Phosphorous is an essential element for the life of organisms, and phosphorus-based compounds have many uses in industry, such as flame retardancy reagents, ingredients in fertilizers, pyrotechnics, etc. Ionic liquids are salts with melting points lower than the boiling point of water. The term “polymerized ionic liquids” (PILs) refers to a class of polyelectrolytes that contain an ionic liquid (IL) species in each monomer repeating unit and are connected by a polymeric backbone to form macromolecular structures. PILs provide a new class of polymeric materials by combining some of the distinctive qualities of ILs in the polymer chain. Ionic liquids have been identified as attractive prospects for a variety of applications due to the high stability (thermal, chemical, and electrochemical) and high mobility of their ions, but their practical applicability is constrained because they lack the benefits of both liquids and solids, suffering from both leakage issues and excessive viscosity. PILs are garnering for developing non-volatile and non-flammable solid electrolytes.
1. Phosphorus
Phosphorus occurs in rocks, soil, plants, and animal tissues. The several allotropes of elemental phosphorus, including the white, red, black, and purple varieties, are also well known. The alteration that best exemplifies the end result of industrial production is white phosphorus (P
4). Phosphorus-rich molecular compounds can be created by functionalization because of P
4’s strong reactivity and unique molecular structure. Both white and yellow are commercially available phosphorus formulations. Small amounts of red phosphorus are present in white phosphorus to form yellow phosphorus. Red phosphorus is created when white phosphorus is heated in the absence of oxygen in an inert atmosphere
[1][2][31,32].
Finite resources of phosphate rock, mineralogical phosphate reserves, and irreplaceable resources cause rising research on the recycling of phosphorus. It is a crucial mineral as a nutrient for many biological processes. However, materials science also uses phosphorus compounds in many different fields. Specifically, recycling the phosphorus from the lithium-ion battery will be an important concept in the future. Nowadays, phosphorus recycling from wastewater treatment residues is a crucial strategy for protecting the world’s phosphorus supplies, and many technologies have been devised to produce pure and effective phosphorus from these wastes, which are basically produced from fertilizers. Additionally, phosphorus-bearing polymer matrices at their end-of-life accumulate in wastewater, sewage sludge, or soils. Phosphorus can be recovered after anaerobic digestion resulting in sewage sludge ashes, mainly in the form of magnesium ammonium phosphate or calcium phosphate. Sludge can be directly used as fertilizer on agricultural soils.
In recent years, the Life Cycle Assessment (LCA) has been performed to determine if recovering dissipated phosphorus by creating phosphate fertilizer based on sludge can be an effective way to prevent phosphorus depletion. Consequently, upstream sludge production was taken into account by allocating some of the environmental costs associated with wastewater treatment to sludge production. It showed that the upstream burden of sludge generation and P recovery was less environmentally benign than that of mineral phosphate fertilizers
[3][33]. However, recovering phosphorus using struvite precipitation
[4][34] and supercritical water oxidation
[5][35] are alternative methods to recover phosphorus if fertilizer production is not desired.
Phosphorus plays a vital role in organic and inorganic chemical synthesis routes, including the Wittig reaction, Staudinger reaction, Arbusov and Michaelis-Arbusov reaction, Mitsunobu reaction, Horner-Wadsworth-Emmons reaction, or as multifunctional phosphine ligands in metal complex catalysts
[1][31]. Additionally, phosphorus is widely used in the study of solid-state chemistry and materials science. It is used in steel
[6][36], light-emitting diodes
[7][37], and even matches. In recent years, phosphorus compounds have played an essential role in the chemical industry, e.g., as desiccants (e.g., phosphorus (V) oxide)
[8][38], in flame retardants
[9][39], additives
[10][40], plasticizers
[11][41], pesticides, or as phosphate in fertilizers
[12][42]. One of the key properties of phosphorus compounds is their thermal resistance and flame retardancy behavior
[13][14][15][43,44,45], which could enhance the safety issue of lithium-ion batteries. Basically, phosphorus compounds in the gas phase act as radical scavengers and solid phase char yield formation to reduce the flammability of the polymer and thus increase the material’s thermal stability.
In short, phosphonium-based PILs show great promise as novel electrolyte materials, although they have not received as much research as their ammonium analogues. Although ILs and PILs have numerous applications in gene delivery
[16][46], antimicrobial coatings
[17][47], gas separation
[18][48], and conductive materials
[19][49], there is a lack of studies on phosphonium-based PILs as electrolytes in LIB applications in the literature.
2. Phosphonium Ionic Liquid Polymer Electrolytes in Lithium-Ion Batteries
2.1. Polymer Electrolytes
Polymer electrolytes are prepared from the dissolution of lithium salt with low lattice energy and bulky anions into the high molecular weight polymer host
[20][50]. The first description of ionic conductivity in polymers with complicated alkali salts was made by Peter V. Wright and Fenton in 1973
[21][51]. The covalent interaction between the polymer backbones and the ionizing groups provides the basis for ionic conduction in polymer electrolytes. The polymer’s electron donor group first creates solvation with the dopant salt’s cation component before facilitating ion separation and an ionic doping mechanism. It produces ionic conductivity as a result
[20][50].
The main requirement when choosing the polymer host is that the polymer must be able to pair with lithium ions as well as dissolve lithium salt to create a polymer electrolyte with high lithium-ion conductivity. The polymer’s polar groups (−O, −S, −N, −P, etc.) work well as building blocks to dissolve lithium salts. Polyethylene oxide (PEO) and its derivatives are the main subjects of most polymer electrolyte research activities. The anion and cation of the lithium salt dissociate because of the Coulombic interaction between the single electron pair of oxygens on the PEO segment and the lithium ion
[22][52]. Lithium salt dissolves into the PEO matrix, while PEO serves as a solvent in the process. Other atoms, such as the nitrogen in the imide (−C=O)NR−(C=O)) and the sulfur in the thiol (−SH), as well as the oxygen atom (−CH
2CH
2O−) on the PEO chain, also play a similar role. Li
+ cations migrate along the polymer segment, or jump from one segment to another, from one coordination point to another, when exposed to an electric field.
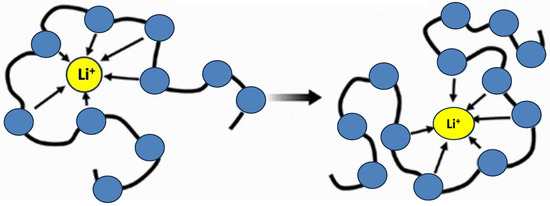
Figure 14 illustrates the ion transport mechanism of polymer electrolytes like PEO
[23][53].
Figure 14. Structural representation of Li+ transport in PEO-doped polymer electrolytes.
Additionally, the flexibility of the polymer chain, the quantity of lithium ions, and the primary electric charge all affect ionic conductivity
[24][54]. Lithium salt’s low lattice energy aids in the dissociation of a polymer with a high dielectric constant
[24][54]. It appears necessary to use a fully amorphous polymer considerably above its glass transition temperature to ensure a large conductivity of the polymer electrolyte. Along the polymer length, the Li
+ cations move from one coordination site to another under the influence of an electric field
[25][55].
It is generally known that anions can reduce conductivity by generating nonconductive ion pairs
[26][56]. The lithium-ion transference number (t
Li+) is likewise lowered by the dispersion of anions
[27][28][57,58]. Therefore, one of the main objectives of innovative electrolyte materials is to control anions in order to regulate these characteristics. Some specific methods for controlling anions have been studied, including electrolyte viscosity
[29][59], high dielectric solvents
[30][31][60,61] Lewis acidic electrolytes
[32][33][62,63], single conducting polymers
[34][64], and ionic liquids
[35][65].
2.2. IL-Based Polymer Electrolyte
An ionic liquid is a molten salt at low temperatures, and it often consists of organic cations and inorganic anions
[36][66]. Ionic liquids have excellent thermal stability, great electrochemical stability, and no vapor pressure due to their unique condition
[35][65]. Although ionic liquids have excellent ionic conductivity, their low viscosity prevents them from being used directly as electrolytes. As a solid electrolyte for lithium-ion batteries, the combination of ionic liquid and polymer provides alternatives.
Higher ionic conductivity is produced when IL is added to a polymer, although this is typically accompanied by a loss of mechanical strength, especially at high temperatures. Higher mechanical strength and a smoother continuous electrolyte surface are produced by lower IL concentrations, which are better for ion transport. Therefore, the ionic conductivity and mechanical characteristics are significantly influenced by the amount of IL. Additionally, battery cycling at high temperatures typically results in the breakdown of the IL components, thus lowering performance. It increases the demand that the polymer components maintain high IL contents by one more criterion. The primary categories of IL-based polymer electrolytes include two classes: (1) ILs/polymer doped; and (2) polymeric ionic liquids (PILs)
[22][52].
3. Phosphonium-Based Polymeric Ionic Liquid Electrolyte
Some of the important factors have to be to taken into account when selecting a polymer host for PEs include better coordination with anions, thermal stability, and high oxidation states
[37][88]. These factors are all present in phosphorus-based polar functional groups connected with ILs and form functional polymers. Thus, a new family of phosphonium-based materials with unique properties and intriguing applications has recently emerged because of the introduction of the functional groups associated with ILs into functional polymers.