Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adèle Ehongo, MD, PhD | -- | 1484 | 2023-07-18 13:47:55 | | | |
| 2 | Wendy Huang | Meta information modification | 1484 | 2023-07-19 10:10:26 | | | | |
| 3 | Adèle Ehongo, MD, PhD | + 1 word(s) | 1485 | 2023-07-21 13:34:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ehongo, A.; Bacq, N. Clinical Investigations and Diagnosis of Peripapillary Intrachoroidal Cavitation. Encyclopedia. Available online: https://encyclopedia.pub/entry/46928 (accessed on 07 February 2026).
Ehongo A, Bacq N. Clinical Investigations and Diagnosis of Peripapillary Intrachoroidal Cavitation. Encyclopedia. Available at: https://encyclopedia.pub/entry/46928. Accessed February 07, 2026.
Ehongo, Adèle, Noélie Bacq. "Clinical Investigations and Diagnosis of Peripapillary Intrachoroidal Cavitation" Encyclopedia, https://encyclopedia.pub/entry/46928 (accessed February 07, 2026).
Ehongo, A., & Bacq, N. (2023, July 18). Clinical Investigations and Diagnosis of Peripapillary Intrachoroidal Cavitation. In Encyclopedia. https://encyclopedia.pub/entry/46928
Ehongo, Adèle and Noélie Bacq. "Clinical Investigations and Diagnosis of Peripapillary Intrachoroidal Cavitation." Encyclopedia. Web. 18 July, 2023.
Copy Citation
Peripapillary intrachoroidal cavitation (PICC) is a yellow-orange lesion, located at the outer border of the myopic conus. First described as a localized detachment of the retinal pigment epithelium, its intrachoroidal location was later revealed, justifying its current name. PICC is related to other myopic complications such as posterior staphyloma, but its pathogenesis is not clear to date. Although it has been considered a benign condition, most eyes with PICC show visual field defects, which leads to diagnostic uncertainty as these deficits resemble those seen in glaucoma. Furthermore, eyes with PICC may develop macular detachment with retinoschisis.
peripapillary intrachoroidal cavitation
border tissue
myopic complication
optical coherence tomography
angiography
1. Introduction
Peripapillary intrachoroidal cavitation (PICC) is a myopic complication of which the prevalence is expected to increase, due to the increasing prevalence of myopia.
PICC is a well-circumscribed yellow-orange lobular lesion, located at the outer border of the myopic conus [1]. Advances in optical coherence tomography (OCT) have revealed that PICC is a hyporeflective intrachoroidal thickening [2] with little or no deformation of the overlying plane of Bruch’s membrane [3]. It appears as a neural-based triangular choroidal thickening in OCT sections crossing the optic nerve (ON) head [3][4]. A discontinuity in the border tissue of the choroid is often associated with PICC [3].
Visual field (VF) defects are reported in up to 73.3% of PICCs [5]. These VF deficits are similar to those observed in glaucoma [5], constituting a cause of diagnostic uncertainty. Additionally, macular detachment with or without retinoschisis can complicate the prognosis of PICC [6][7][8][9][10][11]. Knowledge of the PICC enables avoiding misdiagnosis as a metastatic choroidal tumor which can lead to unnecessary and anxiety-inducing investigations [1].
PICC is related to other myopic complications, namely posterior staphyloma and myopic tilted disc [4]. It is more common in eyes with a higher maculopathy category [12].
While the pathogenesis of PICC has been hypothesized for years, recent findings in the biomechanics of the ON have reopened the debate. Indeed, several methods have demonstrated high traction forces exerted by the ON sheaths on the peripapillary region of myopic eyes during ocular movements [13][14][15]. These biomechanical findings renew interest in the pathogenesis of myopic peripapillary changes because these pulling forces have been suggested as promoters of PICC [16].
2. Fundoscopy and OCT
The yellow orange ophthalmoscopic appearance of PICC [1][3][4][17][18] (Figure 1A) is obvious only in 46.7% to 53% of OCT-detected PICCs [4][18][19]. Therefore, OCT is the recommended tool for the screening of PICC.
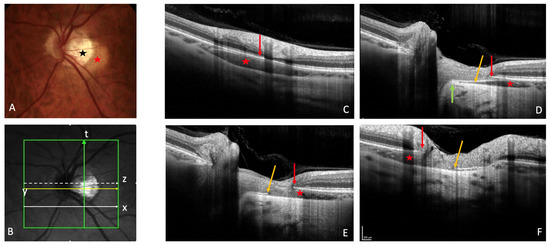
Figure 1. Peripapillary intrachoroidal cavitation (PICC). (A) Fundus picture. PICC is the yellow-orange lesion at the outer border of the myopic conus (black star) (B). Infrared image showing the location of sections (C) to (F). (C–F) PICC is the hyporeflective space behind the plane of Bruch’s membrane (BM). (C) Slice along the line x, below the optic nerve head (ONH). (D–F) Orange arrow = border tissue of the choroid (BT). (D) Section along the arrow y, through the ONH. The BT is continuous between the BM and the sclera (green arrow). (E) Section through the ONH, along the arrow z. The BT is discontinuous between the red and orange arrows. (F) Along the myopic conus (arrow t). The BT shows a discontinuity between the red and orange arrows. (A–F) Red star = PICC. (C–F) Red arrow = BM. The fundus picture was taken using VISUCAM® non-mydriatic camera (PRO NM Carl Zeiss Meditec, Jena, Germany). The device used for OCT is the Spectral Domain OCT Spectralis® OCT HRA-OCT, model S3300 (Heidelberg Engineering GmbH, Heidelberg Germany).
OCT reveals a spectrum of PICC appearances depending on the location and orientation of the section (Figure 1B). At the level of the peripapillary zone, it shows an aspect of hyporeflective intrachoroidal thickening [2] behind the plane of Bruch’s membrane (Figure 1C). If the section is through the ON, the PICC shows a triangular choroidal thickening (Figure 1D) with the base at the ON head. The well-demarcated border tissue of choroid (Figure 1D) may show discontinuity (Figure 1E,F).
The lesion is mainly located below the ON head [1][2][3][5][12][17][18][20][21][22]. However, other peripapillary areas may also be involved [4][10][12][17][21][22], and the PICC may even surround the entire ON head [17][19]. Therefore, Shimada subdivided it into three grades based on its overall circumference around the ON head [17].
Using spectral domain OCT or swept-source OCT, the diagnostic features of PICC are currently well established [2][3][20][21][23], obviating the need for other more invasive modalities such as fluorescein or indocyanine green angiography, the characteristics of which are summarized below.
3. Fluorescein Angiography
The sequence of fluorescein angiography shows early hypofluorescence followed by late hyperfluorescence without dye pooling in the area of PICC [1][2][17][21][24][25][26]. This angiographic sequence may be explained by structural choroidal changes. The early phase (hypofluorescence) results from disorganization, thinning, and loss of normal choroidal architecture while the late phase (hyperfluorescence) without dye pooling is related to the scleral impregnation by the dye, visible through the disorganized choroid [26].
4. Indocyanine Green Angiography
The area of PICC shows hypofluorescence throughout the indocyanine green angiography sequence [2][3][17][24]. This indicates slow or absent choroidal flow.
5. OCT-Angiography
Analysis of highly myopic eyes showed that the vessel density of the radial peripapillary capillary network [27][28] (Figure 2B) and that of the ON head layers [27] were significantly reduced in eyes with (Figure 2A) than in those without PICC. Highly myopic and low myopic eyes also showed a reduced vessel density compared to non-myopic eyes [27].
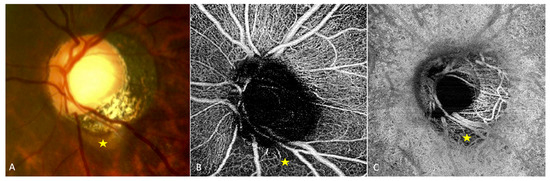
Figure 2. OCT-angiography of a peripapillary intrachoroidal cavitation (PICC). (A–C) Yellow star = PICC. (A) Fundus image with a PICC. (B) En-face OCT-A at the level of the superficial radial peripapillary capillary. Reduced vascular density at the area of PICC is observed. (C) En-face OCT-A at the level of choroid. Reduced vascular density is also seen at the area of PICC. The device used is the PLEX Elite® 9000 SS OCTA (Carl Zeiss Meditec AG, Jena, Germany). A 6 × 6 mm field of view centered on the papilla. The fundus picture was taken using VISUCAM® non-mydriatic camera (PRO NM Carl Zeiss Meditec, Jena, Germany). The device used for OCT-A is the PLEX Elite® 9000 SS OCTA (Carl Zeiss Meditec AG, Jena, Germany). A 6 × 6 mm field of view centered on the papilla.
In a study of 47 glaucomatous eyes with PICC, Kim et al., using En-face OCT-A images at the choroidal layer, described two main vascular features. First, they noted a well demarcated homogeneous area of vessel density reduction matching the location of PICC [22] (Figure 2C). Second, they observed in 89.4% of cases, a choroidal microvasculature dropout (i.e., a focal sector with no visible choroidal and choriocapillary network). These similar patterns were previously reported in two case-reports of non-glaucomatous PICCs [29][30]. Using B scans of the OCT-A, Kim et al. showed that the PICC maintains the choriocapillary signal against the posterior surface of the Bruch’s membrane whereas it shows no signal in the intrachoroidal cavity [22] (Figure 3).
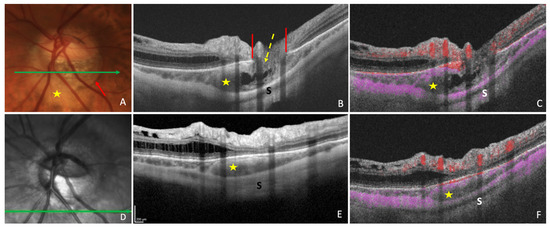
Figure 3. Optical coherence tomography (OCT) and OCT-angiography (OCT-A) in a case of peripapillary intrachoroidal cavitation (PICC). (A) Fundus image with a PICC at the outer border of the conus (red arrow). (B,C) Sections along the green arrow in (A). (B) OCT B-scan disclosing the PICC. Gamma peripapillary atrophy is between the two ends of the Bruch’s membrane (red lines). The retinal layers (dashed yellow arrow) herniate through them. (C) B-scan OCT-A showing the absence of signal inside the choroid. There is a signal (in pink) against Bruch’s membrane, corresponding to the choriocapillaris. Behind the intrachoroidal hyporeflective space, another signal corresponding to the sclera is perceived. The retinal vascular signal is red. (D) Infrared image with the green arrow indicating the location of sections. (E,F) The section is below the optic disc. (F) No signal is seen in the hyporeflective choroidal space between the sclera and the choriocapillaris. A signal is present at the level of choriocapillaris. PICC = yellow star. S = sclera. The fundus picture was taken using VISUCAM® non-mydriatic camera (PRO NM Carl Zeiss Meditec, Jena, Germany). The device used for OCT and infrared image is the Spectral Domain OCT Spectralis® HRA-OCT, model S3300 (Heidelberg Engineering GmbH, Heidelberg Germany). The device used for OCT-angiography is the Swept-source OCT Triton DRI Topcon corporation.
6. Other Modalities
Multimodal imaging [26][31][32] and three-dimensional reconstruction imaging [33] have been used in some cases of PICC, confirming the multiple facets of PICC. Azar et al. used fluorescein angiography and En-face OCT [26]; Chen et al. combined multicolor imaging, ocular B-scan ultrasonography, En-face OCT and enhanced depth imaging OCT [31]; Shen et al. used ocular ultrasonography A and B, fluorescein angiography and OCT [32].
By comparing conventional color fundus photography to multicolor imaging in a case of PICC, Venkatesh et al. found that the multicolor imaging would be less effective than conventional color fundus photography in diagnosing PICC in myopic eyes, which requires confirmation [34].
More recently, Fujimoto et al. developed a method of deep learning-based noise reduction and three-dimensional (3D) rendering of volumetric swept-source OCT images. Using this technology, they calculated the 3D volume parameter of PICC and assessed its value in detecting and understanding this condition. They found that the volume of PICC, as a new 3D parameter, reflects its influence on visual function [35].
References
- Freund, K.B.; Ciardella, A.P.; Yannuzzi, L.A.; Pece, A.; Goldbaum, M.; Kokame, G.T.; Orlock, D. Peripapillary detachment in pathologic myopia. Arch. Ophthalmol. 2003, 121, 197–204. [CrossRef]
- Toranzo, J.; Cohen, S.Y.; Erginay, A.; Gaudric, A. Peripapillary intrachoroidal cavitation in myopia. Am. J. Ophthalmol. 2005, 140, 731–732. [CrossRef] [PubMed]
- Spaide, R.F.; Akiba, M.; Ohno-Matsui, K. Evaluation of peripapillary intrachoroidal cavitation with swept source and enhanced depth imaging optical coherence tomography. Retina 2012, 32, 1037–1044. [CrossRef]
- You, Q.S.; Peng, X.Y.; Chen, C.X.; Xu, L.; Jonas, J.B. Peripapillary intrachoroidal cavitations. The Beijing eye study. PLoS ONE 2013, 8, e78743. [CrossRef] [PubMed]
- Okuma, S.; Mizoue, S.; Ohashi, Y. Visual field defects and changes in macular retinal ganglion cell complex thickness in eyes with intrachoroidal cavitation are similar to those in early glaucoma. Clin. Ophthalmol. 2016, 10, 1217–1222. [CrossRef] [PubMed]
- Shimada, N.; Ohno-Matsui, K.; Iwanaga, Y.; Tokoro, T.; Mochizuki, M. Macular retinal detachment associated with peripapillary detachment in pathologic myopia. Int. Ophthalmol. 2009, 29, 99–102. [CrossRef]
- Rajagopal, J.; Chandra Kumar, H.V.; Ganesh, S. Macular detachment associated with peripapillary detachment in pathologic myopia. Retin. Cases Brief Rep. 2014, 8, 103–106. [CrossRef]
- Yoshizawa, C.; Saito, W.; Noda, K.; Ishida, S. Pars plana vitrectomy for macular schisis associated with peripapillary intrachoroidal cavitation. Ophthalmic Surg. Lasers Imaging Retin. 2014, 45, 350–353. [CrossRef]
- Chen, T.C.; Yang, C.H.; Sun, J.P.; Chen, M.S.; Yang, C.M. Macular retinal detachment associated with intrachoroidal cavitation in myopic patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1437–1446. [CrossRef]
- Ando, Y.; Inoue, M.; Ohno-Matsui, K.; Kusumi, Y.; Iida, T.; Hirakata, A. Macular detachment associated with Intrachoroidal Cavitation in nonpathological myopic eyes. Retina 2015, 35, 1943–1950. [CrossRef]
- Aoki, S.; Imaizumi, H. Vitrectomy for macular retinoschisis associated with peripapillary intrachoroidal cavitations in a moderately myopic eye. Int. J. Retin. Vitr. 2022, 8, 62. [CrossRef]
- Liu, R.; Li, Z.; Xiao, O.; Zhang, J.; Guo, X.; Loong Lee, J.T.; Wang, D.; Lee, P.; Jong, M.; Sankaridurg, P.; et al. Characteristics of peripapillary intrachoroidal cavitation in highly myopic eyes: The Zhongshan Ophthalmic Center-Brien Holden Vision Institute High Myopia Cohort Study. Retina 2021, 41, 1057–1062. [CrossRef]
- Wang, X.; Rumpel, H.; Lim, W.E.; Baskaran, M.; Perera, S.A.; Nongpiur, M.E.; Aung, T.; Milea, D.; Girard, M.J. Finite Element Analysis Predicts Large Optic Nerve Head Strains During Horizontal Eye Movements. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2452–2462. [CrossRef] [PubMed]
- Demer, J.L. Optic Nerve Sheath as a Novel Mechanical Load on the Globe in Ocular Duction. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1826–1838. [CrossRef] [PubMed]
- Chang, M.Y.; Shin, A.; Park, J.; Nagiel, A.; Lalane, R.A.; Schwartz, S.D.; Demer, J.L. Deformation of Optic Nerve Head and Peripapillary Tissues by Horizontal Duction. Am. J. Ophthalmol. 2017, 174, 85–94. [CrossRef]
- Ehongo, A.; Bacq, N.; Kisma, N.; Dugauquier, A.; Alaoui Mhammedi, Y.; Coppens, K.; Bremer, F.; Leroy, K. Analysis of Peripapillary Intrachoroidal Cavitation and Myopic Peripapillary Distortions in Polar Regions by Optical Coherence Tomography. Clin. Ophthalmol. 2022, 16, 2617–2629. [CrossRef]
- Shimada, N.; Ohno-Matsui, K.; Yoshida, T.; Yasuzumi, K.; Kojima, A.; Kobayashi, K.; Futagami, S.; Tokoro, T.; Mochizuki, M. Characteristics of peripapillary detachment in pathologic myopia. Arch. Ophthalmol. 2006, 124, 46–52. [CrossRef]
- Yeh, S.I.; Chang, W.C.; Wu, C.H.; Lan, Y.W.; Hsieh, J.W.; Tsai, S.; Chen, L.J. Characteristics of peripapillary choroidal cavitation detected by optical coherence tomography. Ophthalmology 2013, 120, 544552.[CrossRef] [PubMed]
- Dai, Y.; Jonas, J.B.; Ling, Z.; Wang, X.; Sun, X. Unilateral peripapillary intrachoroidal cavitation and optic disk rotation. Retina 2015, 35, 655–659. [CrossRef] [PubMed]
- Shimada, N.; Ohno-Matsui, K.; Nishimuta, A.; Tokoro, T.; Mochizuki, M. Peripapillary changes detected by optical coherence tomography in eyes with high myopia. Ophthalmology 2007, 114, 2070–2076. [CrossRef][PubMed]
- Wei, Y.H.; Yang, C.M.; Chen, M.S.; Shih, Y.F.; Ho, T.C. Peripapillary intrachoroidal cavitation in high myopia: Reappraisal. Eye 2009, 23, 141–144. [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Lee, E.J.; Kim, T.W. Parapapillary Intrachoroidal Cavitation in Glaucoma: Association withChoroidal Microvas- culature Dropout. Korean J. Ophthalmol. 2021, 35, 44–50. [CrossRef]
- Freund, K.B.; Mukkamala, S.K.; Cooney, M.J. Peripapillary choroidal thickening and cavitation. Arch. Ophthalmol. 2011, 129, 1096–1097. [CrossRef]
- Forte, R.; Pascotto, F.; Cennamo, G.; de Crecchio, G. Evaluation of peripapillary detachment in pathologicmyopia with en face optical coherence tomography. Eye 2008, 22, 158–161. [CrossRef] [PubMed]
- Marticorena-Álvarez, P.; Clement-Fernández, F.; Iglesias-Ussel, L. Peripapillary intrachoroidal cavitation in pathological myopia. Arch. Soc. Esp. Oftalmol. 2014, 89, 316–319. (In English, In Spanish) [CrossRef]
- Azar, G.; Leze, R.; Affortit-Demoge, A.; Faure, C. Peripapillary Intrachoroidal Cavitation in Myopia Evaluated with Multimodal Imaging Comprising “En-Face” Technique. Case Rep. Ophthalmol. Med. 2015, 2015, 890876. [CrossRef]
- Chen, Q.; He, J.; Hua, Y.; Fan, Y. Exploration of peripapillary vessel density in highly myopic eyes with peripapillary intrachoroidal cavitation and its relationship with ocular parameters using optical coherence tomography angiography. Clin. Exp. Ophthalmol. 2017, 45, 884–893. [CrossRef]
- Comune, C.; Montorio, D.; Cennamo, G. Optical coherence tomography angiography in myopic peripapillary intrachoroidal cavitation complicated by choroidal neovascularization. Eur. J. Ophthalmol. 2021, 31, 19201924. [CrossRef] [PubMed]
- Parlak, M.; Ipek, S.C.; Saatci, A.O. Peripapilläre Aufhellung bei hoher Myopie: Nur ein Staphyloma posticum? [Peripapillary whitening in high myopia: Only a staphyloma posticum?]. Ophthalmologe 2020, 117, 379–383. (In German) [CrossRef] [PubMed]
- Mazzaferro, A.; Carnevali, A.; Zucchiatti, I.; Querques, L.; Bandello, F.; Querques, G. Optical coherence tomography angiography features of intrachoroidal peripapillary cavitation. Eur. J. Ophthalmol. 2017, 27,e32–e34. [CrossRef]
- Chen, Y.; Ma, X.; Hua, R. Multi-modality imaging findings of huge intrachoroidal cavitation and myopic peripapillary sinkhole. BMC Ophthalmol. 2018, 18, 24. [CrossRef] [PubMed]
- Shen, K.L.; Foroozan, R.; Weng, C.Y. Peripapillary intrachoroidal cavitation. Clin. Exp. Ophthalmol. 2019, 47, 1200–1202. [CrossRef]
- Chawla, R.; Kumar, A.; Mandal, S. Three-Dimensional Reconstruction Imaging of Peripapillary Intrachoroidal Cavitation in a Myopic Patient. Ophthalmol. Retin. 2019, 3, 928. [CrossRef]
- Venkatesh, R.; Pereira, A.; Gupta, A. Conventional colour fundus photography over multicolour imaging in identifying peripapillary intrachoroidal cavitation in myopic eyes. BMJ Case Rep. 2021, 14, e246837. [CrossRef]
- Fujimoto, S.; Miki, A.; Maruyama, K.; Mei, S.; Mao, Z.; Wang, Z.; Chan, K.; Nishida, K. Three-Dimensional Volume Calculation of Intrachoroidal Cavitation Using Deep-Learning-Based Noise Reduction of Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2022, 11, 1. [CrossRef]
More
Information
Subjects:
Ophthalmology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
792
Revisions:
3 times
(View History)
Update Date:
21 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




