
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rosario Giuffrida | -- | 2263 | 2023-07-13 10:17:38 | | | |
| 2 | Dean Liu | -6 word(s) | 2257 | 2023-07-14 02:32:09 | | | | |
| 3 | Dean Liu | + 4 word(s) | 2261 | 2023-07-31 08:26:04 | | |
Video Upload Options
Being of mesodermal origin, ASCs (adipose-derived mesenchymal stromal cells) can be easily induced to differentiate into chondrocyte-like and osteocyte-like elements and used to repair damaged tissues. Moreover, they can be easily harvested and used for autologous implantation. A plethora of ASC-based strategies are being developed worldwide: they include the transplantation of freshly harvested cells, in vitro expanded cells or predifferentiated cells. Moreover, improving their positive effects, ASCs can be implanted in combination with several types of scaffolds that ensure the correct cell positioning; support cell viability, proliferation and migration; and may contribute to their osteogenic or chondrogenic differentiation. Examples of these strategies are described here, showing the enormous therapeutic potential of ASCs in this field. For safety and regulatory issues, most investigations are still at the experimental stage and carried out in vitro and in animal models. Clinical applications have, however, been reported with promising results and no serious adverse effects.
1. Introduction
2. Adipose-Derived Mesenchymal Stromal Cells (ASCs)
2.1. ASC Osteogenic Differentiation
2.2. ASC Chondrogenic Differentiation
3. ASC-Based Repair Strategies
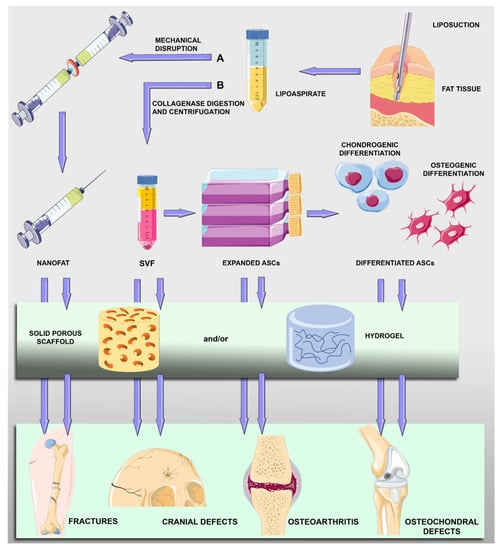
Scaffold-Assisted Strategies
References
- Barba, M.; Cicione, C.; Bernardini, C.; Michetti, F.; Lattanzi, W. Adipose-derived mesenchymal cells for bone regereneration: State of the art. BioMed Res. Int. 2013, 2013, 416391.
- Perdisa, F.; Gostynska, N.; Roffi, A.; Filardo, G.; Marcacci, M.; Kon, E. Adipose-Derived Mesenchymal Stem Cells for the Treatment of Articular Cartilage: A Systematic Review on Preclinical and Clinical Evidence. Stem Cells Int. 2015, 2015, 597652.
- Zakrzewski, W.; Dobrzynski, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68.
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2013, 85, 3–10.
- Mannino, G.; Russo, C.; Maugeri, G.; Musumeci, G.; Vicario, N.; Tibullo, D.; Giuffrida, R.; Parenti, R.; Lo Furno, D. Adult stem cell niches for tissue homeostasis. J. Cell Physiol. 2022, 237, 239–257.
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247.
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403.
- Kumar, R.; Godavarthy, P.S.; Krause, D.S. The bone marrow microenvironment in health and disease at a glance. J. Cell Sci. 2018, 131, jcs201707.
- Zhong, L.; Yao, L.; Seale, P.; Qin, L. Marrow adipogenic lineage precursor: A new cellular component of marrow adipose tissue. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101518.
- Safford, K.M.; Hicok, K.C.; Safford, S.D.; Halvorsen, Y.D.; Wilkison, W.O.; Gimble, J.M.; Rice, H.E. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 2002, 294, 371–379.
- Li, M.; Ikehara, S. Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells Int. 2013, 2013, 132642.
- Arutyunyan, I.; Elchaninov, A.; Makarov, A.; Fatkhudinov, T. Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem Cells Int. 2016, 2016, 6901286.
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. A 2018, 93, 19–31.
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228.
- Dicker, A.; Le Blanc, K.; Astrom, G.; van Harmelen, V.; Gotherstrom, C.; Blomqvist, L.; Arner, P.; Ryden, M. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp. Cell Res. 2005, 308, 283–290.
- Sharma, S.; Muthu, S.; Jeyaraman, M.; Ranjan, R.; Jha, S.K. Translational products of adipose tissue-derived mesenchymal stem cells: Bench to bedside applications. World J. Stem Cells 2021, 13, 1360–1381.
- Heo, J.S.; Choi, Y.; Kim, H.S.; Kim, H.O. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int. J. Mol. Med. 2016, 37, 115–125.
- Calabrese, G.; Giuffrida, R.; Lo Furno, D.; Parrinello, N.L.; Forte, S.; Gulino, R.; Colarossi, C.; Schinocca, L.R.; Giuffrida, R.; Cardile, V.; et al. Potential Effect of CD271 on Human Mesenchymal Stromal Cell Proliferation and Differentiation. Int. J. Mol. Sci. 2015, 16, 15609–15624.
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126.
- Rahman, G.; Frazier, T.P.; Gimble, J.M.; Mohiuddin, O.A. The Emerging Use of ASC/Scaffold Composites for the Regeneration of Osteochondral Defects. Front. Bioeng. Biotechnol. 2022, 10, 893992.
- Mazini, L.; Ezzoubi, M.; Malka, G. Overview of current adipose-derived stem cell (ADSCs) processing involved in therapeutic advancements: Flow chart and regulation updates before and after COVID-19. Stem Cell Res. Ther. 2021, 12, 1.
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295.
- Lo Furno, D.; Graziano, A.C.; Avola, R.; Giuffrida, R.; Perciavalle, V.; Bonina, F.; Mannino, G.; Cardile, V. A Citrus bergamia Extract Decreases Adipogenesis and Increases Lipolysis by Modulating PPAR Levels in Mesenchymal Stem Cells from Human Adipose Tissue. PPAR Res. 2016, 2016, 4563815.
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114, 108765.
- Mannino, G.; Vicario, N.; Parenti, R.; Giuffrida, R.; Lo Furno, D. Connexin expression decreases during adipogenic differentiation of human adipose-derived mesenchymal stem cells. Mol. Biol. Rep. 2020, 47, 9951–9958.
- Mannino, G.; Gennuso, F.; Giurdanella, G.; Conti, F.; Drago, F.; Salomone, S.; Furno, D.L.; Bucolo, C.; Giuffrida, R. Pericyte-like differentiation of human adipose-derived mesenchymal stem cells: An in vitro study. World J. Stem Cells 2020, 12, 1152–1170.
- Mannino, G.; Longo, A.; Gennuso, F.; Anfuso, C.D.; Lupo, G.; Giurdanella, G.; Giuffrida, R.; Lo Furno, D. Effects of High Glucose Concentration on Pericyte-Like Differentiated Human Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 4604.
- Lupo, G.; Agafonova, A.; Cosentino, A.; Giurdanella, G.; Mannino, G.; Lo Furno, D.; Romano, I.R.; Giuffrida, R.; D’Angeli, F.; Anfuso, C.D. Protective Effects of Human Pericyte-like Adipose-Derived Mesenchymal Stem Cells on Human Retinal Endothelial Cells in an In Vitro Model of Diabetic Retinopathy: Evidence for Autologous Cell Therapy. Int. J. Mol. Sci. 2023, 24, 913.
- Guasti, L.; New, S.E.; Hadjidemetriou, I.; Palmiero, M.; Ferretti, P. Plasticity of human adipose-derived stem cells—Relevance to tissue repair. Int. J. Dev. Biol. 2018, 62, 431–439.
- Lo Furno, D.; Pellitteri, R.; Graziano, A.C.; Giuffrida, R.; Vancheri, C.; Gili, E.; Cardile, V. Differentiation of human adipose stem cells into neural phenotype by neuroblastoma- or olfactory ensheathing cells-conditioned medium. J. Cell Physiol. 2013, 228, 2109–2118.
- Lo Furno, D.; Mannino, G.; Giuffrida, R.; Gili, E.; Vancheri, C.; Tarico, M.S.; Perrotta, R.E.; Pellitteri, R. Neural differentiation of human adipose-derived mesenchymal stem cells induced by glial cell conditioned media. J. Cell Physiol. 2018, 233, 7091–7100.
- Russo, C.; Patane, M.; Vicario, N.; Di Bella, V.; Cosentini, I.; Barresi, V.; Gulino, R.; Pellitteri, R.; Russo, A.; Stanzani, S. Olfactory Ensheathing Cells express both Ghrelin and Ghrelin Receptor in vitro: A new hypothesis in favor of a neurotrophic effect. Neuropeptides 2020, 79, 101997.
- Russo, C.; Mannino, G.; Patane, M.; Parrinello, N.L.; Pellitteri, R.; Stanzani, S.; Giuffrida, R.; Lo Furno, D.; Russo, A. Ghrelin peptide improves glial conditioned medium effects on neuronal differentiation of human adipose mesenchymal stem cells. Histochem. Cell Biol. 2021, 156, 35–46.
- Lo Furno, D.; Mannino, G.; Pellitteri, R.; Zappala, A.; Parenti, R.; Gili, E.; Vancheri, C.; Giuffrida, R. Conditioned Media From Glial Cells Promote a Neural-Like Connexin Expression in Human Adipose-Derived Mesenchymal Stem Cells. Front. Physiol. 2018, 9, 1742.
- Mannino, G.; Cristaldi, M.; Giurdanella, G.; Perrotta, R.E.; Lo Furno, D.; Giuffrida, R.; Rusciano, D. ARPE-19 conditioned medium promotes neural differentiation of adipose-derived mesenchymal stem cells. World J. Stem Cells 2021, 13, 1783–1796.
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306.
- Mannino, G.; Russo, C.; Longo, A.; Anfuso, C.D.; Lupo, G.; Lo Furno, D.; Giuffrida, R.; Giurdanella, G. Potential therapeutic applications of mesenchymal stem cells for the treatment of eye diseases. World J. Stem Cells 2021, 13, 632–644.
- Mende, W.; Gotzl, R.; Kubo, Y.; Pufe, T.; Ruhl, T.; Beier, J.P. The Role of Adipose Stem Cells in Bone Regeneration and Bone Tissue Engineering. Cells 2021, 10, 975.
- Marolt Presen, D.; Traweger, A.; Gimona, M.; Redl, H. Mesenchymal Stromal Cell-Based Bone Regeneration Therapies: From Cell Transplantation and Tissue Engineering to Therapeutic Secretomes and Extracellular Vesicles. Front. Bioeng. Biotechnol. 2019, 7, 352.
- Wang, L.; Huang, C.; Li, Q.; Xu, X.; Liu, L.; Huang, K.; Cai, X.; Xiao, J. Osteogenic differentiation potential of adipose-derived stem cells from ovariectomized mice. Cell. Prolif. 2017, 50, e12328.
- Guasti, L.; Prasongchean, W.; Kleftouris, G.; Mukherjee, S.; Thrasher, A.J.; Bulstrode, N.W.; Ferretti, P. High plasticity of pediatric adipose tissue-derived stem cells: Too much for selective skeletogenic differentiation? Stem Cells Transl. Med. 2012, 1, 384–395.
- Calabrese, G.; Giuffrida, R.; Fabbi, C.; Figallo, E.; Lo Furno, D.; Gulino, R.; Colarossi, C.; Fullone, F.; Giuffrida, R.; Parenti, R.; et al. Collagen-Hydroxyapatite Scaffolds Induce Human Adipose Derived Stem Cells Osteogenic Differentiation In Vitro. PLoS ONE 2016, 11, e0151181.
- Lee, S.J.; Kang, S.W.; Do, H.J.; Han, I.; Shin, D.A.; Kim, J.H.; Lee, S.H. Enhancement of bone regeneration by gene delivery of BMP2/Runx2 bicistronic vector into adipose-derived stromal cells. Biomaterials 2010, 31, 5652–5659.
- Brennan, M.A.; Renaud, A.; Guilloton, F.; Mebarki, M.; Trichet, V.; Sensebe, L.; Deschaseaux, F.; Chevallier, N.; Layrolle, P. Inferior In Vivo Osteogenesis and Superior Angiogenesis of Human Adipose-Derived Stem Cells Compared with Bone Marrow-Derived Stem Cells Cultured in Xeno-Free Conditions. Stem Cells Transl. Med. 2017, 6, 2160–2172.
- Stromps, J.P.; Paul, N.E.; Rath, B.; Nourbakhsh, M.; Bernhagen, J.; Pallua, N. Chondrogenic differentiation of human adipose-derived stem cells: A new path in articular cartilage defect management? BioMed Res. Int. 2014, 2014, 740926.
- Wei, Y.; Sun, X.; Wang, W.; Hu, Y. Adipose-derived stem cells and chondrogenesis. Cytotherapy 2007, 9, 712–716.
- Veronesi, F.; Maglio, M.; Tschon, M.; Aldini, N.N.; Fini, M. Adipose-derived mesenchymal stem cells for cartilage tissue engineering: State-of-the-art in in vivo studies. J. Biomed. Mater. Res. A 2014, 102, 2448–2466.
- Gutierrez, R.A.; Fonseca, V.C.; Darling, E.M. Chondrogenesis of Adipose-Derived Stem Cells Using an Arrayed Spheroid Format. Cell Mol. Bioeng. 2022, 15, 587–597.
- Latief, N.; Raza, F.A.; Bhatti, F.U.; Tarar, M.N.; Khan, S.N.; Riazuddin, S. Adipose stem cells differentiated chondrocytes regenerate damaged cartilage in rat model of osteoarthritis. Cell Biol. Int. 2016, 40, 579–588.
- Musumeci, G.; Mobasheri, A.; Trovato, F.M.; Szychlinska, M.A.; Graziano, A.C.; Lo Furno, D.; Avola, R.; Mangano, S.; Giuffrida, R.; Cardile, V. Biosynthesis of collagen I, II, RUNX2 and lubricin at different time points of chondrogenic differentiation in a 3D in vitro model of human mesenchymal stem cells derived from adipose tissue. Acta Histochem. 2014, 116, 1407–1417.
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat grafting: Basic research and clinical applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026.
- Lo Furno, D.; Tamburino, S.; Mannino, G.; Gili, E.; Lombardo, G.; Tarico, M.S.; Vancheri, C.; Giuffrida, R.; Perrotta, R.E. Nanofat 2.0: Experimental evidence for a fat grafting rich in mesenchymal stem cells. Physiol. Res. 2017, 66, 663–671.
- Tamburino, S.; Lombardo, G.A.; Tarico, M.S.; Perrotta, R.E. The Role of Nanofat Grafting in Vulvar Lichen Sclerosus: A Preliminary Report. Arch. Plast. Surg. 2016, 43, 93–95.
- Gorkun, A.A.; Revokatova, D.P.; Zurina, I.M.; Nikishin, D.A.; Bikmulina, P.Y.; Timashev, P.S.; Shpichka, A.I.; Kosheleva, N.V.; Kolokoltsova, T.D.; Saburina, I.N. The Duo of Osteogenic and Angiogenic Differentiation in ADSC-Derived Spheroids. Front. Cell Dev. Biol. 2021, 9, 572727.
- Marolt, D.; Knezevic, M.; Novakovic, G.V. Bone tissue engineering with human stem cells. Stem Cell Res. Ther. 2010, 1, 10.
- Litowczenko, J.; Wozniak-Budych, M.J.; Staszak, K.; Wieszczycka, K.; Jurga, S.; Tylkowski, B. Milestones and current achievements in development of multifunctional bioscaffolds for medical application. Bioact. Mater. 2021, 6, 2412–2438.
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for tissue engineering. Ann. Biomed. Eng. 2014, 42, 323–337.
- Zhang, Z.; Yang, X.; Cao, X.; Qin, A.; Zhao, J. Current applications of adipose-derived mesenchymal stem cells in bone repair and regeneration: A review of cell experiments, animal models, and clinical trials. Front. Bioeng. Biotechnol. 2022, 10, 942128.
- Carvalho, D.N.; Lobo, F.C.M.; Rodrigues, L.C.; Fernandes, E.M.; Williams, D.S.; Mearns-Spragg, A.; Sotelo, C.G.; Perez-Martin, R.I.; Reis, R.L.; Gelinsky, M.; et al. Advanced Polymeric Membranes as Biomaterials Based on Marine Sources Envisaging the Regeneration of Human Tissues. Gels 2023, 9, 247.
- Cao, S.J.; Zhao, Y.; Hu, Y.M.; Zou, L.; Chen, J.D. New perspectives: In-situ tissue engineering for bone repair scaffold. Compos. Part. B Eng. 2020, 202, 108445.
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491.
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludag, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30.
- Huang, Q.; Zou, Y.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.; Dove, A.P.; Du, J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275.
- Haeri, S.M.; Sadeghi, Y.; Salehi, M.; Farahani, R.M.; Mohsen, N. Osteogenic differentiation of human adipose-derived mesenchymal stem cells on gum tragacanth hydrogel. Biologicals 2016, 44, 123–128.
- Ye, J.; Yang, G.; Zhang, J.; Xiao, Z.; He, L.; Zhang, H.; Liu, Q. Preparation and characterization of gelatin-polysaccharide composite hydrogels for tissue engineering. PeerJ 2021, 9, e11022.




