
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Muhammad Syukri Mohamad Misenan | -- | 1455 | 2023-07-10 22:36:18 | | | |
| 2 | Dean Liu | Meta information modification | 1455 | 2023-07-12 02:32:27 | | |
Video Upload Options
Phosphorous is an essential element for the life of organisms, and phosphorus-based compounds have many uses in industry, such as flame retardancy reagents, ingredients in fertilizers, pyrotechnics, etc. Ionic liquids are salts with melting points lower than the boiling point of water. The term “polymerized ionic liquids” (PILs) refers to a class of polyelectrolytes that contain an ionic liquid (IL) species in each monomer repeating unit and are connected by a polymeric backbone to form macromolecular structures. PILs provide a new class of polymeric materials by combining some of the distinctive qualities of ILs in the polymer chain. Ionic liquids have been identified as attractive prospects for a variety of applications due to the high stability (thermal, chemical, and electrochemical) and high mobility of their ions, but their practical applicability is constrained because they lack the benefits of both liquids and solids, suffering from both leakage issues and excessive viscosity. PILs are garnering for developing non-volatile and non-flammable solid electrolytes.
1. Phosphorus
2. Phosphonium Ionic Liquid Polymer Electrolytes in Lithium-Ion Batteries
2.1. Polymer Electrolytes
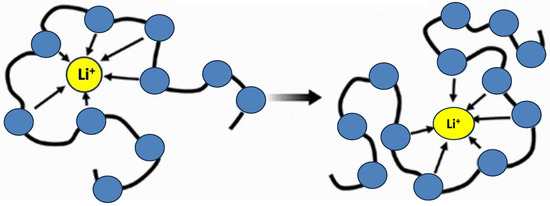
2.2. IL-Based Polymer Electrolyte
3. Phosphonium-Based Polymeric Ionic Liquid Electrolyte
References
- Working Group Phosphorus Chemistry. Gesellschaft Deutscher Chemiker e.V. Available online: www.gdch.de/phosphorchemie (accessed on 9 November 2022).
- Robles, H. Phosphorus; Academic Press: Oxford, UK, 2014; pp. 920–921.
- Pradel, M.; Aissani, L. Environmental impacts of phosphorus recovery from a ‘product’ Life Cycle Assessment perspective: Allocating burdens of wastewater treatment in the production of sludge-based phosphate fertilizers. Sci. Total Environ. 2019, 656, 55–69.
- Bradford-Hartke, Z.; Lane, J.; Lant, P.; Leslie, G. Environmental Benefits and Burdens of Phosphorus Recovery from Municipal Wastewater. Environ. Sci. Technol. 2015, 49, 8611–8622.
- Johansson, K.; Perzon, M.; Fröling, M.; Mossakowska, A.; Svanström, M. Sewage sludge handling with phosphorus utilization—Life cycle assessment of four alternatives. J. Clean. Prod. 2008, 16, 135–151.
- Pound, B.G.; Cox, P.; Mortelmans, K.E. The Use of Quaternary Phosphonium Compounds as Antibacterial Corrosion Inhibitors for Low-Alloy Steel. Corrosion 2018, 74, 694–704.
- Chang, T.-Y.; Chen, Y.; Luo, D.I.; Li, J.X.; Chen, P.L.; Lee, S.; Fang, Z.; Li, W.Q.; Zhang, Y.Y.; Li, M.; et al. Black Phosphorus Mid-Infrared Light-Emitting Diodes Integrated with Silicon Photonic Waveguides. Nano Lett. 2020, 20, 6824–6830.
- Smith, G.F.; Diehl, H. A phosphorus pentoxide desiccant employing exfoliated vermiculite as carrier. Talanta 1962, 9, 84–85.
- Kanat, M.; Eren, T. Synthesis of phosphorus-containing flame retardants and investigation of their flame retardant behavior in textile applications. J. Appl. Polym. Sci. 2019, 136, 47935.
- Tuominen, M.; Karp, H.J.; Itkonen, S.T. Phosphorus-Containing Food Additives in the Food Supply—An Audit of Products on Supermarket Shelves. J. Ren. Nutr. 2022, 32, 30–38.
- Wang, F.; Pan, S.; Zhang, P.; Fan, H.; Chen, Y.; Yan, J. Synthesis and Application of Phosphorus-containing Flame Retardant Plasticizer for Polyvinyl Chloride. Fibers Polym. 2018, 19, 1057–1063.
- Liu, L.; Zheng, X.; Wei, X.; Kai, Z.; Xu, Y. Excessive application of chemical fertilizer and organophosphorus pesticides induced total phosphorus loss from planting causing surface water eutrophication. Sci. Rep. 2021, 11, 23015.
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467.
- Monge, S.; David, G. Phosphorus-Based Polymers; The Royal Society of Chemistry: London, UK, 2014.
- Scharte, B. Phosphorus-based flame retardancy mechanisms-old hat or a starting point for future development? Materials 2010, 3, 4710–4745.
- Rose, V.L.; Mastrotto, F.; Mantovani, G. Phosphonium polymers for gene delivery. Polym. Chem. 2017, 8, 353–360.
- Suer, C.; Demir, C.; Unubol, N.A.; Yalcin, O.; Kocagoz, T.; Eren, T. Antimicrobial Activities of Phosphonium Containing. RSC Adv. 2017, 6, 86151–86157.
- Moghadam, F.; Kamio, E.; Yoshioka, T.; Matsuyama, H. New approach for the fabrication of double-network ion-gel membranes with high CO2/N2 separation performance based on facilitated transport. J. Memb. Sci. 2017, 530, 166–175.
- Hapiot, P.; Lagrost, C. Electrochemical Reactivity in Room-Temperature Ionic Liquids. Chem. Rev. 2008, 108, 2238–2264.
- Sashmitha, K.; Rani, M.U. A Comprehensive Review of Polymer Electrolyte for Lithium-Ion Battery; Springer: Berlin/Heidelberg, Germany, 2022; p. 0123456789.
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 1973, 14, 589.
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522.
- Xue, X.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A Mater. Energy Sustain. 2015, 3, 19218–19253.
- Vincent, C.A. Applications of Electroactive Polymers; Scrosati, B., Ed.; Chapman and Hall: London, UK, 1993; p. 354.
- Wang, W.M. Study on All Solid-State Composite Polymer Electrolyte. Adv. Mater. Res. 2012, 571, 13–16.
- Qiao, B.; Leverick, G.M.; Zhao, W.; Flood, A.H.; Johnson, J.A.; Shao-Horn, Y. Supramolecular Regulation of Anions Enhances Conductivity and Transference Number of Lithium in Liquid Electrolytes. J. Am. Chem. Soc. 2018, 140, 10932–10936.
- Doyle, M.; Fuller, T.F.; Newman, J. The importance of the lithium ion transference number in lithium/polymer cells. Electrochim. Acta 1994, 39, 2073–2081.
- Watanabe, M.; Nagano, S.; Sanui, K.; Ogata, N. Estimation of Li+ transport number in polymer electrolytes by the combination of complex impedance and potentiostatic polarization measurements. Solid State Ion. 1988, 28, 911–917.
- Dudley, J.T.; Wilkinson, D.P.; Thomas, G.; LeVae, R.; Woo, S.; Blom, H.; Horvath, C.; Juzkow, M.W.; Denis, B.; Juric, P.; et al. Conductivity of electrolytes for rechargeable lithium batteries. J. Power Source 1991, 35, 59–82.
- Aurbach, D.; Zaban, A.; Schechter, A.; Ein-Eli, Y.; Zinigrad, E.; Markovsky, B. The Study of Electrolyte Solutions Based on Ethylene and Diethyl Carbonates for Rechargeable Li Batteries: I. Li Metal Anodes. J. Electrochem. Soc. 1995, 142, 2873.
- Huang, M.; Feng, S.; Zhang, W.; Giordano, L.; Chen, M.; Amanchukwu, C.V.; Anandakathir, R.; Shao-Horn, Y.; Johnson, J.A. Fluorinated Aryl Sulfonimide Tagged (FAST) salts: Modular synthesis and structure–property relationships for battery applications. Energy Environ. Sci. 2018, 11, 1326–1334.
- Mathews, K.L.; Budgin, A.M.; Beeram, S.; Joenathan, A.T.; Stein, B.D.; Werner-Zwanziger, U.; Pink, M.; Baker, L.A.; Mahmoud, W.E.; Carini, J.P.; et al. Solid polymer electrolytes which contain tricoordinate boron for enhanced conductivity and transference numbers. J. Mater. Chem. A 2013, 1, 1108–1116.
- Savoie, B.M.; Webb, M.A.; Miller, T.F. III Enhancing Cation Diffusion and Suppressing Anion Diffusion via Lewis-Acidic Polymer Electrolytes. J. Phys. Chem. Lett. 2017, 8, 641–646.
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.P.; Phan, T.N.T.; Bertin, D.; Gigmes, D.; Devaux, D.; et al. Efficient electrolytes for lithium-metal batteries. Nat. Mater. 2013, 12, 452–457.
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629.
- Zhao, Y.; Wu, C.; Peng, G.; Chen, X.; Yao, X.; Bai, Y.; Wu, F.; Chen, S.; Xu, X. A new solid polymer electrolyte incorporating Li10GeP2S12 into a polyethylene oxide matrix for all-solid-state lithium batteries. J. Power Source 2016, 301, 47–53.
- Szczęsna-Chrzan, A.; Marczewski, M.; Syzdek, J.; Kochaniec, M.K.; Smoliński, M.; Marcinek, M. Lithium polymer electrolytes for novel batteries application: The review perspective. Appl. Phys. A Mater. Sci. Process. 2023, 129, 37.




