Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yu Yang | -- | 1447 | 2023-07-20 15:35:49 | | | |
| 2 | Catherine Yang | Meta information modification | 1447 | 2023-07-21 02:37:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jones, A.D.; Morehead, A.T.; Yang, Y. Degradation and Extraction of Organochlorine Pollutants. Encyclopedia. Available online: https://encyclopedia.pub/entry/47058 (accessed on 28 February 2026).
Jones AD, Morehead AT, Yang Y. Degradation and Extraction of Organochlorine Pollutants. Encyclopedia. Available at: https://encyclopedia.pub/entry/47058. Accessed February 28, 2026.
Jones, Aaryn D., Andrew T. Morehead, Yu Yang. "Degradation and Extraction of Organochlorine Pollutants" Encyclopedia, https://encyclopedia.pub/entry/47058 (accessed February 28, 2026).
Jones, A.D., Morehead, A.T., & Yang, Y. (2023, July 20). Degradation and Extraction of Organochlorine Pollutants. In Encyclopedia. https://encyclopedia.pub/entry/47058
Jones, Aaryn D., et al. "Degradation and Extraction of Organochlorine Pollutants." Encyclopedia. Web. 20 July, 2023.
Copy Citation
A subcritical water degradation and extraction method was developed to remediate environmental soils contaminated by highly recalcitrant organochlorine pollutants. Hydrogen peroxide was used to effectively decompose organochlorine pollutants under subcritical water conditions.
subcritical water
oxidation
extraction
phenols
chlorophenols
1. Chlorinated Pollutants
The Environmental Protection Agency (EPA) has compiled a list of persistent, bioaccummulative, and toxic pollutants (PBTs) that pose a threat to both humans and natural ecosystems [1]. One class of compounds on this PBT list that are especially problematic within the soil systems are organochlorine pesticides and insecticides. Chlorinated pesticides, such as DDT, are known for their affinity for the nonpolar soil substrate rather than the polar water column in natural ecosystems. They are therefore quite persistent within the soil and are nearly inert to natural, physical, or chemical degradation, in some cases posing a threat to the integrity of the ecosystem for up to thirty years or more [1][2]. If exposed to mammals, these pollutants are stored and accumulated in the fatty tissues of the organism and will bio-magnify through the food chain. Many of these pollutants are proven or possible carcinogens, and well-documented evidence of the deleterious effects on wildlife has been shown. Due to the combination of these harmful properties, EPA has banned the use of all pollutants found on the PBT list. Although these pesticides are no longer approved for use, many problem areas still exist due to their environmental persistence. Therefore, attempts at soil remediation by removal of the harmful pollutants from the solid matrix should be taken in order to improve the quality of these disturbed ecosystems.
2. Traditional Remediation Methods
Typically, contaminated soils are dealt with by containment or incineration. Containment is simply the removal of the polluted soil and subsequent storage at a monitored hazardous waste site. Containment, however, may pose an eventual threat to the surrounding groundwater and requires long-term monitoring. Incineration is the complete combustion of the polluted soils at temperatures of about 1200 °C. This unfortunately creates a secondary pollution hazard arising from the emissions of the incinerator [3]. Additionally, both of the aforementioned methods are not ideal because they do not allow for the replacement of the soil to the original site.
A popular in situ method of treating polluted soils is bioremediation. This technique attempts to facilitate the biological breakdown of harmful organic pollutants by the available soil microorganisms. In most cases, the ground is tilled or injected with air to maintain the oxygen level. Also, in most cases, the appropriate microorganisms are not naturally present within the soils and must be artificially introduced into the soil.
Although widely sought as an in-situ pollution mitigation method, bioremediation has many disadvantages. The concentration of pollutants is critical to the survival of microorganisms, as a high concentration can be toxic while a low concentration can limit the biological activity of microorganisms. Uncontrollable variations in the environmental conditions at the site such as the natural flux in temperature, amount of sunlight, and frequency of precipitation can also impact microbial growth. Time is also likely to be a disadvantage, as bioremediation can take up to three years or more to completely remove toxins from a polluted site. During bioremediation, the monitoring and maintenance of the site is necessary to measure progress and optimize conditions, which can be quite costly and time consuming. Due to these disadvantages, alternate remediation methods such as extraction and chemical conversion techniques have been explored.
3. Extractions of Contaminated Environmental Solids
Traditional laboratory-scale methods of organic pollutant removal from environmental solids include Soxhlet extraction, liquid–solid extraction, and sonication. These methods not only necessitate the use of large amounts of organic solvents, but they are also time consuming, labor intensive, and essentially not suitable for the remediation of large volumes of polluted environmental solids. The use of organic solvents as an extraction media for polluted soil systems is not optimal because the organic solvents are often harmful themselves and solvent residues can remain on the soil after extraction. The need for cleaner extraction fluids has led to the use of sub- and supercritical fluids as extraction media.
Supercritical fluid extraction (SFE) typically makes use of supercritical carbon dioxide as the extraction fluid. SFE has been used at the industrial scale to extract caffeine from coffee beans and is a promising solvent for many other food preparation techniques due to the non-toxicity of carbon dioxide. Carbon dioxide is very cheap; however, the disadvantage is that the necessary instrumentation for SFE has a very high initial cost. In addition, due to the nonpolar nature of carbon dioxide, the extraction of more polar compounds such as organochlorine pesticides cannot be achieved efficiently without the addition of a small amount of organic modifier.
This once again leads to the need for a clean extraction fluid for soil remediation purposes that can remove both polar and moderately polar pollutants. A solution to this problem has been shown to be the use of subcritical water as an extraction media. Subcritical water is simply heated water that is pressurized to maintain the liquid state. Under these conditions, subcritical water can mimic the physical and chemical properties of commonly used organic solvents, without the hazards associated with these undesirable solvents. Subcritical water has been proven to be a successful extraction fluid at the pilot-scale level for many pollutants commonly found in soils [4][5].
4. Chemical Conversion and Destruction Methods
As mentioned earlier, bioremediation is a popular method used to mitigate soil pollution via the biological transformation of the pollutants. Other methods are currently being studied, such as the chemical alteration of pollutants into innocuous or less persistent byproducts. These environmentally beneficial chemical reactions can be classified as oxidation, reduction, hydrolysis, or dechlorination.
Many types of reactions that facilitate the oxidation of persistent pollutants have been studied. Wet air oxidation has been frequently used to treat wastewater, which simply uses elevated temperatures and the oxygen available in the air to oxidize pollutants. For more persistent pollutants, however, stronger oxidizing agents must be added, such as ozone or hydrogen peroxide. Oxidation with hydrogen peroxide is of particular interest because it can be an extremely powerful oxidizing agent. The oxidative mechanism occurs due to the production of highly oxidizing hydroxyl radicals, which can be catalyzed by metals, UV light, and elevated temperature. Hydrogen peroxide oxidation with the addition of iron catalysts has been studied as an in-situ soil remediation method for gasoline-contaminated soils. Furthermore, the addition of hydrogen peroxide to wastewater or other environmental solids contaminated by polychlorinated biphenyls (PCBs) at elevated temperatures led to a significant reduction in the concentrations of these highly recalcitrant pollutants [6][7][8].
Chemical reactions can also be enhanced by using sub- or supercritical water as a reaction solvent. Supercritical water oxidation is a further development of the aforementioned wet air oxidation. Water in the supercritical state is combined with an oxidant to efficiently destroy pollutants in a matter of minutes. Supercritical water has been shown to effectively remediate soils contaminated with polycyclic aromatic hydrocarbons (PAHs) [9]. The supercritical water oxidation process has several disadvantages, however, which include equipment corrosion, salt deposition, and the high cost of the supercritical water oxidation apparatus.
To avoid the problems associated with supercritical water, a beneficial alternative could be the use of subcritical water as a reaction solvent. Many different types of reactions have been studied in subcritical water due to the relative ease of reaching the necessary conditions and the elimination of traditional organic solvents. Reactions such as oxidation, reduction, dechlorination, and hydrolysis have been performed in subcritical water [10][11][12][13][14][15]. Alkyl aromatic compounds were effectively oxidized to aldehydes, ketones, and acids using molecular oxygen mediated by transition metal catalysts in subcritical water. Several compounds such as methylene chloride and thiodiglycol have been observed to undergo hydrolysis and oxidation under subcritical water conditions [13]. The destruction of TNT in subcritical water was thought to proceed via a hydrolysis mechanism in a study by Hawthorne et. al., while in another study, aldehydes were reduced to alcohols with the addition of sodium formate under subcritical water conditions [5]. Reductive dechlorination of dioxins and pesticides has been performed in subcritical water both with and without the addition of zerovalent iron as a catalyst [14].
5. Organic Degradation Mechanisms
The generic mechanism for the decomposition of phenols utilizing hydroxyl radicals (i.e., Fenton) proposed by Abouseoud et al. [15] is shown below in Figure 1. Other possible byproducts include acetic and formic acids. These reactions are most extensively studied under typical atmospheric pressure conditions and are most effective under acidic conditions [16]. The mechanism of phenols under subcritical water conditions is likely the same, but it is currently less clear how the different properties of the subcritical water and higher temperature and pressure will affect the outcome.
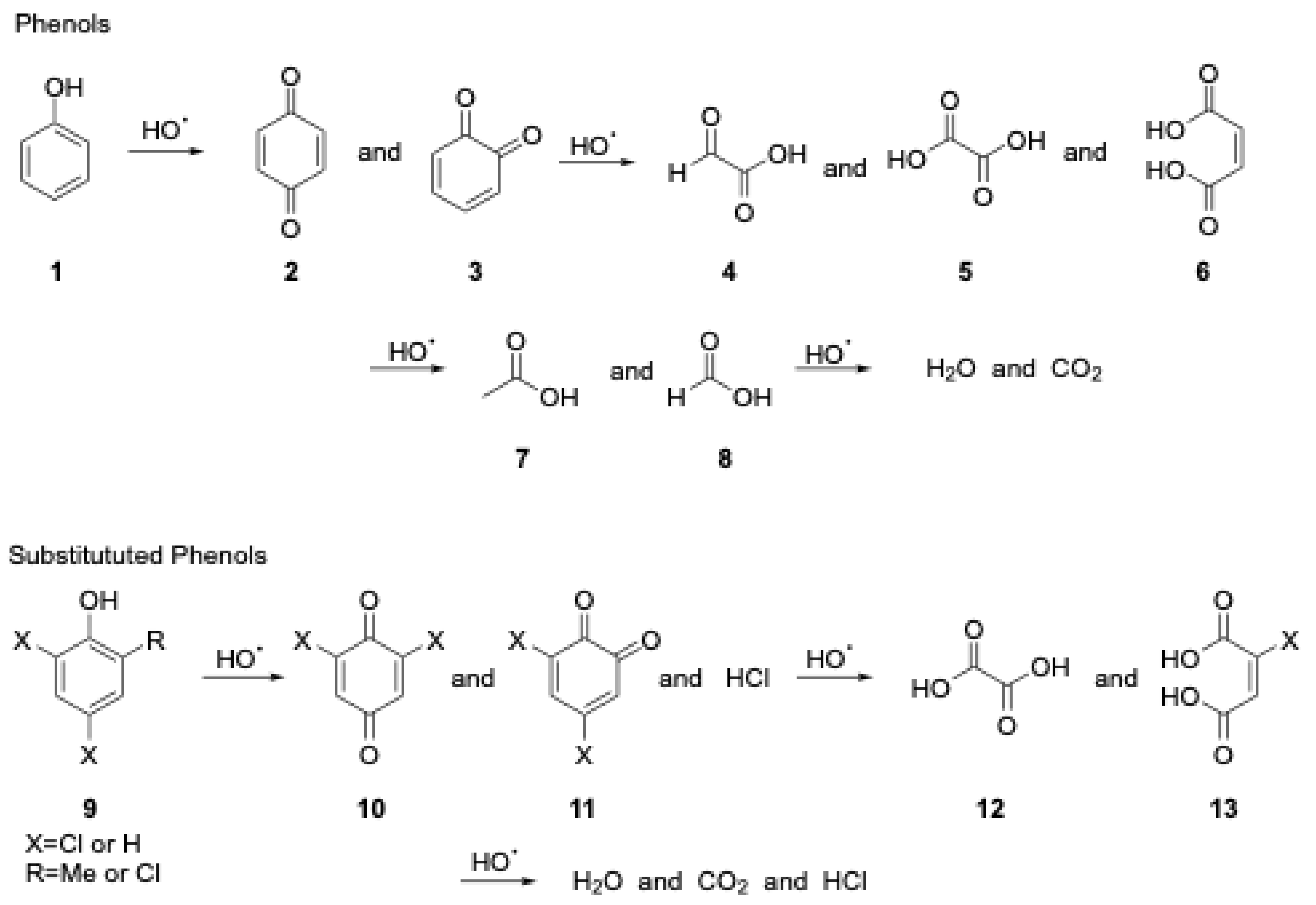
Figure 1. Generic mechanism for decomposition of phenols utilizing hydroxyl radicals.
References
- United States Environmental Protection Agency. Persistent, Bioaccumulative, and Toxic (PBT) Chemicals under TSCA Section 6(h). PBT Program Accomplishments. 2000. Available online: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/persistent-bioaccumulative-and-toxic-pbt-chemicals (accessed on 7 January 2023).
- Forstner, U. Lecture Notes in Earth Sciences: Contaminated Sediments; Springer: Berlin/Heidelberg, Germany, 1989.
- Dean, J.R. Extraction Methods for Environmental Analysis; John Wiley & Sons: Chichester, UK, 1998.
- Lagadec, A.J.M.; Miller, D.J.; Lille, A.V.; Hawthorne, S.B. Pilot-Scale Subcritical Water Remediation of Polycyclic Aromatic Hydrocarbon- and Pesticide-Contaminated Soil. ACS Environ. Sci. Technol. 2000, 34, 1542–1548.
- Hawthorne, S.B.; Lagadec, A.J.M.; Kalderis, D.; Lilke, A.V.; Miller, D.J. Pilot-Scale Destruction of TNT, RDX, and HMX on Contaminated Soils Using Subcritical Water. Environ. Sci. Technol. 2000, 34, 3224–3228.
- Islam, M.N.; Park, J.; Shin, M.; Park, H. Decontamination of PCBs-containing soil using subcritical water extraction process. Chemosphere 2014, 109, 28–33.
- Doctor, N.; Yang, Y. Destruction of Polychlorinated Biphenyls under Subcritical Water Conditions in the Presence of Hydrogen Peroxide or Sodium Hydroxide. Int. J. Chem. Eng. Appl. 2018, 9, 119–122.
- Duffy, J.E.; Anderson, M.A.; Hill, C.G.; Zeltner, W.A. Wet Peroxide Oxidation of Sediments Contaminated with PCBs. ACS Environ. Sci. Technol. 2000, 34, 3199–3204.
- Kronholm, J.; Kalpala, J.; Hartonen, K.; Reikkola, M. Pressurized hot water extraction coupled with supercritical water oxidation in remediation of sand and soil containing PAHs. J. Supercrit. Fluids 2002, 23, 123–134.
- Yabalak, E.; Murat Gizir, A. Treatment of agrochemical wastewater by subcritical water oxidation method: Chemical composition and ion analysis of treated and untreated samples. J. Environ. Sci. Health 2020, 55, 1424–1435.
- Wang, L.; Wang, L.; Miao, Z.; Shao, X.; Chen, J.; Lu, X. Comparison of subcritical water extraction and microwave-assisted water extraction for the determination of chlorophenols in polluted lake sediments. Anal. Methods 2012, 4, 844–848.
- Hashimoto, M.; Taniguchi, S.; Giri, R.R.; Ozaki, H. Oxidative degradation of 2,4-dichlorophenoxyacetic acid (2,4-D) in subcritical and supercritical waters. Water Sci. Technol. 2010, 62, 484–490.
- Lachance, R.; Paschkewitz, J.; DiNaro, J.; Tester, J.W. Thiodiglycol hydrolysis and oxidation in sub- and supercritical water. J. Supercrit. Fluids 1999, 16, 133–147.
- Kubatova, A.; Lagadec, A.J.M.; Hawthorne, S.B. Dechlorination of Lindane, Dieldrin, Tetrachloroethane, Trichloroethene, and PVC in Subcritical Water. ACS Environ. Sci. Technol. 2002, 36, 1337–1343.
- Azizi, A.; Abouseoud, M.; Amrane, A. Phenol Removal by a Sequential Combined Fenton-Enzymatic Process. Nat. Environ. Pollut. Technol. 2017, 16, 321–330.
- Bautista, P.; Mohendano, A.; Amrane, A. An overview of the application of Fenton oxidation to industrial wastewaters treatments. J. Chem. Technol. Biotechnol. 2008, 83, 1323–1338.
More
Information
Subjects:
Green & Sustainable Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
577
Revisions:
2 times
(View History)
Update Date:
21 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





