
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | mumei wang | -- | 2760 | 2023-07-13 15:06:10 | | | |
| 2 | Rita Xu | Meta information modification | 2760 | 2023-07-14 03:25:36 | | |
Video Upload Options
The principal goal of rice (Oryza sativa L.) breeding is to increase the yield. In the past, hybrid rice was mainly indica intra-subspecies hybrids, but its yield has been difficult to improve. The hybridization between the indica and japonica subspecies has stronger heterosis; the utilization of inter-subspecies heterosis is important for long-term improvement of rice yields. However, the different diurnal flower-opening times (DFOTs) between the indica and japonica subspecies seriously reduce the efficiency of cross-pollination and yield and increase the cost of indica–japonica hybrid rice seeds, which has become one of the main constraints for the development of indica–japonica hybrid rice breeding. The DFOT of plants is adapted to their growing environment and is also closely related to species stability and evolution.
1. Introduction
2. Advances in Research on Rice DFOT
2.1. The Structural and the Physiological Basis of Rice Flower Opening
2.1.1. The Structural Basis of Rice Flower Opening
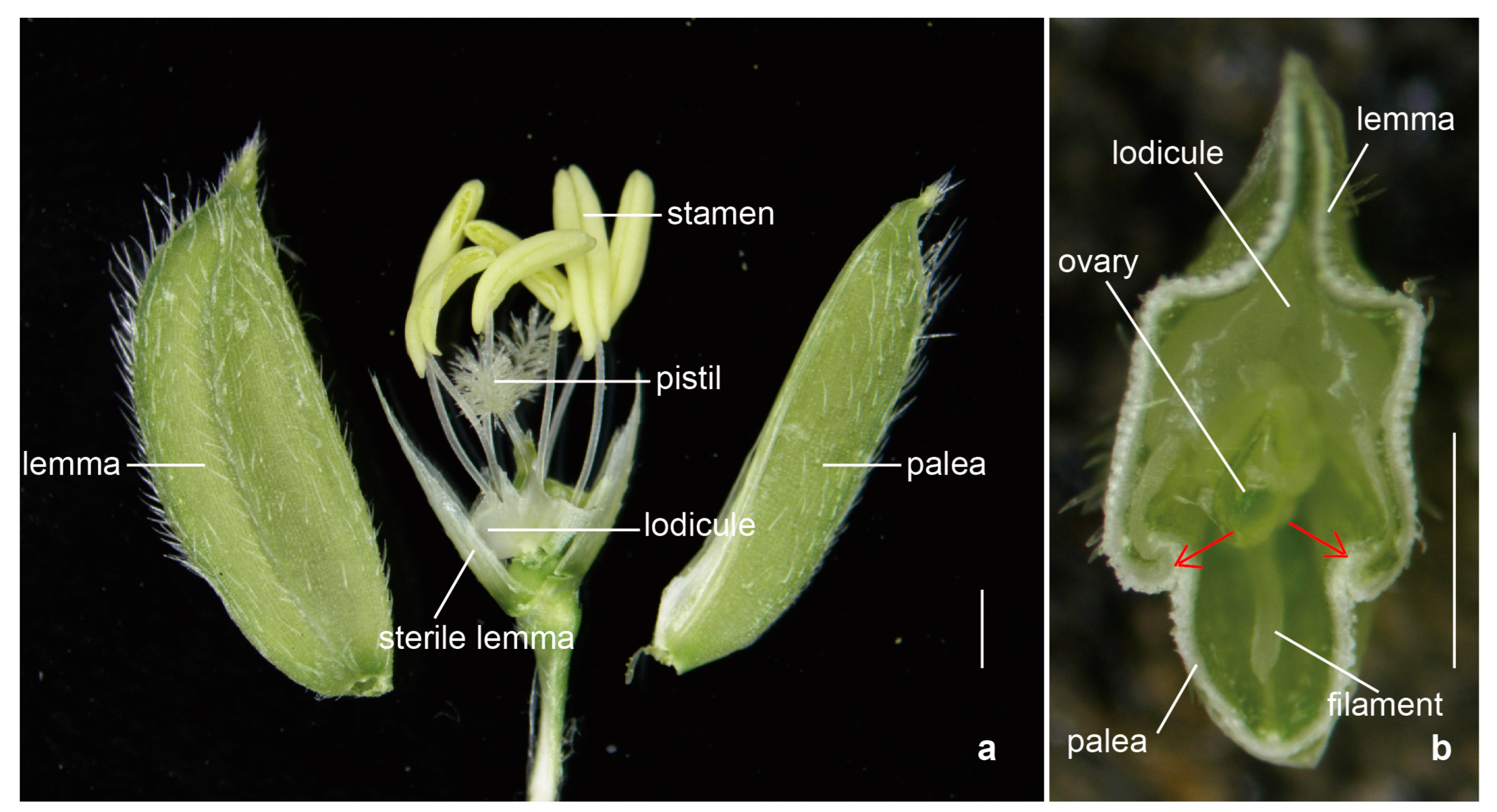
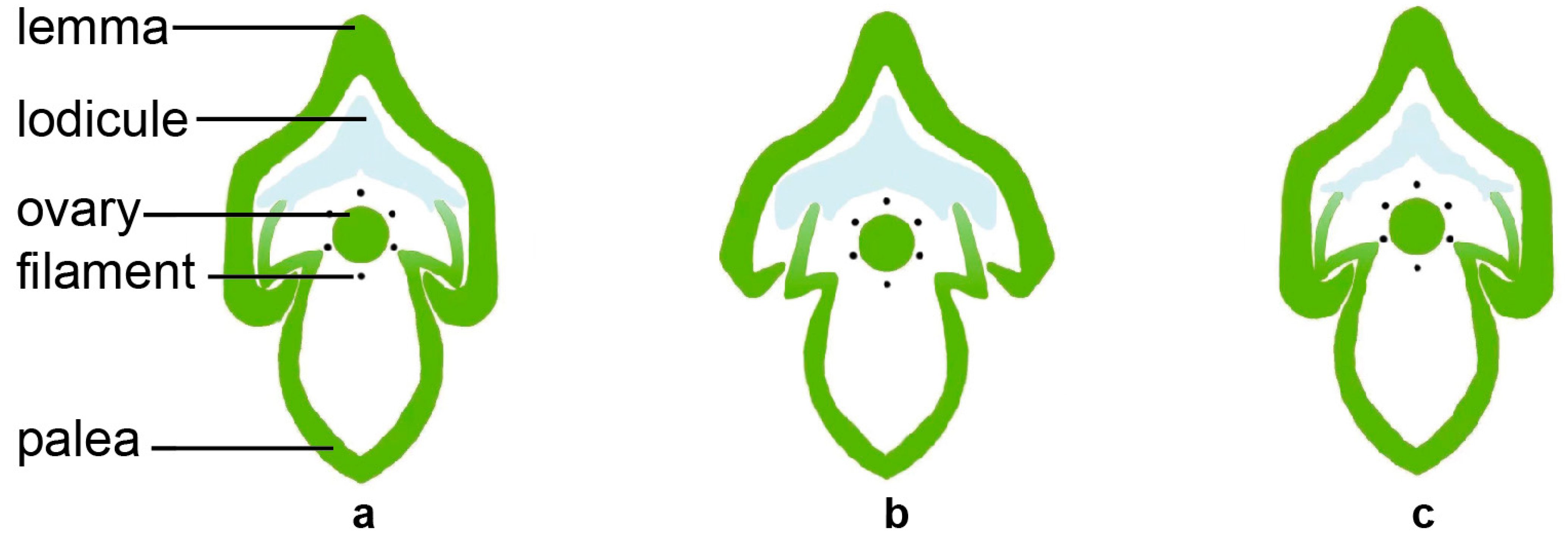
2.1.2. The Physiological Basis of Flower Opening in Rice
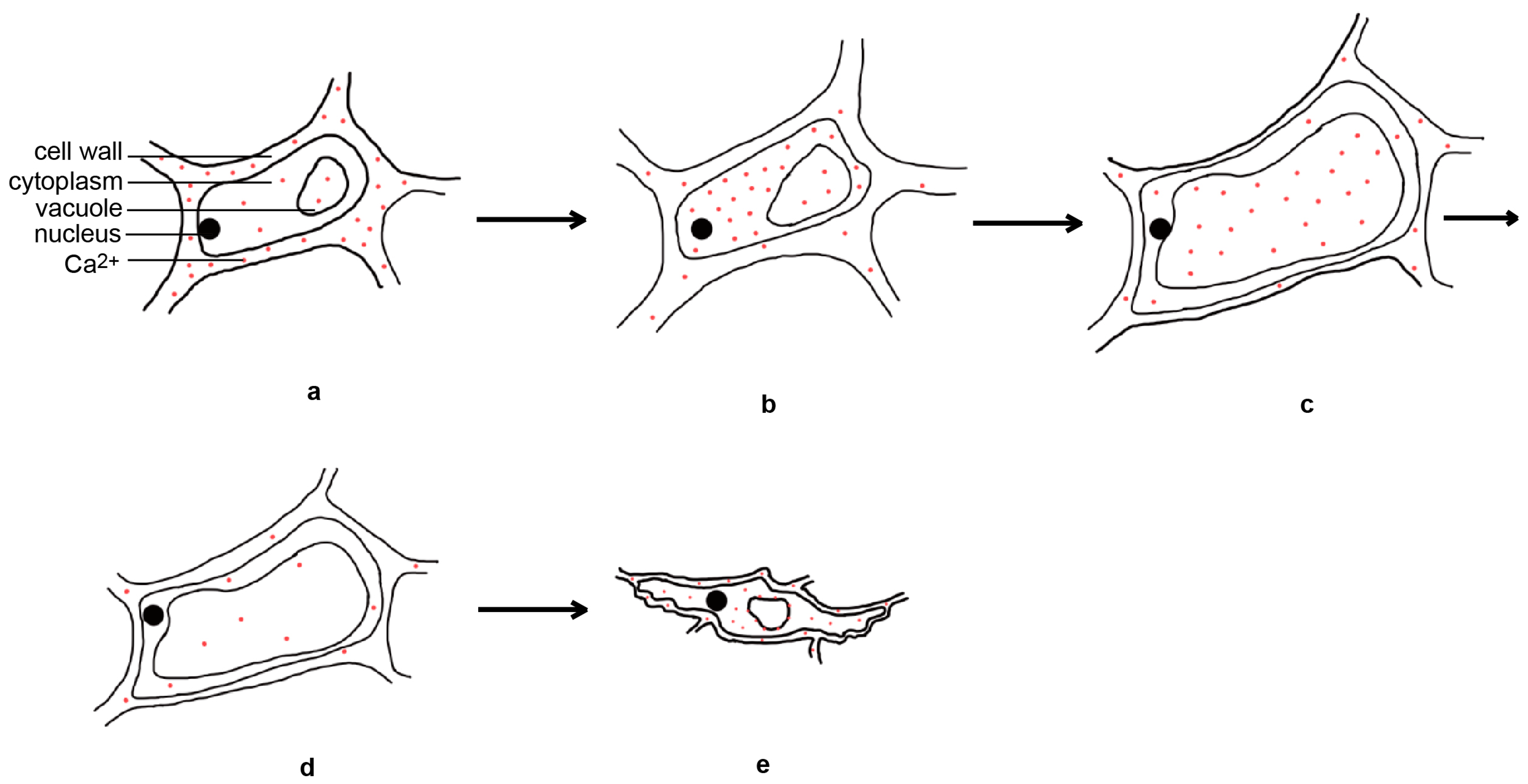
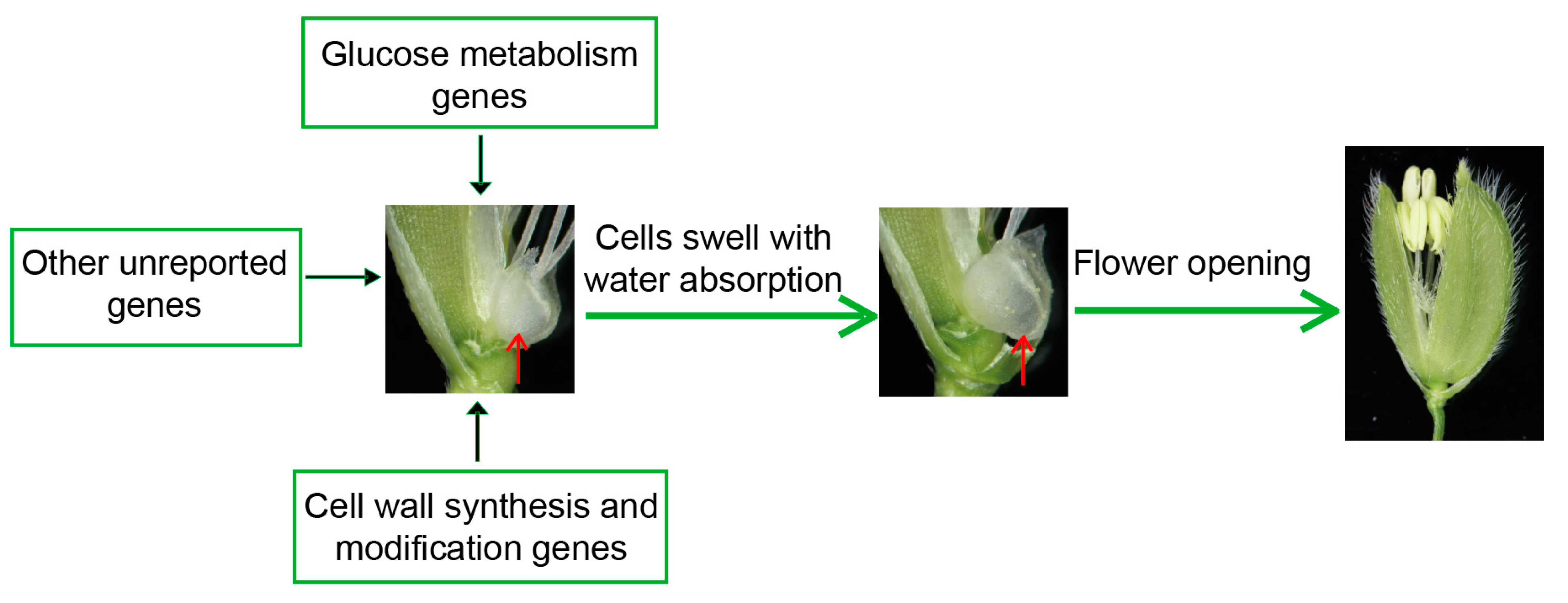
2.2. The Factors Affecting the Regulation of DFOT
2.2.1. The Influence of Genetic Factors on DFOT
2.2.2. The Influence of Plant Hormones and Growth Regulators on DFOT
References
- Qian, Q.; Guo, L.; Smith, S.M.; Li, J. Breeding high-yield superior quality hybrid super rice by rational design. Natl. Sci. Rev. 2016, 3, 283–294.
- Chen, H.; Zhang, Z.; Ni, E.; Lin, J.; Peng, G.; Huang, J.; Zhu, L.; Deng, L.; Yang, F.; Luo, Q.; et al. HMS1 interacts with HMS1I to regulate very-long-chain fatty acid biosynthesis and the humidity-sensitive genic male sterility in rice (Oryza sativa). New Phytol. 2020, 225, 2077–2093.
- Shen, R.; Lan, W.; Liu, X.; Jiang, W.; Jin, W.; Zhao, X.; Xie, X.; Zhu, Q.; Tang, H.; Li, Q.; et al. Genomic structural variation-mediated allelic suppression causes hybrid male sterility in rice. Nat. Commun. 2017, 8, 1310.
- Gou, Y.; Zhu, X.; Wang, H.; Shen, R. Regulation mechanism and breeding application of rice floret-opening-time. J. South China Agric. Univ. 2022, 43, 12.
- Kallugudi, J.; Singh, V.J.; Vinod, K.K.; Krishnan, S.G.; Nandakumar, S.; Dixit, B.K.; Ellur, R.K.; Bollinedi, H.; Nagarajan, M.; Kumar, A.; et al. Population dynamics of wide compatibility system and evaluation of intersubspecific hybrids by indica-japonica hybridization in rice. Plants 2022, 11, 1930.
- Yang, J.; Zhao, X.; Cheng, K.; Du, H.; Ouyang, Y.; Chen, J.; Qiu, S.; Huang, J.; Jiang, Y.; Jiang, L.; et al. A killer-protector system regulates both hybrid sterility and segregation distortion in rice. Science 2012, 337, 1336–1340.
- Zhang, M.; Dai, D.; Li, X.; Zhang, H.; Ma, L. Advances on the study of flowering time trait in hybrid rice. Acta Agric. Nucl. Sin. 2016, 30, 267–274.
- Liu, L.; Zou, Z.; Qian, K.; Xia, C.; He, Y.; Zeng, H.; Zhou, X.; Riemann, M.; Yin, C. Jasmonic acid deficiency leads to scattered floret opening time in cytoplasmic male sterile rice Zhenshan 97A. J. Exp. Bot. 2017, 68, 4613–4625.
- Yoshida, H.; Nagato, Y. Flower development in rice. J. Exp. Bot. 2011, 62, 4719–4730.
- Zhang, L.; Wang, J.; Wang, L.; Fan, H.; Qi, Y.; Song, J. Breeding and application of late japonica CMS line Zhe 08A. J. Zhejiang Agric. Sci. 2017, 58, 1120–1122.
- Cosgrove, D.J. Loosening of plant cell walls by expansins. Nature 2000, 407, 321–326.
- Van Doorn, W.G. flower opening and closure: A review. J. Exp. Bot. 2003, 54, 1801–1812.
- Palin, R.; Geitmann, A. The role of pectin in plant morphogenesis. Biosystems 2012, 109, 397–402.
- Velasquez, S.M.; Ricardi, M.M.; Dorosz, J.G.; Fernandez, P.V.; Nadra, A.D.; Pol-Fachin, L.; Egelund, J.; Gille, S.; Harholt, J.; Ciancia, M.; et al. O-glycosylated cell wall proteins are essential in root hair growth. Science 2011, 322, 1401–1403.
- Hong, L.; Dumond, M.; Zhu, M.; Tsugawa, S.; Li, C.; Boudaoud, A.; Hamant, O.; Roeder, A.H.K. Heterogeneity and robustness in plant morphogenesis: From cells to organs. Annu. Rev. Plant Biol. 2018, 69, 469–495.
- Rui, Y.; Dinneny, J.R. A wall with integrity: Surveillance and maintenance of the plant cell wall under stress. New Phytol. 2020, 25, 1428–1439.
- Zhang, H.; Guo, Z.; Zhuang, Y.; Suo, Y.; Du, J.; Gao, Z.; Pan, J.; Li, L.; Wang, T.; Xiao, L.; et al. MicroRNA775 regulates intrinsic leaf size and reduces cell wall pectin levels by targeting a galactosyltransferase gene in Arabidopsis. Plant Cell 2021, 33, 581–602.
- Wang, M.; Zhu, X.; Peng, G.; Liu, M.; Zhang, S.; Chen, M.; Liao, S.; Wei, X.; Xu, P.; Tan, X.; et al. Methylesterification of cell-wall pectin controls the diurnal flower-opening times in rice. Mol. Plant 2022, 15, 956–972.
- Wang, T.; Park, Y.B.; Cosgrove, D.J.; Hong, M. Cellulose-pectin spatial contacts are inherent to never-dried Arabidopsis primary cell walls: Evidence from solid-state nuclear magnetic resonance. Plant Physiol. 2015, 168, 871–884.
- Atmodjo, M.A.; Sakuragi, Y.; Zhu, X.; Burrell, A.J.; Mohanty, S.S.; Atwood, J.A., 3rd; Orlando, R.; Scheller, H.V.; Mohnen, D. Galacturonosyltransferase (GAUT)1 and GAUT7 are the core of a plant cell wall pectin biosynthetic homogalacturonan: Galacturonosyltransferase complex. Proc. Natl. Acad. Sci. USA 2011, 108, 20225–20230.
- Yan, Z.; Deng, R.; Zhang, H.; Li, J.; Zhu, S. Transcriptome analysis of floret opening and closure both indica and japonica rice. 3 Biotech. 2022, 12, 188.
- Zhang, J.; Zhou, S.; He, F.; Liu, L.; Zhang, Y.; He, J.; Du, X. Expression pattern of the rice α-amylase genes related with the process of floret opening. Sci. Agric. Sin. 2023, 56, 1275–1282.
- Qin, Y.; Yang, J.; Zhao, J. Calcium changes and the response to methyl jasmonate in rice lodicules during anthesis. Protoplasma 2005, 225, 103–112.
- Wang, Z.; Gu, Y.; Gao, Y. Studies on the mechanism of the anthesis of rice V. comparison of lodicule and filament structure between sterile line and fertile line. Acta Agron. Sin. 1994, 20, 5.
- Xiao, Y.; Chen, Y.; Charnikhova, T.; Mulder, P.P.; Heijmans, J.; Hoogenboom, A.; Agalou, A.; Michel, C.; Morel, J.B.; Dreni, L.; et al. OsJAR1 is required for JA-regulated floret opening and anther dehiscence in rice. Plant Mol. Biol. 2014, 86, 19–33.
- Xu, W.; Cai, J.; Yang, Y. Research progress and prospect on utilization of heterosis between indica-japonica rice subspecies. China Rice 2016, 22, 1–7.
- Ma, Z.; Zhan, Z.; Cheng, X.; Gao, J.; He, G.; Liu, D.; Xu, H.; Xu, Z. Flowering time in filial generations of cross between indica and japonica rice and its response to external environment. Hybrid Rice 2011, 26, 70–76.
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697.
- Lyons, R.; Manners, J.M.; Kazan, K. Jasmonate biosynthesis and signaling in monocots: A comparative overview. Plant Cell Rep. 2013, 32, 815–827.
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058.
- He, Y.; Lin, Y.; Zeng, X. Dynamic changes of jasmonic acid biosynthesis in rice florets during natural anthesis. Acta Agron. Sin. 2012, 38, 1891–1899.
- Huang, J.; He, Y.; Zeng, X.; Xiang, M.; Fu, Y. Changes of JA levels in floral organs and expression analysis of JA signaling genes in lodicules before floret opening in rice. Sci. Agric. Sin. 2015, 48, 1219–1227.
- Li, X.; Wang, Y.; Duan, E.; Qi, Q.; Zhou, K.; Lin, Q.; Wang, D.; Wang, Y.; Long, W.; Zhao, Z.; et al. OPEN GLUME1: A key enzyme reducing the precursor of JA, participates in carbohydrate transport of lodicules during anthesis in rice. Plant Cell Rep. 2018, 37, 329–346.
- Kobayasi, K.; Atsuta, Y. Sterility and poor pollination due to early flower opening induced by methyl jasmonate. Plant Prod. Sci. 2009, 13, 29–36.
- Song, P.; Xia, K.; Wu, C.; Bao, D.; Chen, L.; Zhou, X.; Cao, X. Differential response of floret opening in male-sterile and male-fertile rices to methyl jasmonate. Acta Bot. Sin. 2001, 43, 480–485.
- Lin, J.; Tian, X.; Yin, G.; Tang, J.; Yang, Z. Artificial regulation of the flowering time of CMS lines in indica hybrid rice seed production. Sci. Agric. Sin. 2008, 41, 2474–2479.
- Liu, S.; Fu, Y.; He, Y.; Zeng, X. Transcriptome analysis of the impact of exogenous methyl jasmonate on the opening of sorghum florets. PLoS ONE 2021, 16, e0248962.
- Van Doorn, W.G.; Kamdee, C. flower opening and closure: An update. J. Exp. Bot. 2014, 65, 5749–5757.
- Ke, M.; Gao, Z.; Chen, J.; Qiu, Y.; Zhang, L.; Chen, X. Auxin controls circadian flower opening and closure in the waterlily. BMC Plant Biol. 2018, 18, 143.
- Huang, Y.; Zeng, X.; Cao, H. Hormonal regulation of floret closure of rice (Oryza sativa). PLoS ONE 2018, 13, e0198828.
- He, Y.; Zhang, F. Study of regulating effect of auxin on floret opening in rice. Acta Agric. Sin. 2023, 49, 9.
- Yang, T. Ways to improve outcrossing seed setting rate in hybrid rice breeding. Seed Sci. Technol. 2007, 3, 51–53.
- Li, S.; Lv, Y.; Xiao, C. Advances in chemical regulation techniques for seed production and production of hybrid rice. Crop Res. 2011, 25, 400–404.
- Tang, H.; Xiong, Y. Study on the regulation of triacontanol on flowering time of rice sterile lines and maintainers (brief introduction). Guizhou Agric. Sci. 1984, 13, 10–14.
- Liu, D.; Lu, X.; He, M.; Xiao, H.; Fan, X. Effect of plant growth regulator triacontanol (TA) on the yield of rice. Strateg. Study CAE 2002, 11, 82–88.
- Su, Z. Effect of Huaxinling on seed production of hybrid rice. Hybrid Rice 1999, 14, 27.
- Li, S.; Wei, J. Efficacy of Huaxinling on increasing forenoon blooming percentage of seed parents in hybrid rice seed production. Hybrid Rice 1995, 10, 8–9.




