Vanadium pentoxide (V2O5) is a transition metal oxide with features such as high availability, good catalytic activity, unique electrical properties and high conductivity which are appropriate for gas sensing applications.
- V2O5
- gas sensor
- morphology
- sensing mechanism
- toxic gas
1. Introduction

2. Pristine Nanostructured V
2. Pristine Nanostructured V
2
O
5 Gas Sensors
Gas Sensors
Pristine V2O5 gas sensors with different morphologies have been reported in the literature. The gas sensing characteristics of V2O5 hierarchical architectures, especially for hollow spheres are rarely reported in the literature. In this regard, V2O5 hollow spheres (500–550 nm in diameter and a shell thickness of 55 nm) were synthesized through a solvothermal route. The hollow spheres were comprised of nanoplates with thicknesses of 50–80 nm and lengths of 70–120 nm. Moreover, for comparison solid nanostructured spheres were fabricated. The maximum responses (Ra/Rg) to trimethylamine (TEA) at 370 °C were 9.7 for V2O5 hollow spheres and 3.08 for V2O5 solid spheres, respectively. In fact, the hollow sphere hierarchical architecture with a higher surface area offered more adsorption sites for TEA molecules and a higher response for hollow spheres resulted. The sensing mechanism of the sensor to TEA was related to the reduction of V5+ ions to V4+ ions in the presence of TEA. Furthermore, based on XPS studies there was a slight shift to lower binding energy values after exposure of the sensor to TEA gas, confirming formation of V4+ ions. In addition, a color change from yellow to dark blue was observed, further confirming the formation of V4+ ions. V2O5 is an acidic oxide, which is highly suitable for the adsorption of basic molecules, such as TEA and consequently a larger response of the V2O5 hollow spheres sensor to TEA resulted [36][37]. Generally, resistive based gas sensors work at high temperatures, which need external heaters and increase the power consumption. Therefore, development of room temperature gas sensors not only solves above problems, but also integration with flexible substrates become easier. Furthermore, possible risk of explosion during sensing of explosive gases such as H2 gas significantly decreases. Hollow spheres comprising numerous nanocrystals of V2O5 as a shell were synthesized by a facile polyol approach for room temperature hydrogen gas sensing. The surface area of hollow spheres was about 356 m2/g and, therefore, it provided a large active surface area for adsorption of target gases. Furthermore, its porous structure led to further enhancement of gas reactions. Furthermore, the sharp corners as well as the edges of the tiny building blocks of hollow spheres were reported as highly active sites for enhancement of the sensing reactions during hydrogen sensing [37][38]. Another room temperature gas sensor was realized from V2O5 nanoneedles which were synthesized by a physical vapor deposition method [38][39]. The sensor exhibited a response (Ra/Rg) of 2.37 to 140 ppm acetone at room temperature. The relevant reaction was as follows: The most energetically favorable gas reaction is that a surface oxygen atom attacks the carbonyl carbon to form a C-O bond. Acetone contains the carbonyl group and because of the greater electronegativity of oxygen; a carbonyl group is a polar functional group and, therefore, it has a larger dipole moment (D = 2.88), relative to other tested gases, leading to the higher response of the gas sensor to acetone.
Trimethylamine (TMA; (CH3)3N)) is generated from dead fish and, therefore, the concentration of TMA is an indicator of the freshness of fish [39][40]. Furthermore, exposure to TMA vapor can cause nausea, headaches, and irritation to the eyes [40][41]. In this regard, spherical V2O5 hierarchical nanostructures comprised of plenty of nanosheets were produced through a hydrothermal method. The optimal sensor based on spherical V2O5 hierarchical nanostructures displayed a response of ~2.8 to 100 ppm TMA along with fast response/recovery times (5/28 s) at 240 °C. The spherical V2O5 nanostructures were comprised of numerous monocrystalline nanosheets, and therefore they have a unique three-dimensional hierarchical structure which provided plenty of active sites for gas molecules. Accordingly, the reaction between chemisorbed oxygen ions and TMA molecules, resulted in a decrease in electrical resistance and contributed to the sensor signal.
The most energetically favorable gas reaction is that a surface oxygen atom attacks the carbonyl carbon to form a C-O bond. Acetone contains the carbonyl group and because of the greater electronegativity of oxygen; a carbonyl group is a polar functional group and, therefore, it has a larger dipole moment (D = 2.88), relative to other tested gases, leading to the higher response of the gas sensor to acetone.
Trimethylamine (TMA; (CH3)3N)) is generated from dead fish and, therefore, the concentration of TMA is an indicator of the freshness of fish [39][40]. Furthermore, exposure to TMA vapor can cause nausea, headaches, and irritation to the eyes [40][41]. In this regard, spherical V2O5 hierarchical nanostructures comprised of plenty of nanosheets were produced through a hydrothermal method. The optimal sensor based on spherical V2O5 hierarchical nanostructures displayed a response of ~2.8 to 100 ppm TMA along with fast response/recovery times (5/28 s) at 240 °C. The spherical V2O5 nanostructures were comprised of numerous monocrystalline nanosheets, and therefore they have a unique three-dimensional hierarchical structure which provided plenty of active sites for gas molecules. Accordingly, the reaction between chemisorbed oxygen ions and TMA molecules, resulted in a decrease in electrical resistance and contributed to the sensor signal.
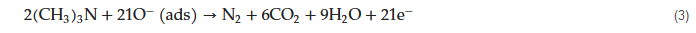 In addition, in a monocrystalline structure free electrons were able to transfer faster than in a polycrystalline structure, which results in fast response and recovery times of gas sensors and improvement of sensing properties [41][42].
Table 1 presents the gas sensing characteristics of pristine V2O5 gas sensors, where different morphologies of V2O5 prepared using various methods have been successful for sensing of different gases.
In addition, in a monocrystalline structure free electrons were able to transfer faster than in a polycrystalline structure, which results in fast response and recovery times of gas sensors and improvement of sensing properties [41][42].
Table 1 presents the gas sensing characteristics of pristine V2O5 gas sensors, where different morphologies of V2O5 prepared using various methods have been successful for sensing of different gases.
|
V2O5 Morphology |
Synthesis Method |
Target Gas |
Conc. (ppm) |
Response (Ra/Rg) or (Rg/Ra) |
Response (Ra/Rg) Or (Rg/Ra) T (°C) |
T (°C) Response time/Recovery Time(s) |
Response Time/Recovery Time(S) Ref. |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Ref. | |||||||||||||||
|
Hollow spheres Solid spheres |
Solvothermal |
C3H | |||||||||||||
|
V2O5 | 9 | N |
/In2O3 core–shells |
Hydrothermal 500 500 |
9.7 3.08 |
n-propylamine370 |
20/83 45/150 |
200 |
4 |
190 |
48/121 |
||||
[ | ] | [ | ] |
Hollow spheres |
Chemical synthesis |
H2 |
200 |
2.8 |
RT |
50/10 |
[37] | ||||
|
MoO3-V2O5 thin films | [ |
Chemical spray pyrolysis | ] | ||||||||||||
NO | 2 |
120 |
80 * |
200 |
118/1182 |
Nanoneedles |
|||||||||
|
(MoO3)0.4(V2O5)0.6 sheet composite |
PVD |
C3H6O |
140 |
2.35 |
RT |
Chemical spray pyrolysis 67/- |
[ |
100 |
115 |
39/453 |
|||||
] | [ | ] |
Hierarchical nanostructures |
||||||||||||
|
Au/V2O5/CuWO | Hydrothermal |
4 composite |
C3 |
Hydrothermal | H9N |
NH3 10–200 |
5 1.13-3.57 |
2.7 240 |
1505/28 |
||||||
35/33 | [ | ] | [98] |
Flower-like |
Hydrothermal |
5 |
|||||||||
|
SnO2/V2O5 composite |
Sol-gel |
C6H6 |
2.25 |
200 |
13/13 |
200 |
10.5 [42 |
275 ][43] |
|||||||
-/- | [ | ] | [99] |
Nanorods |
|||||||||||
|
V2O5/polyvinyl acetate NF composite |
CVD |
Electrospinning NH3 |
NH3 100 |
0.8 235 * |
6 *400 |
-/- |
|||||||||
-/- | [ | ][100] |
Nanorods |
Solvothermal |
|||||||||||
|
V2O5/ZnV2O6 nanobelt composite |
Chemical route |
C2H5OH NH3 |
C2H5OH 500 |
2000 1.04 1.02 |
16.5 |
RT |
240-/- |
-/- |
|||||||
[ | ] | [ | ] |
Spherical |
|||||||||||
|
TiO2/V2 |
Precipitation |
O5 NF composite C |
Electrospinning 2H5OH NH3 |
100 1.04 1.06 |
-/- |
[ |
24.6 45] |
350 [46] |
|||||||
6/7 | [ | ][102] |
Flower-like |
||||||||||||
|
ZnO/V2O5 thin films |
Hydrothermal |
Spray pyrolysis |
1-butylamine |
C7H8 100 |
400 2.6 |
2.3140 |
27 9/49 |
23/28 |
|||||||
[ | ] | [ | ] |
Nanofibers |
Electrospinning |
9.5 |
500 * |
RT |
-/- |
||||||
|
Flower-like Sheet-like |
Hydrothermal |
100 |
3.6 2.8 |
300 |
25/14 17/14 |
||||||||||
|
Nanofibers |
Sol–gel |
NH3 |
2.1 |
11 * |
200 |
50/350 |
|||||||||
|
Flower-like |
DC sputtering |
CH4 |
500 |
100 |
206/247 |
||||||||||
|
Hydrothermal |
C8H10 |
3 |
300 |
44/74 |
|||||||||||
|
Nano stars |
Hydrothermal |
He |
300 |
53 * |
RT |
9/10 |
|||||||||
|
Nanorods |
Chemical spray pyrolysis |
NO2 |
100 |
24.2 * |
200 |
13/140 |
|||||||||
|
Nanofibers |
Chemical spray pyrolysis |
C8H10 |
27 |
RT |
80/50 |
||||||||||
|
Nanowires |
Melt quenching |
C2H5OH |
1000 |
9.09 |
330 |
-/- |
|||||||||
|
Thin film |
Plasma focus method |
H2 |
50 * |
275 |
-/- |
||||||||||
|
Chemical spray pyrolysis |
NO2 |
100 |
41 * |
200 |
20/150 |
* Response = Δ𝑅/𝑅𝑎×100; RT: Room temperature; PVD: Physical vapor deposition; CVD: Chemical vapor deposition.
3. Decorated/Doped V2O5 Gas Sensors
Based on the above section about pristine V2O5 gas sensors, pristine V2O5 nanostructures suffer from low sensitivity and selectivity, which hinder their applications for sensing applications [58][61]. Accordingly, different strategies, such as p-n heterojunction formation [59][62], n-n heterojunction formation [60][61][63,64] and noble metal decoration [62][65] have been proposed to enhance their sensing properties. V2O5 decoration is a good strategy to enhance the sensing properties of gas sensors. V2O5-decorated α-Fe2O3 composite NRs were prepared by an electrospinning technique and they had a high surface area of 30.5 m2/g. The composite exhibited a response (Ra/Rg) of 9 to 100 ppm diethylamine along with good selectivity to diethylamine gas and a fast response time of 2 s at 350 °C [63][66]. Improved sensing performance was related to the formation of V2O5-Fe2O3 heterojunctions and catalytic effect of V2O5 to diethylamine. Porous silicon (PS) is a good candidate for sensing studies due to its offering of a high surface area and a porous structure. In addition it can be simply prepared by a chemical etching process [64][68]. Therefore, composites between V2O5 and PS can be promising for gas sensing studies. In a relevant study, thin V films were decorated on the PS by sputtering at different times of 30 and 60 min and then, the samples were annealed at 600 °C [65][69]. The PS/V2O5 NRs structure provided a better response than pristine PS at 25 °C, and the sensor sputtered for 60 min exhibited the highest response of 7.4 to 2 ppm NO2 gas. Both the PS and V2O5 NRs had plenty of dangling bonds, oxygen vacancies and defects, leading to high adsorption of oxygen molecules even at room temperature. In the interfaces between PS and V2O5, p-n heterojunctions formed and, upon exposure to NO2 gas, the significant modulation of electrical resistance in the heterojunctions led to the appearance of a sensing signal. Graphene and its derivations such as graphene oxide and reduced graphene oxide have high surface areas and unique electrical properties which can be beneficial for gas sensing studies [66][67][70,71]. The initial resistance of the GO is high, limiting its practical applications in pristine form [68][69][72,73]. However, after the reduction GO to RGO, there are some defects, vacancies and functional groups which are useful for gas sensing applications [70][74]. In a relevant study, an RGO surface was decorated with Mn3O4 and V2O5 NOs via a hydrothermal method for detection of H2 gas at 30 °C. The sensor showed a high response (ΔR/Ra × 100) of 174% to 50 ppm H2 gas at room temperature. The sheet-like structure of RGO provided a large surface area for gas sensing reactions. In addition, because of the formation of p-n (RGO-V2O5) and p-p (RGO-Mn3O4) heterojunctions, significant modulation of resistance in the presence of H2 occurred, resulting in the generation of a sensing signal [71][75]. In another study, a V2O5 film was prepared by a reactive sputtering technique and then, RGO was decorated over the V2O5 thin film by a drop casting method for NO2 sensing studies. The sensor showed a response of 50.7% to 100 ppm NO2 gas at 150 °C. However, its recovery time was long (778 s). Formation and modulation of the p-n heterojunction at the interface of RGO and V2O5 was the main reason for the detection of NO2 gas. Moreover, the presence of active sites such as oxygen functional groups on the RGO surface improved the sensing response [72][76]. Not only can V2O5-decoration be a useful technique to enhance the gas sensing properties, but decoration of other metal oxides or noble metals on the surface of gas sensor can also be a good technique to improve the gas sensing properties of V2O5-based gas sensors. A P-type CuO with excellent catalytic activity is extensively used for sensing studies [73][77]. The work function of CuO is 5.3 eV, which is different to that of V2O5 (4.7 eV). Therefore, when heterojunctions form between the CuO and V2O5, enhanced gas response can be expected. In this context, hollow nanostructures using CuO decorated V2O5 nano-strings of pearls were fabricated through an electrospinning method. The V2O5/CuO sensors demonstrated a response (Ra/Rg ) of 8.8 to 500 ppm acetone at 440 °C, which was more than three times higher than that of bare V2O5 NFs. The improved performance was related to the generation of CuO/V2O5 p-n heterojunctions, which provided plenty of resistance modulation sources and upon exposure to acetone gas higher response was resulted [74][78]. Table 2 shows the gas sensing properties of decorated or doped V2O5-based gas sensors, where different synthesis methods along with different morphologies and various materials have been reported to realize gas sensors for the sensing of toxic gases.|
Sensor |
Synthesis Method |
Target Gas |
Conc. (ppm) |
Response (Ra/Rg) or (Rg/Ra) |
Working Temp. (°C) |
Response Time/Recovery Time(s) |
Ref. |
|---|---|---|---|---|---|---|---|
|
Pd decorated porous Si/V2O5 nanopillars |
DC sputtering |
NO2 |
2 |
4.5 |
RT |
-/- |
|
|
Ru-decorated layer structure V2O5 |
Hydrothermal |
NH3 |
130 |
4 * |
RT |
~2/~12 |
|
|
V2O5-decorated α-Fe2O3 nanorods |
Electrospinning |
C4H11N |
300 |
9 |
350 |
2/40 |
|
|
V2O5 decorated SnO2 NWs |
VLS/ALD |
NO2 |
200 ppb |
3.6 |
250 |
-/- |
|
|
Porous Si/V2O5 NR composite |
Galvanostatic electrochemical etching |
NO2 |
2 |
7.4 |
RT |
-/- |
|
|
rGO/Mn3O4/V2O5 nanocomposite |
Hydrothermal |
H2 |
50 |
175 |
RT |
82/92 |
|
|
Pd-decorated CuO NWs |
UV irradiation |
H2S |
100 |
1.962 |
100 |
-/- |
|
|
V2O5/CuO nano-string of pearls |
Electrospinning |
C3H6O |
500 |
9 |
440 |
~40/~100 |
|
|
CuO-decorated V2O5 NWs |
Hydrothermal and wet-deposition |
H2S |
23 |
31.86 |
220 |
130/218 |
|
|
SnO2 NP-decorated V2O5 NWs |
Hydrothermal |
C2H5OH |
1000 |
1.3 |
RT |
-/- |
|
|
Fe2O3 activated V2O5 nanotubes |
Hydrolysis |
C2H5OH |
1000 |
2.2 |
270 |
-/- |
|
|
TiO2-decorated V2O5 NWs |
Hydrothermal |
O3 |
1.25 |
2.6 * |
300 |
~180/~180 |
|
|
RGO-decorated V2O5 thin film |
Reactive sputtering and drop casting |
NO2 |
100 |
50.7 |
150 |
-/- |
|
|
Au NP-decorated V2O5 |
Two-step in-situ reduction of Au and thermal oxidization as V2O5 |
Amines |
100 |
7.5 |
240 |
90/35 |
|
|
Pd-decorated V2O5 thin film |
DC magnetron reactive sputtering |
H2 |
100 |
5.7 |
100 |
~6/14.8 |
|
|
V2O5- doped SnO2 NFs |
Electrospinning |
C6H6 |
25 |
6.32 |
325 |
3/47 |
* Response = Δ𝑅/𝑅𝑎×100; RT: Room temperature; VLS: Vapor-liquid-solid; NR; Nanorod; NP; Nanoparticle; NF; Nanofiber.
4. Nanocomposites/Nanohybrids of V2O5 Gas Sensors
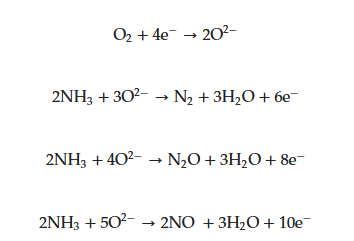
|
Sensing Material |
Synthesis Method |
Target Gas |
Conc. (Ppm) |
|---|
* Response = Δ𝑅/𝑅𝑎×100.
