biswas, P.; Kumar, M.; Roy, M.S.; Nanda, K.K.; Krupanidhi, S.B.; Kumar, M. No2 gas sensing performance enhancement based on reduced graphene oxide decorated v2o5 thin films. Nanotechnology 2019, 30, 224001.
- Kim, J.-Y.; Lee, J.-H.; Kim, J.-H.; Mirzaei, A.; Woo Kim, H.; Kim, S.S. Realization of h2s sensing by pd-functionalized networked cuo nanowires in self-heating mode. Sens. Actuators B Chem. 2019, 299, 126965.
- Wu, J.; Xing, X.; Zhu, Z.; Zheng, L.; Chen, J.; Wang, C.; Yang, D. Electrospun hollow cuo modified v2o5 nano-string of pearls with improved acetone sensitivity. Chem. Phys. Lett. 2019, 727, 19–24.
- Ko, W.C.; Kim, K.M.; Kwon, Y.J.; Choi, H.; Park, J.K.; Jeong, Y.K. Ald-assisted synthesis of v2o5 nanoislands on sno2 nanowires for improving no2 sensing performance. Appl. Surf. Sci. 2020, 509, 144821.
- Yeh, B.-Y.; Jian, B.-S.; Wang, G.-J.; Tseng, W.J. Cuo/v2o5 hybrid nanowires for highly sensitive and selective h2s gas sensor. RSC Adv. 2017, 7, 49605–49612.
- Wang, R.; Yang, S.; Deng, R.; Chen, W.; Liu, Y.; Zhang, H.; Zakharova, G.S. Enhanced gas sensing properties of v2o5 nanowires decorated with sno2 nanoparticles to ethanol at room temperature. RSC Adv. 2015, 5, 41050–41058.
- Jin, W.; Dong, B.; Chen, W.; Zhao, C.; Mai, L.; Dai, Y. Synthesis and gas sensing properties of fe2o3 nanoparticles activated v2o5 nanotubes. Sens. Actuators B Chem. 2010, 145, 211–215.
- Avansi, W.; Catto, A.C.; da Silva, L.F.; Fiorido, T.; Bernardini, S.; Mastelaro, V.R.; Aguir, K.; Arenal, R. One-dimensional v2o5/tio2 heterostructures for chemiresistive ozone sensors. ACS Appl. Nano Mater. 2019, 2, 4756–4764.
- Yang, X.; Wang, W.; Wang, C.; Xie, H.; Fu, H.; An, X.; Jiang, X.; Yu, A. Synthesis of au decorated v2o5 microflowers with enhanced sensing properties towards amines. Powder Technol. 2018, 339, 408–418.
- Sanger, A.; Kumar, A.; Kumar, A.; Jaiswal, J.; Chandra, R. A fast response/recovery of hydrophobic pd/v2o5 thin films for hydrogen gas sensing. Sens. Actuators B Chem. 2016, 236, 16–26.
- Feng, C.; Li, X.; Wang, C.; Sun, Y.; Zheng, J.; Lu, G. Facile synthesis benzene sensor based on v2o5-doped sno2 nanofibers. RSC Adv. 2014, 4, 47549–47555.
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Neri, G. oxide-shell nanomaterials for gas-sensing applications: A review. J. Nanopart. Res. 2015, 17, 371.
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. How shell thickness can affect the gas sensing properties of nanostructured materials: Survey of literature. Sens. Actuators B Chem. 2018, 258, 270–294.
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Low power-consumption co gas sensors based on au-functionalized sno2-zno core-shell nanowires. Sens. Actuators B Chem. 2018, 267, 597–607.
- Shah, A.H.; Liu, Y.; Nguyen, V.T.; Zakharova, G.S.; Mehmood, I.; Chen, W. Enhanced ultra-stable n-propylamine sensing behavior of v2o5/in2o3 core–shell nanorods. RSC Adv. 2015, 5, 54412–54419.
- Mane, A.A.; Nikam, S.A.; Moholkar, A.V. No2 gas sensing properties of sprayed composite porous moo3-v2o5 thin films. Mater. Chem. Phys. 2018, 216, 294–304.
- Mane, A.A.; Maldar, P.S.; Desai, S.P.; Moholkar, A.V. Gas sensing properties of (moo3)0.4(v2o5)0.6 microsheets: Effect of pd sensitization. Vacuum 2017, 144, 135–144.
- Naderi, H.; Hajati, S.; Ghaedi, M.; Dashtian, K.; Sabzehmeidani, M.M. Sensitive, selective and rapid ammonia-sensing by gold nanoparticle-sensitized v2o5/cuwo4 heterojunctions for exhaled breath analysis. Appl. Surf. Sci. 2020, 501, 144270.
- Zhang, F.; Wang, X.; Dong, J.; Qin, N.; Xu, J. Selective btex sensor based on a sno2/v2o5 composite. Sens. Actuators B Chem. 2013, 186, 126–131.
- Modafferi, V.; Trocino, S.; Donato, A.; Panzera, G.; Neri, G. Electrospun v2o5 composite fibers: Synthesis, characterization and ammonia sensing properties. Thin Solid Film 2013, 548, 689–694.
- Xiao, B.; Huang, H.; Yu, X.; Song, J.; Qu, J. Facile synthesis of layered v2o5/znv2o6 heterostructures with enhanced sensing performance. Appl. Surf. Sci. 2018, 447, 569–575.
- Wang, Y.; Zhou, Y.; Meng, C.; Gao, Z.; Cao, X.; Li, X.; Xu, L.; Zhu, W.; Peng, X.; Zhang, B.; et al. A high-response ethanol gas sensor based on one-dimensional tio2/v2o branched nanoheterostructures. Nanotechnology 2016, 27, 425503.
- Nagaraju, P.; Vijayakumar, Y.; Reddy, M.V.R.; Deshpande, U.P. Effect of vanadium pentoxide concentration in zno/v2o5 nanostructured composite thin films for toluene detection. RSC Adv. 2019, 9, 16515–16524.




