Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dhanush Haspula | -- | 3952 | 2023-07-10 17:54:26 | | | |
| 2 | Camila Xu | Meta information modification | 3952 | 2023-07-11 03:13:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Haspula, D.; Cui, Z. Hypothalamus and the Brainstem in Health and Obesity. Encyclopedia. Available online: https://encyclopedia.pub/entry/46612 (accessed on 12 January 2026).
Haspula D, Cui Z. Hypothalamus and the Brainstem in Health and Obesity. Encyclopedia. Available at: https://encyclopedia.pub/entry/46612. Accessed January 12, 2026.
Haspula, Dhanush, Zhenzhong Cui. "Hypothalamus and the Brainstem in Health and Obesity" Encyclopedia, https://encyclopedia.pub/entry/46612 (accessed January 12, 2026).
Haspula, D., & Cui, Z. (2023, July 10). Hypothalamus and the Brainstem in Health and Obesity. In Encyclopedia. https://encyclopedia.pub/entry/46612
Haspula, Dhanush and Zhenzhong Cui. "Hypothalamus and the Brainstem in Health and Obesity." Encyclopedia. Web. 10 July, 2023.
Copy Citation
The hypothalamus and brainstem are critical components of the homeostatic system that regulates appetite and energy balance. These key brain regions comprise of distinct neuronal populations and nuclei which exerts tremendous control over several facets of energy balance. Importantly, several of these neuronal populations exhibit both overlapping and also contrasting metabolic roles, thereby enabling the CNS to fine tune metabolic functions under physiological conditions.
obesity
hypothalamus
appetite
glucose homeostasis
weight-loss drugs
1. Hypothalamus
The hypothalamus is one of the most well-studied brain regions in metabolism. Apart from regulating a broad range of thermoregulatory, reproductive, and cardiovascular functions, it also exerts tremendous influence on several aspects of energy balance. The hypothalamus is composed of multiple nuclei located adjacent to the third ventricle. These nuclei comprise a distinct subpopulation of neurons, capable of altering energy intake and/or energy expenditure via anabolic or catabolic functions. The complex nexus of hypothalamic neuronal interconnections can integrate responses from peripheral signals (hormones, nutrients, and metabolites), to modulate appetite centrally, and to influence lipid and glucose metabolism, peripherally. Additionally, they also have reciprocal projections to and from extrahypothalamic nuclei located in the brainstem, midbrain, and forebrain which can also alter synaptic activity in the hypothalamic metabolic circuits. Consequently, via integration and coordination of responses from the brain and periphery, hypothalamic nuclei are key regulators of energy homeostasis.
1.1. Arcuate Nucleus (ARC)
The ARC is considered as one of the most important brain regions involved in the regulation of appetite and energy expenditure. Located near the median eminence, a region enriched in fenestrated capillaries, the ARC is accessible to circulating hormones, nutrients and metabolites, thus, serving as an ideal relay center to communicate circulating peripheral signals to the brain. The ARC comprises two distinct neuronal subpopulations that have opposing roles in energy homeostasis, the anabolic neuropeptideY/Agouti-related protein (NPY/AGRP) neurons and the catabolic, pro-opiomelanocortin (POMC) and cocaine-and amphetamine-regulated transcript (CART) or POMC/CART neurons (referred to henceforth as AGRP and POMC neurons). Both these neurons are first order neurons, which have glucose and nutrient sensing capabilities, in addition to receiving input from circulating hormones and satiety signals [1][2][3][4]. These counterregulatory neuronal populations are modulated by energy status. Food deprivation rapidly activates AGRP neurons and inhibits POMC neurons [5][6][7]. AGRP neurons release both NPY, which is an agonist for the Y1-5 receptors, and AGRP, an inverse agonist for melanocortin receptors [8][9]. Ablation of AGRP neurons results in a dramatic reduction in feeding, while acute activation results in a robust increase in food intake, weight gain, and altered autonomic outflow to several organs and tissues [10][11][12][13]. NPY was one of the first orexigenic neuropeptides to be identified, and subsequent functional studies revealed a potent, albeit fleeting, appetite-stimulating effect [14]. More recently, it has been revealed that NPY-mediated effects on feeding are mediated via the Y1 receptor, while its effects on energy expenditure are driven via the Y2 receptor [15]. Although both of these orexigenic neuropeptides, NPY and AGRP, have complimentary roles in triggering a hyperphagic response and reducing energy expenditure, the longer-lasting or sustained effect of these neurons on food intake is dependent on AGRP release, while the more rapid effect on food intake is dependent on NPY secretion [13][14][16]. Additionally, AGRP neurons also release GABA, which plays an integral role in AGRP-mediated effects on appetite and energy balance [14][17]. Furthermore, diet-induced obesity blunts AGRP responsiveness to circulating hormones [18]. In stark contrast to AGRP neurons, POMC neurons have a pronounced catabolic effect due to their ability to release the anorectic neuropeptide, α-melanocyte-stimulating hormone (α-MSH), a major satiety neuropeptide which is an agonist of melanocortin receptors [19]. Ablation of POMC neurons was reported to result in a mild obesity phenotype characterized by both reduced and increased food intake [20][21]. Interestingly, only chronic, but not acute chemogenetic activation of these neurons results in suppression of food intake, suggesting a role for POMC in maintaining long-term energy homeostasis [21]. POMC neurons have been reported to exhibit functional and spatial heterogeneity characterized by differences in both molecular architecture and anatomical projections to distinct brain regions, suggesting a more complex neural network involved in metabolic control [22][23][24]. POMC neuronal activity is also regulated by AGRP neurons. Anatomic and functional evidence indicates that GABA-releasing AGRP neurons are involved in inhibiting POMC neuronal activity and α-MSH release [25][26][27]. Apart from α-MSH, it is also to be noted that POMC neurons also release β-endorphin, which binds to the μ-opioid receptors. Both these POMC-derived neuropeptides have functionally antagonistic roles in the regulation of energy balance [28]. Both hypothalamic AGRP and POMC neurons are known to express the μ-opioid receptors (MOR). In the case of POMC neurons, the MORs function as autoinhibitory receptors that are activated by the release of β-endorphins [29]. Interestingly, while α-MSH is predominantly involved in suppressing appetite, β-endorphin were shown to play a major role in promoting a palatability-driven feeding response [30][31]. Naltrexone, a MOR antagonist which has been shown to suppress feeding on a short-term basis, has been shown to have stimulatory effects on POMC neurons in both rodents and humans [32][33]. More about their therapeutic utility will be covered in a later section.
Both AGRP and POMC neurons also express receptors for insulin (IR) and leptin (LepR). Leptin depolarizes and increases firing frequency of POMC neurons, while hyperpolarizing and inhibiting AGRP/NPY neuronal activity and neuropeptide release [34][35][36][37][38]. Mechanistic studies revealed that deletion of Rho-kinase 1, a protein kinase involved in cytoskeletal reorganization and neuropeptide release, in both AGRP and POMC neuronal populations resulted in leptin resistance and obesity [39][40]. Collectively, these data point to a crucial central mechanism by which leptin can induce a negative energy balance. Studies investigating the role of insulin signaling in both AGRP and POMC neurons on appetite regulation have yielded contradictory results. While some studies reported on little-to-no effect on appetite and body weight change with IR deletion in AGRP neurons, others have described a more nuanced role of AGRP-specific insulin signaling on regulating meal size [41][42]. A context-dependent appetite suppression role is reported for insulin signaling in the AGRP neurons, which is characterized by acute repression of feeding bouts without altering total calorie intake, and the suppression of highly palatable high-fat-diet food over standard chow [42]. In the case of POMC neurons, while the deletion of LepR results in mild obesity, knockout of IR in these neurons had no significant effect on body weight [43][44]. Furthermore, both AGRP and POMC neurons are modulated by postprandial signals, such as ghrelin, incretins, and amylin, to regulate food intake [45][46][47][48][49]. Apart from having integral roles in appetite and satiety regulation, these neuronal populations are also involved in maintaining glucose homeostasis as chemogenetic activation of AGRP and POMC neurons revealed distinct roles of G protein activation on food intake and glycemic control [11][13][21][50]. Additionally, both AGRP and POMC neurons can also regulate energy balance via the hypothalamic–pituitary–thyroid (HPT) axis. HPT axis is well-known to stimulate energy expenditure. Thyroid hormones play an important role in maintenance of homeothermia, and stimulation of the thyroid axis is known to increase energy expenditure via thermogenesis [51]. ICV administration of NPY has been shown to suppress circulating levels of thyroid hormones [52]. Interestingly, the melanocortin system has also been shown to regulate the HPT axis. Both in vivo and in vitro studies revealed that α-MSH can stimulate the HPT axis by increasing the levels of thyroid stimulating hormone (TSH), while AGRP on the other hand inhibits it [53][54]. For more information on the role of the melanocortin system in regulating the HPT axis, readers can refer to other reviews on this topic [55].
Another key aspect of the ARC neurons, especially POMC, is that they exhibit sexual dimorphism. Higher number of POMC neurons and increased neural activity were observed in female animals when compared to their male counterparts [56]. Disruption of key genes in POMC neurons in female mice resulted in the development of obesity [56][57][58][59]. More recently, POMC-specific alteration of certain highly expressed CNS genes, resulted in changes in glucoregulation and energy balance in female mice only [60][61][62].
ARC is highly susceptible to synaptic plasticity in response to the hormonal milieu. Both AGRP and POMC neurons have been described as exhibiting some level of synaptic rewiring under periods of food deprivation and overfeeding conditions [63]. Particularly, the melanocortin system has been reported to exhibit synaptic remodeling under both extreme metabolic changes, such as starvation and overfeeding, but also under physiological feeding states which results in modest metabolic changes [64][65][66]. Plasticity of the ARC has important implications in obesity, as diet-induced obesity has been demonstrated to suppress hypothalamic remodeling and neurogenesis resulting in reduced neuronal turnover [67]. It was also demonstrated to result in reactive gliosis in the ARC with altered synaptic architecture of the NPY and POMC neurons [68]. High fat diet (HFD)-induced neurogenesis is not restricted to the neuronal populations alone in the ARC. HFD activated neurogenesis in the median eminence however leads to energy storage, while prevention of it results in a reduction in weight gain [69]. Stimulation of neurogenesis in response to HFD is observed in female mice and not in males, suggesting a sexual dimorphic nature of hypothalamic neurogenesis [70].
1.2. Paraventricular Nucleus (PVH)
The PVH serves as an important convergence/termination point for orexigenic and anorexigenic projections arising from the ARC and other hypothalamic regions. Neurons present in this region express two different types of melanocortin receptors subtypes (MC3R and MC4R) that can be activated by the melanocortin peptide, α-MSH [71][72]. α-MSH and AGRP, released from the ARC projections, can modulate PVH neuronal activity by either activating or antagonizing the melanocortin receptors, respectively [9][72][73][74]. Thus, these neurons provide counterregulatory inputs to fine tune energy balance in response to changes in the levels of circulating signals. PVH neurons express single-minded 1 (Sim1), a transcriptional factor required for PVH development and the maintenance of energy homeostasis [75][76]. Sim1 neurons have pronounced effects on satiety and energy homeostasis as both sim1 heterozygous mice, and inducible Sim1-deficient mice, exhibit hyperphagia leading to obesity [76][77]. A major subset of Sim1 neurons in the PVH express MC4R [43][72]. Mutations in the MC4R gene are a leading cause of monogenic forms of obesity, and MC4R variants have been linked to increased obesity in certain populations [78][79][80][81]. The MC4R/Sim1 neurons, located in the PVH, together with the POMC neuronal projections, arising from the ARC, form the melanocortin pathway in the hypothalamus. Stimulation of MC4R neurons in the PVH results in pronounced satiation effects and thereby can induce a negative energy balance and confer protection against obesity [43][82][83][84]. Interestingly, short-term administration of MC4R agonists can also increase resting energy expenditure and shift substrate utilization towards increased fat oxidation in obese individuals suggesting additional mechanisms through which the melanocortin pathway and Sim1 neurons induce a negative energy balance [85]. Knockdown of MC4R results in potential disruption of synaptic plasticity and attenuation of long-term potentiation in the PVH [86]. Perturbation of MC4R signaling in the PVH alone, or in both PVH and DMV results in hyperphagic obesity with reduced energy expenditure and defects in insulin sensitivity [82][87]. It is to be noted that MC4R-expressing neurons are not just located in the hypothalamic nuclei, but also located in the brainstem, intermediolateral cell column of the spinal cord, and autonomic neurons where they not only exert prominent cardiovascular effects, but also regulate metabolic functions including thermogenesis, glucose homeostasis and energy expenditure [88][89][90][91]. Interestingly, PVH not only comprises MC4R neurons, but also contains other neuronal populations such as prodynorphin-expressing neurons, which lack MC4R. These neuronal populations have comparable effects to the PVH-MC4R expressing neurons on regulating satiety [92]. Several such anatomically distinct neuronal populations have been identified in the PVH as having appetite-regulatory roles, which further highlights the complexity of this nucleus [93][94][95][96]. For a more detailed review on the pathophysiological roles of MC4R neurons, readers can refer to excellent reviews on this topic [97][98].
1.3. Ventromedial Nucleus of the Hypothalamus (VMH)
Despite having an inauspicious history in metabolism research, the VMH is still appreciated as one of the principal satiety centers in the brain [99]. Early studies have highlighted an important role of VMH in suppressing appetite [100][101]. Apart from regulating food intake, VMH neurons have also been associated with improvements in several metabolic parameters and conferring protection against obesity [102][103]. A major subset of VMH neurons express steroidogenic factor 1 (SF1), often serving as a biomarker to distinguish VMH from other hypothalamic nuclei. Similar in function to the POMC neurons, activated SF1 neurons elicit pronounced anorexigenic effects with increased energy expenditure [104][105]. These neurons not only provide excitatory input directly onto the POMC neurons, but also project to the paraventricular thalamus to induce an aversive effect and suppress appetite [106][107]. Deletion of LepR from SF1 neurons also resulted in a similar degree of weight gain in mice when compared with LepR-specific KO in POMC neurons, suggesting important roles of leptin signaling in both sets of neuronal populations [43][108]. Moreover, SF1 neurons have distinct projections to other regions of the brain involved in negating insulin-induced hypoglycemia [109]. This will be covered in a later section. For a more detailed review on the role of SF1 neurons in metabolic disorders, the readers can refer to the review by Fosch et al. [110].
1.4. Dorsomedial Hypothalamus (DMH)
Another hypothalamic nucleus that affects feeding response is the DMH. DMH lesion in both young and older rats produced a hypophagic response with reduced body weight [111]. Interestingly, the DMH expresses NPY, which shows altered levels in various models of obesity [112][113][114]. Overexpression of NPY in the DMH results in an increase in food intake, weight gain, and an obese phenotype under high-fat-diet conditions, while knockdown of NPY ameliorated these effects in obese mice [115]. Inhibitory GABAergic neurons projecting to the PVH have been proposed as a key mechanism for eliciting a DMH-mediated orexigenic response [116]. Additionally, DMH neurons project to the ARC where they inhibit POMC neurons during fasting suggesting parallel neural circuits from DMH to regulate appetite [7]. The DMH may also be involved in the regulation of food intake by other hormones and peptides, as intra-DMH administration of the appetite-suppressing hormone, cholecystokinin (CCK), resulted in a suppression of food intake [117][118]. Interestingly, under refeeding conditions, excitatory glutamatergic projections are also activated by a subset of DMH glutamatergic neurons leading to reduced food intake [119]. A recently published study reported on DMH having bidirectional effects on food intake, which receive key leptin-responsive projections from the AGRP neurons [120]. Thus, it is likely that DMH projections could participate in the fine tuning of energy intake by activating distinct inhibitory and excitatory projections to other hypothalamic nuclei.
2. Brainstem
The brainstem exerts significant control over autonomous biological functions. The medulla is a key brainstem structure which has prominent cardioregulatory and metabolic functions, via specialized cardiovascular and satiety centers, respectively. The medullary cardiovascular centers have well-established roles in the homeostatic regulation of blood pressure via the baroreflex [121]. Although the brainstem is not as well-investigated as its counterpart, the hypothalamus, in metabolism, studies dating back to the 1970s highlighted the importance of the caudal brainstem in mediating satiety and glucoregulatory responses [122][123]. Importantly, specialized medullary regions serve as crucial integration points between the CNS and the digestive tract. They receive visceral afferent input from gastrointestinal sensory neurons, the latter conveying satiety signals in response to a meal. Additionally, the brainstem also comprises a circumventricular organ, area postrema (AP), which allows access to satiety signals. These signals in turn can modulate adjacent and supra-adjacent neuronal populations located in the brainstem. These proximally located neuronal populations in the caudal brainstem, in conjunction with the AP, are key structures in mediating postprandial satiety [124][125].
Dorsal Vagal Complex (DVC)
The caudal brainstem not only expresses receptors for circulating pressor peptides, but it can also be modulated by metabolic cues and thus exerts control over energy homeostasis [126][127][128][129][130]. The DVC located in the hindbrain is designated as the brainstem satiety center. The DVC comprises the AP, the nucleus of the solitary tract (NTS), and the dorsal motor nucleus of the vagus nerve (DMV). The NTS serves as the primary hub for ascending neural signals from the nodose ganglia, which contains cell bodies for several vagal afferents that densely innervate the gastrointestinal tract (GIT) [131]. The sensory vagal nerve terminals in the GIT are heterogenous in nature conveying both chemosensory, from nutrients and gut hormones, and mechanosensory signals to the brainstem [131][132]. Postprandial gut hormones and nutrients suppress food intake by transmitting information via the sensory vagal afferent terminals to the NTS, a crucial entry point in the brain for visceral information [133][134]. CCK, one of the first gut peptides to be identified to mediate satiety, elicits its actions by acting on the CCK-A receptors that are abundantly expressed on the vagal afferents and the cell bodies of the nodose ganglia [133][135][136][137]. Additionally other receptors involved in regulating satiety, such as the LepR, are also expressed on these cell bodies [138]. As a result, circulating signals such as leptin can also act along with CCK on the nodose ganglia, to synergistically suppress food intake [139][140]. Another class of gut hormones, the incretins, also exert prominent effects on satiety and glucose homeostasis. The incretins, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), are released in response to a meal, and they act on their corresponding receptors (GLP1R and GIPR) located in pancreatic islets where they promote insulin release. These receptors are also expressed in non-islet cells, where they exert prominent metabolic actions independent of direct effects on pancreatic insulin secretion [141]. In the GIT, the GLP1R is expressed on mechanosensitive vagal sensory neurons, and its activation results in pronounced inhibition of food intake, while its knockdown is associated with an increased meal size [142][143].
Interestingly, profiling of G-protein coupled receptors in the GIT revealed a lack of receptor expression for GIP and ghrelin on vagal afferents, potentially highlighting other CNS-dependent mechanisms to alter food intake [144]. Similar to the ARC, the AP lacks a well-defined blood–brain barrier, as a result is accessible to various satiety signals and circulating hormones. Since the NTS is located in close proximity to the fourth ventricle, it serves as a crucial node for integrating signals from the gut and circulation. Satiety signals, such as CCK, GLP-1, and their analogs, have been shown to inhibit appetite by acting on their corresponding receptors localized to the brainstem neurons in the satiety center [145][146][147]. The GLP1R is highly expressed in the NTS, and knockdown of preproglucagon, a precursor for GLP1, in the brainstem results in hyperphagia and increased adiposity, suggesting a crucial role for central GLP1R in mediating satiety [148]. Interestingly, GIPR agonism not only enhances the anorectic effect of GLP1R agonism, but recent studies suggest that it improves tolerability of PYY analogs by modulating the brainstem neural circuits and blocking its anorectic effect [149][150][151]. Thereby, understanding of incretins-mediated modulation of the brainstem neural circuitry has significant implications for the development of weight loss drugs with an improved side effect profile. The role of incretins in the hypothalamic and brainstem neural circuitry in the regulation of energy homeostasis will be discussed in a later section. Other pancreatic and gut-derived postprandial signals, such as amylin and PYY, also act on neuronal populations in the AP and NTS to promote satiety [152][153][154][155][156]. Additionally, leptin-mediated signaling in the NTS also activates the satiation neural circuitry to suppress food intake and regulate energy balance [157][158][159].
Projections from the NTS extend to other brain regions involved in appetite control and food aversion behaviors, where they suppress appetite by triggering either a positive or negative valence [160][161]. The latter may well be dependent on both the molecular architecture of the neural circuit, and the brain regions innervated by it. For instance, NTS projections to calcitonin gene-related protein (CGRP) expressing neurons located in higher brain regions, are strongly involved in mediating anorexia and reducing body weight [162][163]. However, they can exert opposing motivational valences, since projections to specific brain regions can generate both a positive valence (NTS to PVH projection) and a negative valence (NTS to PBN projection); the latter aversive response triggered by the activation of CGRP neurons in the PBN [164][165][166]. In stark contrast to the CCK neurons, calcitonin receptor expressing neurons from the NTS do not activate CGRP neurons, and hence produce a non-aversive suppression of food intake despite projecting to the PBN [166]. Other neuronal populations such as GLP-1 expressing neurons, which are primarily located in the caudal NTS, have projections to the VTA where they regulate intake of highly palatable food [167][168].
The NTS neurons also comprise a small, but metabolically relevant, population of POMC expressing neurons, accounting for about 10% of the total POMC neuronal population [169][170]. Interestingly, while they are activated by postprandial visceral afferents from the gut, they do not co-express several of the other neuropeptide markers observed in the NTS, suggesting a distinct hub of neurons involved in mediating satiety [171][172]. These neuronal populations are functionally similar to the POMC neurons in the ARC, but they exhibit different kinetics in terms of suppression of food intake. ARC-POMC neurons are involved in long-term suppression, while the NTS-POMC neurons mediate short-term feeding responses [21]. The latter neurons are potentially involved in a more rapid feeding suppression via circulating satiety signals. NTS-POMC neurons have been shown to be crucial for the acute appetite-suppressing effect of lorcaserin, indicative of their clinical relevance [173]. More recently, this effect of lorcaserin was also shown to be meditated via the GLP-1 neurons in the brainstem, in addition to the NTS-POMC neurons [174].
In addition to the regulation of food intake, the hindbrain circuitry also has important roles in glucose sensing and modulation of systemic glucose via vagal efferents [175][176]. Neuropeptide FF (NPFF), a key analgesic peptide which has been demonstrated to have a role in substrate utilization and regulation of energy balance, is strongly expressed in the caudal brainstem, mainly localized in the DVC [177]. More recently, a study reported on impairments in glucose homeostasis in mice deficient in NPFF, further highlighting the glucoregulatory role of the DVC [178]. The role of the various satiety signals in regulating glucose and lipid metabolism via brainstem circuits will be covered in more detail in later sections.
The sections so far highlight the pivotal roles of hypothalamic orexigenic and anorexigenic neuronal populations, along with the brainstem satiety center, in the regulation of energy intake and expenditure. A schematic summarizing this is shown in Figure 1. While the NTS integrates multiple metabolic cues to promote satiation, the ARC neuronal populations are able to exert both short- and long-term effects on energy homeostasis in response to energy demands.
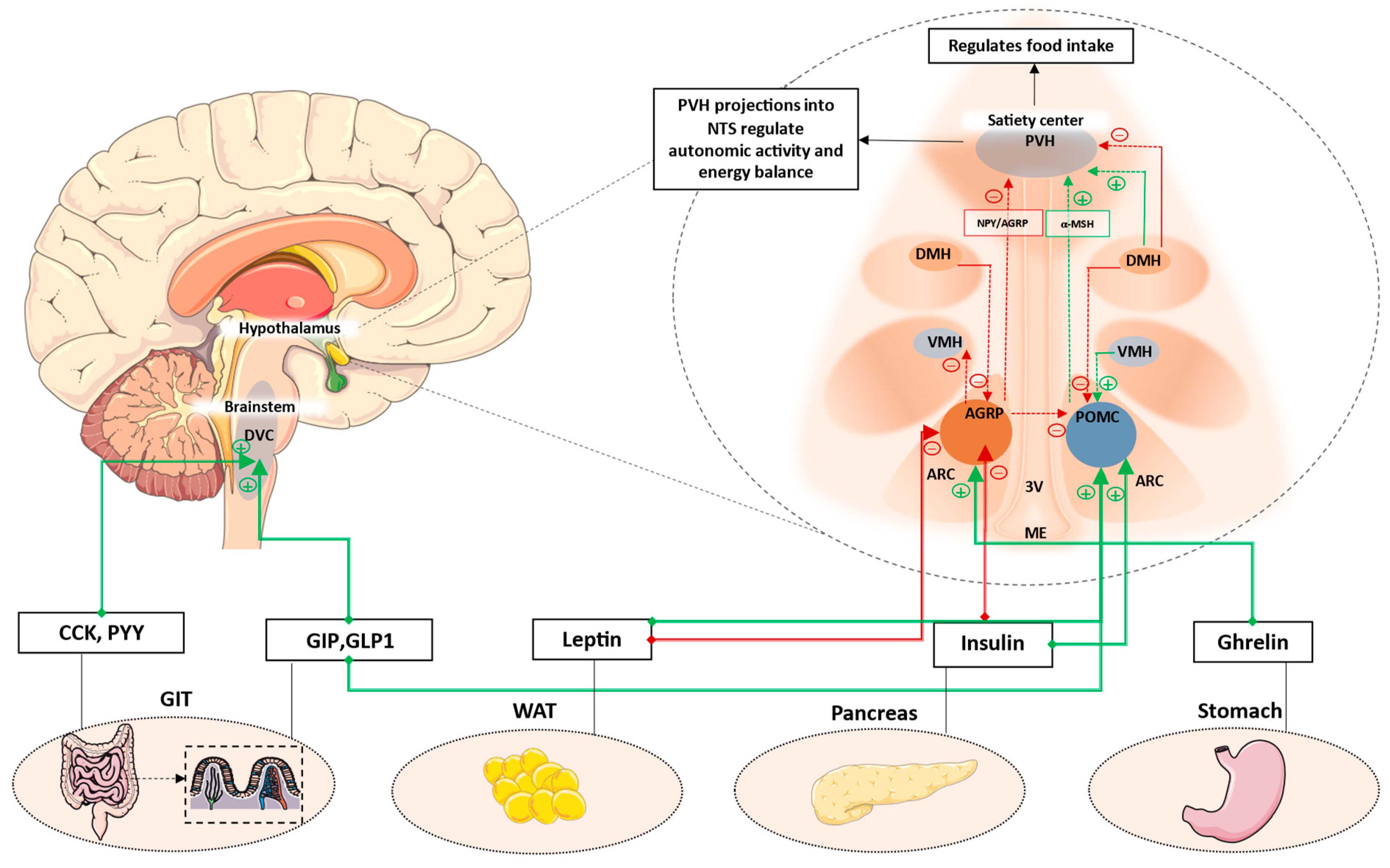
Figure 1. Key hypothalamic nuclei involved in the regulation of appetite and energy balance. ARC, comprising AGRP and POMC neurons, is located next to the median eminence. This region comprises permeable capillaries, thereby allowing access to circulating signals. These signals can modulate ARC neuronal populations, which then have extensive projections to PVH and other hypothalamic nuclei. PVH is the major hypothalamic satiety center. POMC neurons activate MC4R neurons in the PVH to decrease appetite, while AGRP neurons inhibit PVH-MC4R neurons to increase appetite. Additionally, AGRP neurons also inhibit POMC neurons via stimulation of inhibitory GABAergic input to POMC neurons. Anorexigenic signals such as leptin and GLP1 increase satiety by acting on POMC neurons, whereas orexigenic signals such as ghrelin can increase appetite by acting on AGRP neurons. Other hypothalamic neuronal populations have extensive projections to and from adjacent nuclei. While DMH has predominantly inhibitory projections to PVH and POMC, it also has been shown to also have activate inhibitory GABAergic neurons projecting to the AGRP neurons in the ARC. VMH mainly has excitatory projections to the POMC neurons, while AGRP neurons has inhibitory projections to VMH. Additionally, postprandial satiety signals from the enteroendocrine cells of the GIT can also act on DVC located in the brainstem to suppress appetite. AGRP: Agouti-related protein; POMC: Pro-opiomelanocortin; ARC: Arcuate nucleus; ME: median eminence; VMH: Ventromedial nucleus of the hypothalamus; DMH: Dorsomedial hypothalamus; PVH: Paraventricular nucleus; 3V: Third ventricle; DVC: Dorsal vagal complex; CCK: cholecystokinin; GIP: Glucose-dependent insulinotropic polypeptide; GLP1: Glucagon-like peptide-1; WAT: White adipose tissue; GIT: Gastrointestinal tract. Green dotted lines/arrows represent activation. Red dotted lines/arrows represent inhibition.
References
- Parton, L.E.; Ye, C.P.; Coppari, R.; Enriori, P.J.; Choi, B.; Zhang, C.Y.; Xu, C.; Vianna, C.R.; Balthasar, N.; Lee, C.E.; et al. Glucose Sensing by POMC Neurons Regulates Glucose Homeostasis and Is Impaired in Obesity. Nature 2007, 449, 228–232.
- Belgardt, B.F.; Okamura, T.; Brüning, J.C. Hormone and Glucose Signalling in POMC and AgRP Neurons. J. Physiol. 2009, 587, 5305–5314.
- Roh, E.; Song, D.K.; Kim, M.S. Emerging Role of the Brain in the Homeostatic Regulation of Energy and Glucose Metabolism. Exp. Mol. Med. 2016, 48, e216.
- Yoon, N.A.; Diano, S. Hypothalamic Glucose-Sensing Mechanisms. Diabetologia 2021, 64, 985–993.
- Yang, Y.; Atasoy, D.; Su, H.H.; Sternson, S.M. Hunger States Switch a Flip-Flop Memory Circuit via a Synaptic AMPK-Dependent Positive Feedback Loop. Cell 2011, 146, 992–1003.
- Millington, G.W.M. The Role of Proopiomelanocortin (POMC) Neurones in Feeding Behaviour. Nutr. Metab. 2007, 4, 1–16.
- Rau, A.R.; Hentges, S.T. GABAergic Inputs to POMC Neurons Originating from the Dorsomedial Hypothalamus Are Regulated by Energy State. J. Neurosci. 2019, 39, 6449–6459.
- O’Shea, D.; Morgan, D.G.A.; Meeran, K.; Edwards, C.M.B.; Turton, M.D.; Choi, S.J.; Heath, M.M.; Gunn, I.; Taylor, G.M.; Howard, J.K.; et al. Neuropeptide Y Induced Feeding in the Rat Is Mediated by a Novel Receptor. Endocrinology 1997, 138, 196–202.
- Nijenhuis, W.A.J.; Oosterom, J.; Adan, R.A.H. AgRP(83-132) Acts as an Inverse Agonist on the Human-Melanocortin-4 Receptor. Mol. Endocrinol. 2001, 15, 164–171.
- Luquet, S.; Perez, F.A.; Hnasko, T.S.; Palmiter, R.D. NPY/AgRP Neurons Are Essential for Feeding in Adult Mice but Can Be Ablated in Neonates. Science 2005, 310, 683–685.
- Krashes, M.J.; Koda, S.; Ye, C.P.; Rogan, S.C.; Adams, A.C.; Cusher, D.S.; Maratos-Flier, E.; Roth, B.L.; Lowell, B.B. Rapid, Reversible Activation of AgRP Neurons Drives Feeding Behavior in Mice. J. Clin. Investig. 2011, 121, 1424–1428.
- Joly-Amado, A.; Denis, R.G.P.; Castel, J.; Lacombe, A.; Cansell, C.; Rouch, C.; Kassis, N.; Dairou, J.; Cani, P.D.; Ventura-Clapier, R.; et al. Hypothalamic AgRP-Neurons Control Peripheral Substrate Utilization and Nutrient Partitioning. EMBO J. 2012, 31, 4276–4288.
- Nakajima, K.I.; Cui, Z.; Li, C.; Meister, J.; Cui, Y.; Fu, O.; Smith, A.S.; Jain, S.; Lowell, B.B.; Krashes, M.J.; et al. Gs-Coupled GPCR Signalling in AgRP Neurons Triggers Sustained Increase in Food Intake. Nat. Commun. 2016, 7, 10268.
- Krashes, M.J.; Shah, B.P.; Koda, S.; Lowell, B.B. Rapid versus Delayed Stimulation of Feeding by the Endogenously Released AgRP Neuron Mediators GABA, NPY, and AgRP. Cell Metab. 2013, 18, 588–595.
- Qi, Y.; Lee, N.J.; Ip, C.K.; Enriquez, R.; Tasan, R.; Zhang, L.; Herzog, H. NPY Derived from AGRP Neurons Controls Feeding via Y1 and Energy Expenditure and Food Foraging Behaviour via Y2 Signalling. Mol. Metab. 2022, 59, 101455.
- Luo, N.; Marcelin, G.; Liu, S.M.; Schwartz, G.; Chua, S. Neuropeptide Y and Agouti-Related Peptide Mediate Complementary Functions of Hyperphagia and Reduced Energy Expenditure in Leptin Receptor Deficiency. Endocrinology 2011, 152, 883–889.
- Tong, Q.; Ye, C.P.; Jones, J.E.; Elmquist, J.K.; Lowell, B.B. Synaptic Release of GABA by AgRP Neurons Is Required for Normal Regulation of Energy Balance. Nat. Neurosci. 2008, 11, 998–1000.
- Deem, J.D.; Faber, C.L.; Morton, G.J. AgRP Neurons: Regulators of Feeding, Energy Expenditure, and Behavior. FEBS J 2022, 289, 2362–2381.
- D’agostino, G.; Diano, S. Alpha-Melanocyte Stimulating Hormone: Production and Degradation. J. Mol. Med. 2010, 88, 1195–1201.
- Greenman, Y.; Kuperman, Y.; Drori, Y.; Asa, S.L.; Navon, I.; Forkosh, O.; Gil, S.; Stern, N.; Chen, A. Postnatal Ablation of POMC Neurons Induces an Obese Phenotype Characterized by Decreased Food Intake and Enhanced Anxiety-like Behavior. Mol. Endocrinol. 2013, 27, 1091–1102.
- Zhan, C.; Zhou, J.; Feng, Q.; Zhang, J.-e.; Lin, S.; Bao, J.; Wu, P.; Luo, M. Acute and Long-Term Suppression of Feeding Behavior by POMC Neurons in the Brainstem and Hypothalamus, Respectively. J. Neurosci. 2013, 33, 3624–3632.
- Toda, C.; Santoro, A.; Kim, J.D.; Diano, S. POMC Neurons: From Birth to Death. Annu. Rev. Physiol. 2017, 79, 209–236.
- Quarta, C.; Claret, M.; Zeltser, L.M.; Williams, K.W.; Yeo, G.S.H.; Tschöp, M.H.; Diano, S.; Brüning, J.C.; Cota, D. POMC Neuronal Heterogeneity in Energy Balance and beyond: An Integrated View. Nat. Metab. 2021, 3, 299–308.
- Saucisse, N.; Mazier, W.; Simon, V.; Binder, E.; Catania, C.; Bellocchio, L.; Romanov, R.A.; Léon, S.; Matias, I.; Zizzari, P.; et al. Functional Heterogeneity of POMC Neurons Relies on MTORC1 Signaling. Cell Rep. 2021, 37, 109800.
- Wu, Q.; Howell, M.P.; Cowley, M.A.; Palmiter, R.D. Starvation after AgRP Neuron Ablation Is Independent of Melanocortin Signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 2687–2692.
- Chen, S.R.; Chen, H.; Zhou, J.J.; Pradhan, G.; Sun, Y.; Pan, H.L.; Li, D.P. Ghrelin Receptors Mediate Ghrelin-Induced Excitation of Agouti-Related Protein/Neuropeptide Y but Not pro-Opiomelanocortin Neurons. J. Neurochem. 2017, 142, 512–520.
- Rau, A.R.; Hentges, S.T. The Relevance of AgRP Neuron-Derived GABA Inputs to POMC Neurons Differs for Spontaneous and Evoked Release. J. Neurosci. 2017, 37, 7362–7372.
- Dutia, R.; Meece, K.; Dighe, S.; Kim, A.J.; Wardlaw, S.L. β-Endorphin Antagonizes the Effects of α-MSH on Food Intake and Body Weight. Endocrinology 2012, 153, 4246–4255.
- Kelly, M.J.; Loose, M.D.; Ronnekleiv, O.K. Opioids Hyperpolarize Beta-Endorphin Neurons via Mu-Receptor Activation of a Potassium Conductance. Neuroendocrinology 1990, 52, 268–275.
- Koch, M.; Varela, L.; Kim, J.G.; Kim, J.D.; Hernández-Nuño, F.; Simonds, S.E.; Castorena, C.M.; Vianna, C.R.; Elmquist, J.K.; Morozov, Y.M.; et al. Hypothalamic POMC Neurons Promote Cannabinoid-Induced Feeding. Nature 2015, 519, 45–50.
- Tolentino, L.; Iqbal, A.; Rahman, S.; Lutfy, K. The Role of Beta-Endorphin in Food Deprivation-Mediated Increases in Food Intake and Binge-Eating. Brain Sciences 2023, 13, 212.
- Panigrahi, S.K.; Meece, K.; Wardlaw, S.L. Effects of Naltrexone on Energy Balance and Hypothalamic Melanocortin Peptides in Male Mice Fed a High-Fat Diet. J. Endocr. Soc. 2019, 3, 590–601.
- Gordon, R.J.; Panigrahi, S.K.; Meece, K.; Atalayer, D.; Smiley, R.; Wardlaw, S.L. Effects of Opioid Antagonism on Cerebrospinal Fluid Melanocortin Peptides and Cortisol Levels in Humans. J. Endocr. Soc. 2017, 1, 1235–1246.
- Elias, C.F.; Kelly, J.F.; Lee, C.E.; Ahima, R.S.; Drucker, D.J.; Saper, C.B.; Elmquist, J.K. Chemical Characterization of Leptin-Activated Neurons in the Rat Brain. J. Comp. Neurol. 2000, 423, 261–281.
- Cowley, M.A.; Smart, J.L.; Rubinstein, M.; Cerdán, M.G.; Diano, S.; Horvath, T.L.; Cone, R.D.; Low, M.J. Leptin Activates Anorexigenic POMC Neurons through a Neural Network in the Arcuate Nucleus. Nature 2001, 411, 480–484.
- Korner, J.; Savontaus, E.; Chua, S.C.; Leibel, R.L.; Wardlaw, S.L. Leptin Regulation of Agrp and Npy MRNA in the Rat Hypothalamus. J. Neuroendocrinol. 2001, 13, 959–966.
- Takahashi, K.A.; Cone, R.D. Fasting Induces a Large, Leptin-Dependent Increase in the Intrinsic Action Potential Frequency of Orexigenic Arcuate Nucleus Neuropeptide Y/Agouti-Related Protein Neurons. Endocrinology 2005, 146, 1043–1047.
- Baver, S.B.; Hope, K.; Guyot, S.; Bjørbaek, C.; Kaczorowski, C.; O’Connell, K.M.S. Leptin Modulates the Intrinsic Excitability of AgRP/NPY Neurons in the Arcuate Nucleus of the Hypothalamus. J. Neurosci. 2014, 34, 5486–5496.
- Huang, H.; Kong, D.; Byun, K.H.; Ye, C.; Koda, S.; Lee, D.H.; Oh, B.C.; Lee, S.W.; Lee, B.; Zabolotny, J.M.; et al. Rho-Kinase Regulates Energy Balance by Targeting Hypothalamic Leptin Receptor Signaling. Nat. Neurosci. 2012, 15, 1391–1398.
- Huang, H.; Lee, S.H.; Ye, C.; Lima, I.S.; Oh, B.C.; Lowell, B.B.; Zabolotny, J.M.; Kim, Y.B. ROCK1 in AgRP Neurons Regulates Energy Expenditure and Locomotor Activity in Male Mice. Endocrinology 2013, 154, 3660–3670.
- Koch, L.; Wunderlich, F.T.; Seibler, J.; Könner, A.C.; Hampel, B.; Irlenbusch, S.; Brabant, G.; Kahn, C.R.; Schwenk, F.; Brüning, J.C. Central Insulin Action Regulates Peripheral Glucose and Fat Metabolism in Mice. J. Clin. Investig. 2008, 118, 2132–2147.
- Dodd, G.T.; Kim, S.J.; Méquinion, M.; Xirouchaki, C.E.; Brüning, J.C.; Andrews, Z.B.; Tiganis, T. Insulin Signaling in AgRP Neurons Regulates Meal Size to Limit Glucose Excursions and Insulin Resistance. Sci. Adv. 2021, 7, eabf4100.
- Balthasar, N.; Dalgaard, L.T.; Lee, C.E.; Yu, J.; Funahashi, H.; Williams, T.; Ferreira, M.; Tang, V.; McGovern, R.A.; Kenny, C.D.; et al. Divergence of Melanocortin Pathways in the Control of Food Intake and Energy Expenditure. Cell 2005, 123, 493–505.
- Könner, A.C.; Janoschek, R.; Plum, L.; Jordan, S.D.; Rother, E.; Ma, X.; Xu, C.; Enriori, P.; Hampel, B.; Barsh, G.S.; et al. Insulin Action in AgRP-Expressing Neurons Is Required for Suppression of Hepatic Glucose Production. Cell Metab. 2007, 5, 438–449.
- Andrews, Z.B.; Liu, Z.W.; Walllingford, N.; Erion, D.M.; Borok, E.; Friedman, J.M.; Tschöp, M.H.; Shanabrough, M.; Cline, G.; Shulman, G.I.; et al. UCP2 Mediates Ghrelin’s Action on NPY/AgRP Neurons by Lowering Free Radicals. Nature 2008, 454, 846–851.
- Guan, X.; Shi, X.; Li, X.; Chang, B.; Wang, Y.; Li, D.; Chan, L. GLP-2 Receptor in POMC Neurons Suppresses Feeding Behavior and Gastric Motility. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E853.
- Su, Z.; Alhadeff, A.L.; Betley, J.N. Nutritive, Post-Ingestive Signals Are the Primary Regulators of AgRP Neuron Activity. Cell Rep. 2017, 21, 2724–2736.
- Lutz, T.A.; Coester, B.; Whiting, L.; Dunn-Meynell, A.A.; Boyle, C.N.; Bouret, S.G.; Levin, B.E.; Le Foll, C. Amylin Selectively Signals onto POMC Neurons in the Arcuate Nucleus of the Hypothalamus. Diabetes 2018, 67, 805–817.
- Gouveia, A.; de Oliveira Beleza, R.; Steculorum, S.M. AgRP Neuronal Activity across Feeding-Related Behaviours. Eur. J. Neurosci. 2021, 54, 7458–7475.
- Üner, A.G.; Keçik, O.; Quaresma, P.G.F.; De Araujo, T.M.; Lee, H.; Li, W.; Kim, H.J.; Chung, M.; Bjørbæk, C.; Kim, Y.B. Role of POMC and AgRP Neuronal Activities on Glycaemia in Mice. Sci. Rep. 2019, 9, 13068.
- Iwen, K.A.; Oelkrug, R.; Brabant, G. Effects of Thyroid Hormones on Thermogenesis and Energy Partitioning. J. Mol. Endocrinol. 2018, 60, R157–R170.
- Fekete, C.; Kelly, J.; Mihály, E.; Sarkar, S.; Rand, W.M.; Légrádi, G.; Emerson, C.H.; Lechan, R.M. Neuropeptide Y Has a Central Inhibitory Action on the Hypothalamic-Pituitary-Thyroid Axis. Endocrinology 2001, 142, 2606–2613.
- Kim, M.S.; Small, C.J.; Stanley, S.A.; Morgan, D.G.A.; Seal, L.J.; Kong, W.M.; Edwards, C.M.B.; Abusnana, S.; Sunter, D.; Ghatei, M.A.; et al. The Central Melanocortin System Affects the Hypothalamo-Pituitary Thyroid Axis and May Mediate the Effect of Leptin. J. Clin. Investig. 2000, 105, 1005–1011.
- Fekete, C.; Sarkar, S.; Rand, W.M.; Harney, J.W.; Emerson, C.H.; Bianco, A.C.; Lechan, R.M. Agouti-Related Protein (AGRP) Has a Central Inhibitory Action on the Hypothalamic-Pituitary-Thyroid (HPT) Axis; Comparisons between the Effect of AGRP and Neuropeptide Y on Energy Homeostasis and the HPT Axis. Endocrinology 2002, 143, 3846–3853.
- Martin, N.M.; Smith, K.L.; Bloom, S.R.; Small, C.J. Interactions between the Melanocortin System and the Hypothalamo–Pituitary–Thyroid Axis. Peptides 2006, 27, 333–339.
- Wang, C.; He, Y.; Xu, P.; Yang, Y.; Saito, K.; Xia, Y.; Yan, X.; Hinton, A.; Yan, C.; Ding, H.; et al. TAp63 Contributes to Sexual Dimorphism in POMC Neuron Functions and Energy Homeostasis. Nat. Commun. 2018, 9, 1544.
- Xu, Y.; Nedungadi, T.P.; Zhu, L.; Sobhani, N.; Irani, B.G.; Davis, K.E.; Zhang, X.; Zou, F.; Gent, L.M.; Hahner, L.D.; et al. Distinct Hypothalamic Neurons Mediate Estrogenic Effects on Energy Homeostasis and Reproduction. Cell Metab. 2011, 14, 453–465.
- Yang, Y.; van der Klaauw, A.A.; Zhu, L.; Cacciottolo, T.M.; He, Y.; Stadler, L.K.J.; Wang, C.; Xu, P.; Saito, K.; Hinton, A.; et al. Steroid Receptor Coactivator-1 Modulates the Function of Pomc Neurons and Energy Homeostasis. Nat. Commun. 2019, 10, 1718.
- Xu, A.W.; Ste-Marie, L.; Kaelin, C.B.; Barsh, G.S. Inactivation of Signal Transducer and Activator of Transcription 3 in Proopiomelanocortin (Pomc) Neurons Causes Decreased Pomc Expression, Mild Obesity, and Defects in Compensatory Refeeding. Endocrinology 2007, 148, 72–80.
- Yu, M.; Bean, J.C.; Liu, H.; He, Y.; Yang, Y.; Cai, X.; Yu, K.; Pei, Z.; Liu, H.; Tu, L.; et al. SK3 in POMC Neurons Plays a Sexually Dimorphic Role in Energy and Glucose Homeostasis. Cell Biosci. 2022, 12, 170.
- Pei, Z.; He, Y.; Bean, J.C.; Yang, Y.; Liu, H.; Yu, M.; Yu, K.; Hyseni, I.; Cai, X.; Liu, H.; et al. Gabra5 Plays a Sexually Dimorphic Role in POMC Neuron Activity and Glucose Balance. Front. Endocrinol. 2022, 13, 889122.
- Li, Y.; Zhu, S.; Du, D.; Li, Q.; Xie, K.; Chen, L.; Feng, X.; Wu, X.; Sun, Z.; Zhou, J.; et al. TLR4 in POMC Neurons Regulates Thermogenesis in a Sex-Dependent Manner. J. Lipid Res. 2023, 64, 100368.
- Nuzzaci, D.; Laderrière, A.; Lemoine, A.; Nédélec, E.; Pénicaud, L.; Rigault, C.; Benani, A. Plasticity of the Melanocortin System: Determinants and Possible Consequences on Food Intake. Front. Endocrinol. 2015, 6, 143.
- Benani, A.; Hryhorczuk, C.; Gouazé, A.; Fioramonti, X.; Brenachot, X.; Guissard, C.; Krezymon, A.; Duparc, T.; Colom, A.; Nédélec, E.; et al. Food Intake Adaptation to Dietary Fat Involves PSA-Dependent Rewiring of the Arcuate Melanocortin System in Mice. J. Neurosci. 2012, 32, 11970–11979.
- Liu, T.; Kong, D.; Shah, B.P.; Ye, C.; Koda, S.; Saunders, A.; Ding, J.B.; Yang, Z.; Sabatini, B.L.; Lowell, B.B. Fasting Activation of AgRP Neurons Requires NMDA Receptors and Involves Spinogenesis and Increased Excitatory Tone. Neuron 2012, 73, 511–522.
- Nuzzaci, D.; Cansell, C.; Liénard, F.; Nédélec, E.; Ben Fradj, S.; Castel, J.; Foppen, E.; Denis, R.; Grouselle, D.; Laderrière, A.; et al. Postprandial Hyperglycemia Stimulates Neuroglial Plasticity in Hypothalamic POMC Neurons after a Balanced Meal. Cell Rep. 2020, 30, 3067–3078.e5.
- McNay, D.E.G.; Briançon, N.; Kokoeva, M.V.; Maratos-Flier, E.; Flier, J.S. Remodeling of the Arcuate Nucleus Energy-Balance Circuit Is Inhibited in Obese Mice. J. Clin. Investig. 2012, 122, 142.
- Horvath, T.L.; Sarman, B.; García-Cáceres, C.; Enriori, P.J.; Sotonyi, P.; Shanabrough, M.; Borok, E.; Argente, J.; Chowen, J.A.; Perez-Tilve, D.; et al. Synaptic Input Organization of the Melanocortin System Predicts Diet-Induced Hypothalamic Reactive Gliosis and Obesity. Proc. Natl. Acad. Sci. USA 2010, 107, 14875–14880.
- Lee, D.A.; Bedont, J.L.; Pak, T.; Wang, H.; Song, J.; Miranda-Angulo, A.; Takiar, V.; Charubhumi, V.; Balordi, F.; Takebayashi, H.; et al. Tanycytes of the Hypothalamic Median Eminence Form a Diet-Responsive Neurogenic Niche. Nat. Neurosci. 2012, 15, 700–702.
- Lee, D.A.; Yoo, S.; Pak, T.; Salvatierra, J.; Velarde, E.; Aja, S.; Blackshaw, S. Dietary and Sex-Specific Factors Regulate Hypothalamic Neurogenesis in Young Adult Mice. Front. Neurosci. 2014, 8, 157.
- Jacobowitz, D.M.; O’Donohue, T.L. α-Melanocyte Stimulating Hormone: Immunohistochemical Identification and Mapping in Neurons of Rat Brain. Proc. Natl. Acad. Sci. USA 1978, 75, 6300–6304.
- Mountjoy, K.G.; Mortrud, M.T.; Low, M.J.; Simerly, R.B.; Cone, R.D. Localization of the Melanocortin-4 Receptor (MC4-R) in Neuroendocrine and Autonomic Control Circuits in the Brain. Mol. Endocrinol. 1994, 8, 1298–1308.
- Lu, D.; Willard, D.; Patel, I.R.; Kadwell, S.; Overton, L.; Kost, T.; Luther, M.; Chen, W.; Woychik, R.P.; Wilkison, W.O.; et al. Agouti Protein Is an Antagonist of the Melanocyte-Stimulating-Hormone Receptor. Nature 1994, 371, 799–802.
- Ollmann, M.M.; Wilson, B.D.; Yang, Y.K.; Kerns, J.A.; Chen, Y.; Gantz, I.; Barsh, G.S. Antagonism of Central Melanocortin Receptors in Vitro and in Vivo by Agouti-Related Protein. Science 1997, 278, 135–138.
- Michaud, J.L.; Rosenquist, T.; May, N.R.; Fan, C.M. Development of Neuroendocrine Lineages Requires the BHLH-PAS Transcription Factor SIM1. Genes Dev. 1998, 12, 3264–3275.
- Michaud, J.L.; Boucher, F.; Melnyk, A.; Gauthier, F.; Goshu, E.; Lévy, E.; Mitchell, G.A.; Himms-Hagen, J.; Fan, C.M. Sim1 Haploinsufficiency Causes Hyperphagia, Obesity and Reduction of the Paraventricular Nucleus of the Hypothalamus. Hum. Mol. Genet. 2001, 10, 1465–1473.
- Tolson, K.P.; Gemelli, T.; Meyer, D.; Yazdani, U.; Kozlitina, J.; Zinn, A.R. Inducible Neuronal Inactivation of Sim1 in Adult Mice Causes Hyperphagic Obesity. Endocrinology 2014, 155, 2436–2444.
- Hinney, A.; Schmidt, A.; Nottebom, K.; Heibült, O.; Becker, I.; Ziegler, A.; Gerber, G.; Sina, M.; Görg, T.; Mayer, H.; et al. Several Mutations in the Melanocortin-4 Receptor Gene Including a Nonsense and a Frameshift Mutation Associated with Dominantly Inherited Obesity in Humans. J. Clin. Endocrinol. Metab. 1999, 84, 1483–1486.
- Vaisse, C.; Clement, K.; Durand, E.; Hercberg, S.; Guy-Grand, B.; Froguel, P. Melanocortin-4 Receptor Mutations Are a Frequent and Heterogeneous Cause of Morbid Obesity. J. Clin. Investig. 2000, 106, 253–262.
- Farooqi, I.S.; Keogh, J.M.; Yeo, G.S.H.; Lank, E.J.; Cheetham, T.; O’Rahilly, S. Clinical Spectrum of Obesity and Mutations in the Melanocortin 4 Receptor Gene. N. Engl. J. Med. 2003, 348, 1085–1095.
- Loos, R.J.F.; Lindgren, C.M.; Li, S.; Wheeler, E.; Hua Zhao, J.; Prokopenko, I.; Inouye, M.; Freathy, R.M.; Attwood, A.P.; Beckmann, J.S.; et al. Common Variants near MC4R Are Associated with Fat Mass, Weight and Risk of Obesity. Nat. Genet. 2008, 40, 768–775.
- Li, Y.Q.; Shrestha, Y.; Pandey, M.; Chen, M.; Kablan, A.; Gavrilova, O.; Offermanns, S.; Weinstein, L.S. Gq/11α and Gsα Mediate Distinct Physiological Responses to Central Melanocortins. J. Clin. Investig. 2016, 126, 40–49.
- Garfield, A.S.; Li, C.; Madara, J.C.; Shah, B.P.; Webber, E.; Steger, J.S.; Campbell, J.N.; Gavrilova, O.; Lee, C.E.; Olson, D.P.; et al. A Neural Basis for Melanocortin-4 Receptor Regulated Appetite. Nat. Neurosci. 2015, 18, 863.
- Matsumura, S.; Miyakita, M.; Miyamori, H.; Kyo, S.; Shima, D.; Yokokawa, T.; Ishikawa, F.; Sasaki, T.; Jinno, T.; Tanaka, J.; et al. Stimulation of GSsignaling in MC4R Cells by DREADD Increases Energy Expenditure, Suppresses Food Intake, and Increases Locomotor Activity in Mice. Am. J. Physiol. Endocrinol. Metab. 2022, 322, E436–E445.
- Chen, K.Y.; Muniyappa, R.; Abel, B.S.; Mullins, K.P.; Staker, P.; Brychta, R.J.; Zhao, X.; Ring, M.; Psota, T.L.; Cone, R.D.; et al. RM-493, a Melanocortin-4 Receptor (MC4R) Agonist, Increases Resting Energy Expenditure in Obese Individuals. J. Clin. Endocrinol. Metab. 2015, 100, 1639–1645.
- Wang, X.; Cui, X.; Li, Y.; Li, F.; Li, Y.; Dai, J.; Hu, H.; Wang, X.; Sun, J.; Yang, Y.; et al. MC4R Deficiency Causes Dysregulation of Postsynaptic Excitatory Synaptic Transmission as a Crucial Culprit for Obesity. Diabetes 2022, 71, 2331–2343.
- Podyma, B.; Sun, H.; Wilson, E.A.; Carlson, B.; Pritikin, E.; Gavrilova, O.; Weinstein, L.S.; Chen, M. The Stimulatory G Protein Gsα Is Required in Melanocortin 4 Receptor–Expressing Cells for Normal Energy Balance, Thermogenesis, and Glucose Metabolism. J. Biol. Chem. 2018, 293, 10993.
- Kuo, J.J.; Silva, A.A.; Hall, J.E. Hypothalamic Melanocortin Receptors and Chronic Regulation of Arterial Pressure and Renal Function. Hypertension 2003, 41, 768–774.
- Sohn, J.W.; Harris, L.E.; Berglund, E.D.; Liu, T.; Vong, L.; Lowell, B.B.; Balthasar, N.; Williams, K.W.; Elmquist, J.K. Melanocortin 4 Receptors Reciprocally Regulate Sympathetic and Parasympathetic Preganglionic Neurons. Cell 2013, 152, 612–619.
- Iwasa, M.; Kawabe, K.; Sapru, H.N. Activation of Melanocortin Receptors in the Intermediolateral Cell Column of the Upper Thoracic Cord Elicits Tachycardia in the Rat. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H885.
- Berglund, E.D.; Liu, T.; Kong, X.; Sohn, J.W.; Vong, L.; Deng, Z.; Lee, C.E.; Lee, S.; Williams, K.W.; Olson, D.P.; et al. Melanocortin 4 Receptors in Autonomic Neurons Regulate Thermogenesis and Glycemia. Nat. Neurosci. 2014, 17, 911–913.
- Li, M.M.; Madara, J.C.; Steger, J.S.; Krashes, M.J.; Balthasar, N.; Campbell, J.N.; Resch, J.M.; Conley, N.J.; Garfield, A.S.; Lowell, B.B. The Paraventricular Hypothalamus Regulates Satiety and Prevents Obesity via Two Genetically Distinct Circuits. Neuron 2019, 102, 653–667.
- An, J.J.; Liao, G.Y.; Kinney, C.E.; Sahibzada, N.; Xu, B. Discrete BDNF Neurons in the Paraventricular Hypothalamus Control Feeding and Energy Expenditure. Cell Metab. 2015, 22, 175–188.
- Li, C.; Navarrete, J.; Liang-Guallpa, J.; Lu, C.; Funderburk, S.C.; Chang, R.B.; Liberles, S.D.; Olson, D.P.; Krashes, M.J. Defined Paraventricular Hypothalamic Populations Exhibit Differential Responses to Food Contingent on Caloric State. Cell Metab. 2019, 29, 681–694.e5.
- Varela, L.; Horvath, T.L. Parallel Paths in PVH Control of Feeding. Neuron 2019, 102, 514–516.
- An, J.J.; Kinney, C.E.; Tan, J.W.; Liao, G.Y.; Kremer, E.J.; Xu, B. TrkB-Expressing Paraventricular Hypothalamic Neurons Suppress Appetite through Multiple Neurocircuits. Nat. Commun. 2020, 11, 1729.
- Krashes, M.J.; Lowell, B.B.; Garfield, A.S. Melanocortin-4 Receptor–Regulated Energy Homeostasis. Nat. Neurosci. 2016, 19, 206–219.
- Baldini, G.; Phelan, K.D. The Melanocortin Pathway and Control of Appetite-Progress and Therapeutic Implications. J. Endocrinol. 2019, 241, R1–R33.
- King, B.M. The Rise, Fall, and Resurrection of the Ventromedial Hypothalamus in the Regulation of Feeding Behavior and Body Weight. Physiol. Behav. 2006, 87, 221–244.
- Becker, E.E.; Kissileff, H.R. Inhibitory Controls of Feeding by the Ventromedial Hypothalamus. Am. J. Physiol. 1974, 226, 383–396.
- Maes, H. Time Course of Feeding Induced by Pentobarbital-Injections into the Rat’s VMH. Physiol. Behav. 1980, 24, 1107–1114.
- Gaur, A.; Pal, G.K.; Ananthanarayanan, P.H.; Pal, P. Role of Ventromedial Hypothalamus in High Fat Diet Induced Obesity in Male Rats: Association with Lipid Profile, Thyroid Profile and Insulin Resistance. Ann. Neurosci. 2014, 21, 104–107.
- Wang, Q.; Zhang, B.; Stutz, B.; Liu, Z.W.; Horvath, T.L.; Yang, X. Ventromedial Hypothalamic OGT Drives Adipose Tissue Lipolysis and Curbs Obesity. Sci. Adv. 2022, 8, eabn8092.
- Viskaitis, P.; Irvine, E.E.; Smith, M.A.; Choudhury, A.I.; Alvarez-Curto, E.; Glegola, J.A.; Hardy, D.G.; Pedroni, S.M.A.; Paiva Pessoa, M.R.; Fernando, A.B.P.; et al. Modulation of SF1 Neuron Activity Coordinately Regulates Both Feeding Behavior and Associated Emotional States. Cell Rep. 2017, 21, 3559–3572.
- Coutinho, E.A.; Okamoto, S.; Ishikawa, A.W.; Yokota, S.; Wada, N.; Hirabayashi, T.; Saito, K.; Sato, T.; Takagi, K.; Wang, C.C.; et al. Activation of SF1 Neurons in the Ventromedial Hypothalamus by DREADD Technology Increases Insulin Sensitivity in Peripheral Tissues. Diabetes 2017, 66, 2372–2386.
- Sternson, S.M.; Shepherd, G.M.G.; Friedman, J.M. Topographic Mapping of VMH → Arcuate Nucleus Microcircuits and Their Reorganization by Fasting. Nat. Neurosci. 2005, 8, 1356–1363.
- Zhang, J.; Chen, D.; Sweeney, P.; Yang, Y. An Excitatory Ventromedial Hypothalamus to Paraventricular Thalamus Circuit That Suppresses Food Intake. Nat. Commun. 2020, 11, 6326.
- Dhillon, H.; Zigman, J.M.; Ye, C.; Lee, C.E.; McGovern, R.A.; Tang, V.; Kenny, C.D.; Christiansen, L.M.; White, R.D.; Edelstein, E.A.; et al. Leptin Directly Activates SF1 Neurons in the VMH, and This Action by Leptin Is Required for Normal Body-Weight Homeostasis. Neuron 2006, 49, 191–203.
- Meek, T.H.; Nelson, J.T.; Matsen, M.E.; Dorfman, M.D.; Guyenet, S.J.; Damian, V.; Allison, M.B.; Scarlett, J.M.; Nguyen, H.T.; Thaler, J.P.; et al. Functional Identification of a Neurocircuit Regulating Blood Glucose. Proc. Natl. Acad. Sci. USA 2016, 113, E2073–E2082.
- Fosch, A.; Zagmutt, S.; Casals, N.; Rodríguez-Rodríguez, R. New Insights of SF1 Neurons in Hypothalamic Regulation of Obesity and Diabetes. Int. J. Mol. Sci. 2021, 22, 22.
- Bellinger, L.L.; Bernardis, L.L. The Dorsomedial Hypothalamic Nucleus and Its Role in Ingestive Behavior and Body Weight Regulation: Lessons Learned from Lesioning Studies. Physiol. Behav. 2002, 76, 431–442.
- Kesterson, R.A.; Huszar, D.; Lynch, C.A.; Simerly, R.B.; Cone, R.D. Induction of Neuropeptide Y Gene Expression in the Dorsal Medial Hypothalamic Nucleus in Two Models of the Agouti Obesity Syndrome. Mol. Endocrinol. 1997, 11, 630–637.
- Guan, X.M.; Yu, H.; Van Der Ploeg, L.H.T. Evidence of Altered Hypothalamic Pro-Opiomelanocortin/Neuropeptide Y MRNA Expression in Tubby Mice. Mol. Brain Res. 1998, 59, 273–279.
- Bi, S.; Ladenheim, E.E.; Schwartz, G.J.; Moran, T.H. A Role for NPY Overexpression in the Dorsomedial Hypothalamus in Hyperphagia and Obesity of OLETF Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R254–R260.
- Yang, L.; Scott, K.A.; Hyun, J.; Tamashiro, K.L.; Tray, N.; Moran, T.H.; Bi, S. Role of Dorsomedial Hypothalamic Neuropeptide Y in Modulating Food Intake and Energy Balance. J. Neurosci. 2009, 29, 179–190.
- Otgon-Uul, Z.; Suyama, S.; Onodera, H.; Yada, T. Optogenetic Activation of Leptin- and Glucose-Regulated GABAergic Neurons in Dorsomedial Hypothalamus Promotes Food Intake via Inhibitory Synaptic Transmission to Paraventricular Nucleus of Hypothalamus. Mol. Metab. 2016, 5, 709–715.
- Chen, J.; Scott, K.A.; Zhao, Z.; Moran, T.H.; Bi, S. Characterization of the Feeding Inhibition and Neural Activation Produced by Dorsomedial Hypothalamic Cholecystokinin Administration. Neuroscience 2008, 152, 178–188.
- Rust, V.A.; Crosby, K.M. Cholecystokinin Acts in the Dorsomedial Hypothalamus of Young Male Rats to Suppress Appetite in a Nitric Oxide-Dependent Manner. Neurosci. Lett. 2021, 764, 136295.
- Imoto, D.; Yamamoto, I.; Matsunaga, H.; Yonekura, T.; Lee, M.L.; Kato, K.X.; Yamasaki, T.; Xu, S.; Ishimoto, T.; Yamagata, S.; et al. Refeeding Activates Neurons in the Dorsomedial Hypothalamus to Inhibit Food Intake and Promote Positive Valence. Mol. Metab. 2021, 54, 101366.
- Han, Y.; He, Y.; Harris, L.; Xu, Y.; Wu, Q. Identification of a GABAergic Neural Circuit Governing Leptin Signaling Deficiency-Induced Obesity. Elife 2023, 12, e82649.
- Haspula, D.; Clark, M.A. Neuroinflammation and Sympathetic Overactivity: Mechanisms and Implications in Hypertension. Auton. Neurosci. 2018, 210, 10–17.
- Grill, H.J.; Norgren, R. Chronically Decerebrate Rats Demonstrate Satiation but Not Bait Shyness. Science 1978, 201, 267–269.
- Dirocco, R.J.; Grill, H.J. The Forebrain Is Not Essential for Sympathoadrenal Hyperglycemic Response to Glucoprivation. Science 1979, 204, 1112–1114.
- Jeong, J.K.; Dow, S.A.; Young, C.N. Sensory Circumventricular Organs, Neuroendocrine Control, and Metabolic Regulation. Metabolites 2021, 11, 494.
- Watts, A.G.; Kanoski, S.E.; Sanchez-Watts, G.; Langhans, W. The Physiological Control of Eating: Signals, Neurons, and Networks. Physiol. Rev. 2022, 102, 689–813.
- Grill, H.J.; Hayes, M.R. The Nucleus Tractus Solitarius: A Portal for Visceral Afferent Signal Processing, Energy Status Assessment and Integration of Their Combined Effects on Food Intake. Int. J. Obes. 2009, 33 (Suppl. 1), S11–S15.
- Kandalam, U.; Sarmiento, N.; Haspula, D.; Clark, M.A. Angiotensin III Induces Signal Transducer and Activator of Transcription 3 and Interleukin-6 MRNA Levels in Cultured Rat Astrocytes. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 758–767.
- Haspula, D.; Clark, M.A. MAPK Activation Patterns of AT1R and CB1R in SHR versus Wistar Astrocytes: Evidence of CB1R Hypofunction and Crosstalk between AT1R and CB1R. Cell. Signal. 2017, 40, 81–90.
- Haspula, D.; Clark, M.A. Molecular Basis of the Brain Renin Angiotensin System in Cardiovascular and Neurologic Disorders: Uncovering a Key Role for the Astroglial Angiotensin Type 1 Receptor AT1R. J. Pharmacol. Exp. Ther. 2018, 366, 251–264.
- O’Connor, A.T.; Haspula, D.; Alanazi, A.Z.; Clark, M.A. Roles of Angiotensin III in the Brain and Periphery. Peptides 2022, 153, 170802.
- Moura-Assis, A.; Friedman, J.M.; Velloso, L.A. Gut-to-Brain Signals in Feeding Control. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E326.
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A Gut-Brain Neural Circuit for Nutrient Sensory Transduction. Science 2018, 361, eaat5236.
- Owyang, C.; Heldsinger, A. Vagal Control of Satiety and Hormonal Regulation of Appetite. J. Neurogastroenterol. Motil. 2011, 17, 338–348.
- Berthoud, H.R.; Neuhuber, W.L. Vagal Mechanisms as Neuromodulatory Targets for the Treatment of Metabolic Disease. Ann. N. Y. Acad. Sci. 2019, 1454, 42–55.
- Gibbs, J.; Young, R.C.; Smith, G.P. Cholecystokinin Decreases Food Intake in Rats. J. Comp. Physiol. Psychol. 1973, 84, 488–495.
- Widdop, R.E.; Krstew, E.; Mercer, L.D.; Carlsberg, M.; Beart, P.M.; Jarrott, B. Electrophysiological and Autoradiographical Evidence for Cholecystokinin A Receptors on Rat Isolated Nodose Ganglia. J. Auton. Nerv. Syst. 1994, 46, 65–73.
- Schwartz, G.J.; Moran, T.H. CCK Elicits and Modulates Vagal Afferent Activity Arising from Gastric and Duodenal Sites. Ann. N. Y. Acad. Sci. 1994, 713, 121–128.
- Leon Mercado, L.; Caron, A.; Wang, Y.; Burton, M.; Gautron, L. Identification of Leptin Receptor–Expressing Cells in the Nodose Ganglion of Male Mice. Endocrinology 2019, 160, 1307–1322.
- Barrachina, M.D.; Martínez, V.; Wang, L.; Wei, J.Y.; Taché, Y. Synergistic Interaction between Leptin and Cholecystokinin to Reduce Short-Term Food Intake in Lean Mice. Proc. Natl. Acad. Sci. USA 1997, 94, 10455.
- Brierley, D.I.; de Lartigue, G. Reappraising the Role of the Vagus Nerve in GLP-1-Mediated Regulation of Eating. Br. J. Pharmacol. 2022, 179, 584–599.
- Campbell, J.E.; Drucker, D.J. Pharmacology, Physiology, and Mechanisms of Incretin Hormone Action. Cell Metab. 2013, 17, 819–837.
- Krieger, J.P.; Arnold, M.; Pettersen, K.G.; Lossel, P.; Langhans, W.; Lee, S.J. Knockdown of GLP-1 Receptors in Vagal Afferents Affects Normal Food Intake and Glycemia. Diabetes 2016, 65, 34–43.
- Bai, L.; Mesgarzadeh, S.; Ramesh, K.S.; Huey, E.L.; Liu, Y.; Gray, L.A.; Aitken, T.J.; Chen, Y.; Beutler, L.R.; Ahn, J.S.; et al. Genetic Identification of Vagal Sensory Neurons That Control Feeding. Cell 2019, 179, 1129–1143.e23.
- Egerod, K.L.; Petersen, N.; Timshel, P.N.; Rekling, J.C.; Wang, Y.; Liu, Q.; Schwartz, T.W.; Gautron, L. Profiling of G Protein-Coupled Receptors in Vagal Afferents Reveals Novel Gut-to-Brain Sensing Mechanisms. Mol. Metab. 2018, 12, 62–75.
- Baumgartner, I.; Pacheco-López, G.; Rüttimann, E.B.; Arnold, M.; Asarian, L.; Langhans, W.; Geary, N.; Hillebrand, J.J.G. Hepatic-Portal Vein Infusions of Glucagon-like Peptide-1 Reduce Meal Size and Increase c-Fos Expression in the Nucleus Tractus Solitarii, Area Postrema and Central Nucleus of the Amygdala in Rats. J. Neuroendocrinol. 2010, 22, 557–563.
- Campos, C.A.; Wright, J.S.; Czaja, K.; Ritter, R.C. CCK-Induced Reduction of Food Intake and Hindbrain MAPK Signaling Are Mediated by NMDA Receptor Activation. Endocrinology 2012, 153, 2633–2646.
- Punjabi, M.; Arnold, M.; Rüttimann, E.; Graber, M.; Geary, N.; Pacheco-López, G.; Langhans, W. Circulating Glucagon-like Peptide-1 (GLP-1) Inhibits Eating in Male Rats by Acting in the Hindbrain and Without Inducing Avoidance. Endocrinology 2014, 155, 1690–1699.
- Barrera, J.G.; Jones, K.R.; Herman, J.P.; D’Alessio, D.A.; Woods, S.C.; Seeley, R.J. Hyperphagia and Increased Fat Accumulation in Two Models of Chronic CNS Glucagon-Like Peptide-1 Loss of Function. J. Neurosci. 2011, 31, 3904–3913.
- Finan, B.; Ma, T.; Ottaway, N.; Müller, T.D.; Habegger, K.M.; Heppner, K.M.; Kirchner, H.; Holland, J.; Hembree, J.; Raver, C.; et al. Unimolecular Dual Incretins Maximize Metabolic Benefits in Rodents, Monkeys, and Humans. Sci. Transl. Med. 2013, 5, 209ra151.
- Zhang, C.; Vincelette, L.K.; Reimann, F.; Liberles, S.D. A Brainstem Circuit for Nausea Suppression. Cell Rep. 2022, 39, 110953.
- Samms, R.J.; Cosgrove, R.; Snider, B.M.; Furber, E.C.; Droz, B.A.; Briere, D.A.; Dunbar, J.; Dogra, M.; Alsina-Fernandez, J.; Borner, T.; et al. GIPR Agonism Inhibits PYY-Induced Nausea-Like Behavior. Diabetes 2022, 71, 1410–1423.
- Lutz, T.A.; Mollet, A.; Rushing, P.A.; Riediger, T.; Scharrer, E. The Anorectic Effect of a Chronic Peripheral Infusion of Amylin Is Abolished in Area Postrema/Nucleus of the Solitary Tract (AP/NTS) Lesioned Rats. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1005–1011.
- Halatchev, I.G.; Cone, R.D. Peripheral Administration of PYY(3-36) Produces Conditioned Taste Aversion in Mice. Cell Metab. 2005, 1, 159–168.
- Woods, S.C.; Lutz, T.A.; Geary, N.; Langhans, W. Pancreatic Signals Controlling Food Intake; Insulin, Glucagon and Amylin. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1219.
- Braegger, F.E.; Asarian, L.; Dahl, K.; Lutz, T.A.; Boyle, C.N. The Role of the Area Postrema in the Anorectic Effects of Amylin and Salmon Calcitonin: Behavioral and Neuronal Phenotyping. Eur. J. Neurosci. 2014, 40, 3055–3066.
- Coester, B.; Le Foll, C.; Lutz, T.A. Viral Depletion of Calcitonin Receptors in the Area Postrema: A Proof-of-Concept Study. Physiol. Behav. 2020, 223, 112992.
- Scott, M.M.; Williams, K.W.; Rossi, J.; Lee, C.E.; Elmquist, J.K. Leptin Receptor Expression in Hindbrain Glp-1 Neurons Regulates Food Intake and Energy Balance in Mice. J. Clin. Investig. 2011, 121, 2413–2421.
- Kanoski, S.E.; Zhao, S.; Guarnieri, D.J.; DiLeone, R.J.; Yan, J.; De Jonghe, B.C.; Bence, K.K.; Hayes, M.R.; Grill, H.J. Endogenous Leptin Receptor Signaling in the Medial Nucleus Tractus Solitarius Affects Meal Size and Potentiates Intestinal Satiation Signals. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E496–E503.
- Cheng, W.; Ndoka, E.; Hutch, C.; Roelofs, K.; MacKinnon, A.; Khoury, B.; Magrisso, J.; Kim, K.S.; Rhodes, C.J.; Olson, D.P.; et al. Leptin Receptor-Expressing Nucleus Tractus Solitarius Neurons Suppress Food Intake Independently of GLP1 in Mice. JCI Insight. 2020, 5, e134359.
- Venkatraman, A.; Edlow, B.L.; Immordino-Yang, M.H. The Brainstem in Emotion: A Review. Front. Neuroanat. 2017, 11, 15.
- Vander Weele, C.M.; Siciliano, C.A.; Matthews, G.A.; Namburi, P.; Izadmehr, E.M.; Espinel, I.C.; Nieh, E.H.; Schut, E.H.S.; Padilla-Coreano, N.; Burgos-Robles, A.; et al. Dopamine Enhances Signal-to-Noise Ratio in Cortical-Brainstem Encoding of Aversive Stimuli. Nature 2018, 563, 397–401.
- Wu, Q.; Clark, M.S.; Palmiter, R.D. Deciphering a Neuronal Circuit That Mediates Appetite. Nature 2012, 483, 594–597.
- Roman, C.W.; Derkach, V.A.; Palmiter, R.D. Genetically and Functionally Defined NTS to PBN Brain Circuits Mediating Anorexia. Nat. Commun. 2016, 7, 11905.
- D’Agostino, G.; Lyons, D.J.; Cristiano, C.; Burke, L.K.; Madara, J.C.; Campbell, J.N.; Garcia, A.P.; Land, B.B.; Lowell, B.B.; Dileone, R.J.; et al. Appetite Controlled by a Cholecystokinin Nucleus of the Solitary Tract to Hypothalamus Neurocircuit. eLife 2016, 5, e12225.
- Roman, C.W.; Sloat, S.R.; Palmiter, R.D. A Tale of Two Circuits: CCKNTS Neuron Stimulation Controls Appetite and Induces Opposing Motivational States by Projections to Distinct Brain Regions. Neuroscience 2017, 358, 316–324.
- Cheng, W.; Gonzalez, I.; Pan, W.; Tsang, A.H.; Adams, J.; Ndoka, E.; Gordian, D.; Khoury, B.; Roelofs, K.; Evers, S.S.; et al. Calcitonin Receptor Neurons in the Mouse Nucleus Tractus Solitarius Control Energy Balance via the Non-Aversive Suppression of Feeding. Cell Metab. 2020, 31, 301–312.e5.
- Larsen, P.J.; Tang-Christensen, M.; Holst, J.J.; Ørskov, C. Distribution of Glucagon-like Peptide-1 and Other Preproglucagon-Derived Peptides in the Rat Hypothalamus and Brainstem. Neuroscience 1997, 77, 257–270.
- Alhadeff, A.L.; Rupprecht, L.E.; Hayes, M.R. GLP-1 Neurons in the Nucleus of the Solitary Tract Project Directly to the Ventral Tegmental Area and Nucleus Accumbens to Control for Food Intake. Endocrinology 2012, 153, 647–658.
- Palkovits, M.; Eskay, R.L. Distribution and Possible Origin of β-Endorphin and ACTH in Discrete Brainstem Nuclei of Rats. Neuropeptides 1987, 9, 123–137.
- Bronstein, D.M.; Schafer, M.K.H.; Watson, S.J.; Akil, H. Evidence That Beta-Endorphin Is Synthesized in Cells in the Nucleus Tractus Solitarius: Detection of POMC MRNA. Brain Res 1992, 587, 269–275.
- Appleyard, S.M.; Bailey, T.W.; Doyle, M.W.; Jin, Y.H.; Smart, J.L.; Low, M.J.; Andresen, M.C. Proopiomelanocortin Neurons in Nucleus Tractus Solitarius Are Activated by Visceral Afferents: Regulation by Cholecystokinin and Opioids. J. Neurosci. 2005, 25, 3578–3585.
- Georgescu, T.; Lyons, D.; Doslikova, B.; Garcia, A.P.; Marston, O.; Burke, L.K.; Chianese, R.; Lam, B.Y.H.; Yeo, G.S.H.; Rochford, J.J.; et al. Neurochemical Characterization of Brainstem Pro-Opiomelanocortin Cells. Endocrinology 2020, 161, bqaa032.
- D’Agostino, G.; Lyons, D.; Cristiano, C.; Lettieri, M.; Olarte-Sanchez, C.; Burke, L.K.; Greenwald-Yarnell, M.; Cansell, C.; Doslikova, B.; Georgescu, T.; et al. Nucleus of the Solitary Tract Serotonin 5-HT2C Receptors Modulate Food Intake. Cell Metab. 2018, 28, 619–630.e5.
- Wagner, S.; Brierley, D.I.; Leeson-Payne, A.; Jiang, W.; Chianese, R.; Lam, B.Y.H.; Dowsett, G.K.C.; Cristiano, C.; Lyons, D.; Reimann, F.; et al. Obesity Medication Lorcaserin Activates Brainstem GLP-1 Neurons to Reduce Food Intake and Augments GLP-1 Receptor Agonist Induced Appetite Suppression. Mol. Metab. 2023, 68, 101665.
- Ritter, S.; Dinh, T.T.; Zhang, Y. Localization of Hindbrain Glucoreceptive Sites Controlling Food Intake and Blood Glucose. Brain Res. 2000, 856, 37–47.
- Boychuk, C.R.; Smith, K.C.; Peterson, L.E.; Boychuk, J.A.; Butler, C.R.; Derera, I.D.; McCarthy, J.J.; Smith, B.N. A Hindbrain Inhibitory Microcircuit Mediates Vagally-Coordinated Glucose Regulation. Sci. Rep. 2019, 9, 2722.
- Zhang, L.; Koller, J.; Ip, C.K.; Gopalasingam, G.; Bajaj, N.; Lee, N.J.; Enriquez, R.F.; Herzog, H. Lack of Neuropeptide FF Signalling in Mice Leads to Reduced Repetitive Behavior, Altered Drinking Behavior, and Fuel Type Selection. FASEB J. 2021, 35, e21980.
- Zhang, L.; Koller, J.; Gopalasingam, G.; Qi, Y.; Herzog, H. Central NPFF Signalling Is Critical in the Regulation of Glucose Homeostasis. Mol. Metab. 2022, 62, 101525.
More
Information
Subjects:
Neurosciences; Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
821
Revisions:
2 times
(View History)
Update Date:
11 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




