
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jesús Argente | -- | 2163 | 2023-07-13 13:34:05 | | | |
| 2 | Jessie Wu | Meta information modification | 2163 | 2023-07-14 03:45:26 | | |
Video Upload Options
The growth hormone (GH)/insulin-like growth factor (IGF) axis plays fundamental roles during development, maturation, and aging. Members of this axis, composed of various ligands, receptors, and binding proteins, are regulated in a tissue- and time-specific manner that requires precise control that is not completely understood. Some of the most recent advances in understanding the implications of this axis in human growth are derived from the identifications of new mutations in the gene encoding the pregnancy-associated plasma protein PAPP-A2 protease that liberates IGFs from their carrier proteins in a selective manner to allow binding to the IGF receptor 1. The identification of three nonrelated families with mutations in the PAPP-A2 gene has shed light on how this protease affects human physiology.
1. Pregnancy-Associated Plasma Protein A2 Mouse Models: What Are We Learning?
| Constitutive Pappa2 KO | Constitutive Pappa2 KO | Constitutive Induction of Pappa2 KO | Conditional Pappa2 KO in Osteoblasts | Constitutive Human PAPP-A2 KI | |
|---|---|---|---|---|---|
| Main references | Conover et al. 2011 | Christians et al. 2013, 2015a, 2019; Rogowska et al. 2021; Rubio et al. 2021 | Christians et al. 2015a | Amiri and Christians, 2015 | Fujimoto et al. 2019 |
| Auxological parameters | |||||
| Body weight (BW) | Ns 1 in males Reduction in females |
Reduction | ns | Reduction | Reduction |
| Body length | Ns in males Reduction in females |
Reduction | -- | Reduction in tail | Reduction |
| Organ size | Ns in males Increase in females |
ns | -- | -- | Increase in liver |
| Body composition | -- | -- | -- | -- | Higher fat mass. Lower lean mass |
| Hormonal parameters | |||||
| Free Igf1 | Decrease | Decrease | -- | -- | Decrease |
| Total Igf1 | Increase | Increase | -- | -- | Increase |
| Igfbp5 | -- | Increase in plasma Ns in liver and kidney Increase in ovaries Ns in tibia |
ns | ns | -- |
| Igfbp3 | -- | Decrease in serum Ns in liver and kidney Increase in tibia |
Decrease | -- | Increase |
| Igfals | -- | Ns in tibia | -- | -- | Increase |
| Energy metabolism | |||||
| Glucose tolerance | -- | ns | -- | -- | Intolerant |
| Insulin sensitivity | -- | ns | -- | -- | Resistant |
| Adiposity | ns | -- | -- | -- | |
| BW loss | -- | Increase in fasting | -- | -- | -- |
| Caloric intake | -- | Increase in HCHD 2 | -- | -- | -- |
| Allometric parameters | |||||
| Femur length | ns | Reduction | -- | Reduction | Reduction |
| Femur weight | -- | Reduction | -- | -- | -- |
| Other bone dimensions | -- | Defects | -- | Defects | -- |
| Bone shape | -- | Defects (pelvic girdle and mandible) | -- | Defects (pelvic girdle and mandible) | -- |
| Bone mineral properties | |||||
| Bone mineral content | Decreases in trabecular and cortical femur | Decrease in trabecular femur Increase in cortical femur |
-- -- |
-- -- |
Decrease in trabecular femur Ns in cortical femur |
| Bone mineral composition | -- | Alterations in male femur | -- | -- | -- |
| Collagen maturity | Decrease in female femur | -- | -- | -- | |
| Bone remodeling | |||||
| Bone formation and resorption | -- | Decreases in female serum Increases in female tibia |
-- | -- | -- |
2. Expression Pattern of Pappa2 in Mice
3. Available Models and Their Differences in Growth and Body Weight
4. Differences in Hormonal Alterations in Available Models of Pappa2 Deficiency
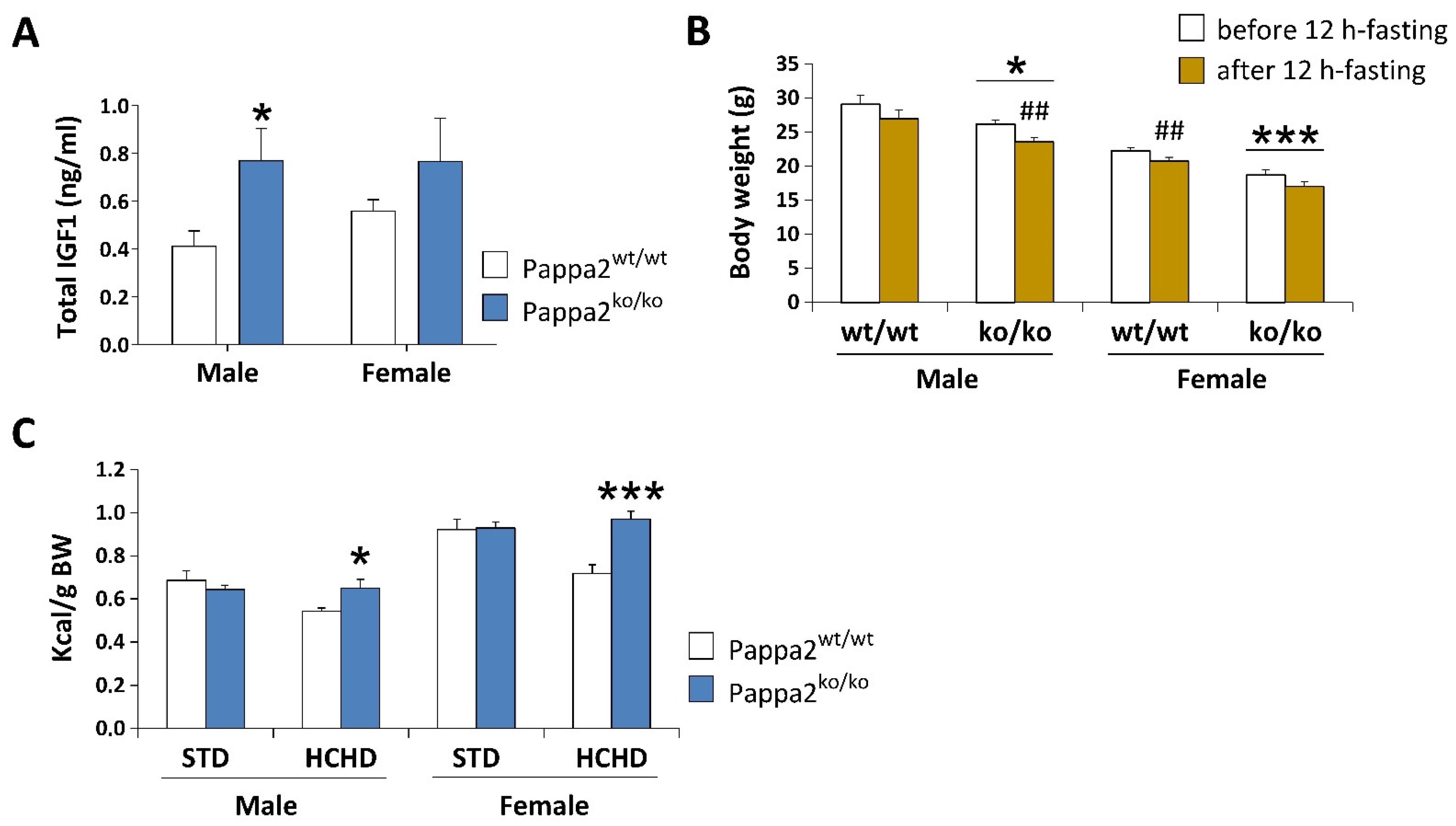
5. Metabolic Disturbance in Available Models of Pappa2 Deficiency
References
- Fujimoto, M.; Andrew, M.; Dauber, A. Disorders caused by genetic defects associated with GH-dependent genes: PAPPA2 defects. Mol. Cell. Endocrinol. 2020, 518, 110967.
- Farr, M.; Strübe, J.; Geppert, H.-G.; Kocourek, A.; Mahne, M.; Tschesche, H. Pregnancy-associated plasma protein-E (PAPP-E). Biochim. Biophys. Acta BBA-Gene Struct. Expr. 2000, 1493, 356–362.
- Page, N.; Butlin, D.; Lomthaisong, K.; Lowry, P. The Characterization of Pregnancy Associated Plasma Protein-E and the Identification of an Alternative Splice Variant. Placenta 2001, 22, 681–687.
- Wagner, P.K.; Otomo, A.; Christians, J.K. Regulation of pregnancy-associated plasma protein A2 (PAPPA2) in a human placental trophoblast cell line (BeWo). Reprod. Biol. Endocrinol. 2011, 9, 48.
- Conover, C.A.; Boldt, H.B.; Bale, L.K.; Clifton, K.B.; Grell, J.A.; Mader, J.R.; Mason, E.J.; Powell, D.R. Pregnancy-Associated Plasma Protein-A2 (PAPP-A2): Tissue Expression and Biological Consequences of Gene Knockout in Mice. Endocrinology 2011, 152, 2837–2844.
- Christians, J.K.; Hoeflich, A.; Keightley, P.D. PAPPA2, an Enzyme That Cleaves an Insulin-Like Growth-Factor-Binding Protein, Is a Candidate Gene for a Quantitative Trait Locus Affecting Body Size in Mice. Genetics 2006, 173, 1547–1553.
- Wang, J.; Qiu, Q.; Haider, M.; Bell, M.; Gruslin, A.; Christians, J.K. Expression of pregnancy-associated plasma protein A2 during pregnancy in human and mouse. J. Endocrinol. 2009, 202, 337–345.
- Chen, Y.; Lv, H.; Li, L.; Wang, E.; Zhang, L.; Zhao, Q. Expression of PAPP-A2 and IGF Pathway-Related Proteins in the Hip Joint of Normal Rat and Those with Developmental Dysplasia of the Hip. Int. J. Endocrinol. 2019, 2019, 7691531.
- Amiri, N.; Christians, J.K. PAPP-A2 expression by osteoblasts is required for normal postnatal growth in mice. Growth Horm. IGF Res. 2015, 25, 274–280.
- Kjaer-Sorensen, K.; Engholm, D.H.; Jepsen, M.R.; Morch, M.G.; Weyer, K.; Hefting, L.L.; Skov, L.L.; Laursen, L.S.; Oxvig, C. Papp-a2 modulates development of cranial cartilage and angiogenesis in zebrafish embryos. J. Cell Sci. 2014, 127 Pt 23, 5027–5037.
- Fujimoto, M.; Andrew, M.; Liao, L.; Zhang, D.; Yildirim, G.; Sluss, P.; Kalra, B.; Kumar, A.; Yakar, S.; Hwa, V.; et al. Low IGF-I Bioavailability Impairs Growth and Glucose Metabolism in a Mouse Model of Human PAPPA2 p.Ala1033Val Mutation. Endocrinology 2019, 160, 1363–1376.
- Christians, J.K.; de Zwaan, D.; Fung, S.H.Y. Pregnancy Associated Plasma Protein A2 (PAPP-A2) Affects Bone Size and Shape and Contributes to Natural Variation in Postnatal Growth in Mice. PLoS ONE 2013, 8, e56260.
- Christians, J.K.; Bath, A.K.; Amiri, N. Pappa2 deletion alters IGFBPs but has little effect on glucose disposal or adiposity. Growth Horm. IGF Res. 2015, 25, 232–239.
- Rubio, L.; Vargas, A.; Rivera, P.; López-Gambero, A.; Tovar, R.; Christians, J.; Martín-De-Las-Heras, S.; de Fonseca, F.R.; Chowen, J.; Argente, J.; et al. Recombinant IGF-1 Induces Sex-Specific Changes in Bone Composition and Remodeling in Adult Mice with Pappa2 Deficiency. Int. J. Mol. Sci. 2021, 22, 4048.
- Dauber, A.; Muñoz-Calvo, M.T.; Barrios, V.; Domené, H.M.; Kloverpris, S.; Serra-Juhé, C.; Desikan, V.; Pozo, J.; Muzumdar, R.; Martos-Moreno, G.Á.; et al. Mutations in pregnancy-associated plasma protein A2 cause short stature due to low IGF-I availability. EMBO Mol. Med. 2016, 8, 363–374.
- Rogowska, M.D.; Pena, U.N.V.; Binning, N.; Christians, J.K. Recovery of the maternal skeleton after lactation is impaired by advanced maternal age but not by reduced IGF availability in the mouse. PLoS ONE 2021, 16, e0256906.
- Christians, J.K.; King, A.Y.; Rogowska, M.D.; Hessels, S.M. Pappa2 deletion in mice affects male but not female fertility. Reprod. Biol. Endocrinol. 2015, 13, 109.
- Carvalho, A.L.; DeMambro, V.E.; Guntur, A.R.; Le, P.; Nagano, K.; Baron, R.; de Paula, F.J.A.; Motyl, K.J. High fat diet attenuates hyperglycemia, body composition changes, and bone loss in male streptozotocin-induced type 1 diabetic mice. J. Cell. Physiol. 2018, 233, 1585–1600.
- Yanagihara, G.R.; Shimano, R.C.; Tida, J.A.; Yamanaka, J.S.; Fukada, S.Y.; Issa, J.P.M.; Tavares, J.M.R.S. Influence of High-Fat Diet on Bone Tissue: An Experimental Study in Growing Rats. J. Nutr. Health Aging 2017, 21, 1337–1343.
- Qiao, J.; Wu, Y.-W.; Ren, Y.-Z. The impact of a high fat diet on bones: Potential mechanisms. Food Funct. 2021, 12, 963–975.
- Kawai, M.; Rosen, C.J. The IGF-I regulatory system and its impact on skeletal and energy homeostasis. J. Cell. Biochem. 2010, 111, 14–19.




