
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bożena Romanowska-Dixon | -- | 1862 | 2023-07-06 12:54:12 | | | |
| 2 | Conner Chen | -29 word(s) | 1833 | 2023-07-10 05:44:05 | | | | |
| 3 | Conner Chen | Meta information modification | 1833 | 2023-07-10 05:44:30 | | |
Video Upload Options
Ruthenium-106 brachytherapy emits beta radiation (Ru-106 gives off radiation in the form of high energy electrons called beta particles as it decays into rhodium-106 and then into palladium-106, which isn't radioactive) and is used for tumors up to 5 mm in height due to the limited range of radiation penetration, but in some centers, it is used to treat thicker tumors. Therefore, ruthenium is intended for the treatment of small and some medium-sized tumors.
1. Introduction
Uveal melanoma (UM) is the most common primary intraocular malignancy in adults, with the highest incidence of 5–10 per million Whites per year. The eyeball is the most common extracutaneous location of melanoma [1][2][3][4][5][6][7][8]. Usually, it is located in the choroid (90%), and less often in the ciliary body (6%) and iris (4%) [9]. The peak of incidence falls in the sixth to the seventh and, to a lesser extent, the third decade of life [2]. Uveal melanoma metastasizes distantly via blood vessels, most often to the liver (93%), lungs (24%) and bones (16%). It can also spread locally and infiltrate extraocular structures. Extraocular infiltration is associated with a higher risk of distant metastases and worse prognosis. Melanoma metastases are found in most patients at the time of their death [10][11]. They can even be detected several years after successful treatment of the primary cancer, which may be associated with their presence, but also the inability to detect them at the time of therapy [1]. The prognosis in patients with confirmed distant metastases is very poor, and the 1- and 3-year survival rates are approximately 21% and 4%, respectively [12]. Unfortunately, there are currently no effective treatments for distant metastases, which are less susceptible to chemotherapy than skin melanoma metastases [13]. Recently, there have been studies presenting therapies with the use of Tebentafusp and giving hope for improving this situation [14]. This bispecific glycoprotein 100 peptide-HLA-directed CD3 T-cell engager was approved by the Food and Drug Administration (FDA) in 2022 for patients with unresectable or metastatic uveal melanoma. In the same year, it received a positive response from the European Union Committee for Medicinal Products for Human Use for the treatment of uveal melanoma [15]. UM shows specific chromosomal abnormalities. The most significant is the complete or partial loss of chromosome 3. Other common genetic changes are deletions in 1p, 6q, 8p and 9p, and additions in 1q, 6p and 8q [16]. There have been publications describing familial cases of UM belonging to the familial BAP1-associated cancer predisposition syndrome inherited in an autosomal dominant manner [16][17].
The treatment uses surgical methods, which include, tumor endoresection (during pars plana vitrectomy) or egzoresection (under tha scleral flap), enucleation, and conservative methods, such as brachytherapy (BT), proton therapy (PT), stereotactic radiotherapy (SRT), stereotactic radiosurgery (SRS), transpupillary thermotherapy (TTT) and photodynamic therapy (PDT) and combined methods. Many factors are taken into account when planning a treatment strategy, including the size of the tumor, its location and the general condition of the patient. Initially, the treatment of choice in uveal melanoma was enucleation. Over time, brachytherapy began to play a dominant role [1][4][18]. The increase in the popularity of brachytherapy was largely influenced by the publication of the results of the Collaborative Ocular Melanoma Study (COMS), which showed the lack of an advantage of enucleation over brachytherapy in terms of distant metastases and survival in the case of small- and medium-sized tumors [3][11]. In a COMS study, tumor size was defined as follows: small: 1.5–2.4 mm in height and 5–16 mm in diameter; medium: 2.5–10 mm in apical height and ≤16 mm in diameter; and large: >10 mm in apical height and >16 mm in diameter; these terms will be used to describe tumors of these dimensions in the remainder, unless otherwise noted [3,11]. Brachytherapy has gradually become the most commonly used treatment for choroidal melanoma. It involves suturing a plate containing a radioactive isotope to the sclera in the place where the base of the tumor is located. After a few days to inactivate the tumor, the applicator is removed from the sclera [1][19]. In Europe and Asia, the most commonly used radioactive isotope is ruthenium (106Ru), which emits mainly beta radiation (Figure 1). In North America, iodine (125 I) is dominant, which emits mainly gamma radiation (Figure 2). Less frequently used isotopes are Pd-103, Sr-90, Ir-192, Cs-131, Au-198 and others. There are different sizes (10-25mm diameter) and shapes of plates, e.g., round, or with a notch for the optic nerve, dedicated to the treatment of tumors located close to the optic disc. The time of exposure to radiation is several days and depends on the thickness of the tumor and the activity of the applicator. Treatment planning is carried out using specialized computer systems (Figure 3 and Figure 4) [9][18][19]. Local tumor control, depending on the source of radiation, ranges from 59–98% for ruthenium-106 [20] to 76–100% for iodine-125 [21]. Brachytherapy can also be combined with other methods, e.g., TTT, and this combination is called “sandwich therapy” [1][18][22]. This method is dedicated especially to tumours located close to the optic disc. TTT, and this combination is called “sandwich therapy” [1][18][22].
a.

b.

Figure 1. a. and b. Ruthenium-106 applicator.

Figure 2. Iodine-125 applicator (metal shell and polymer insert with spaces for iodine seeds).
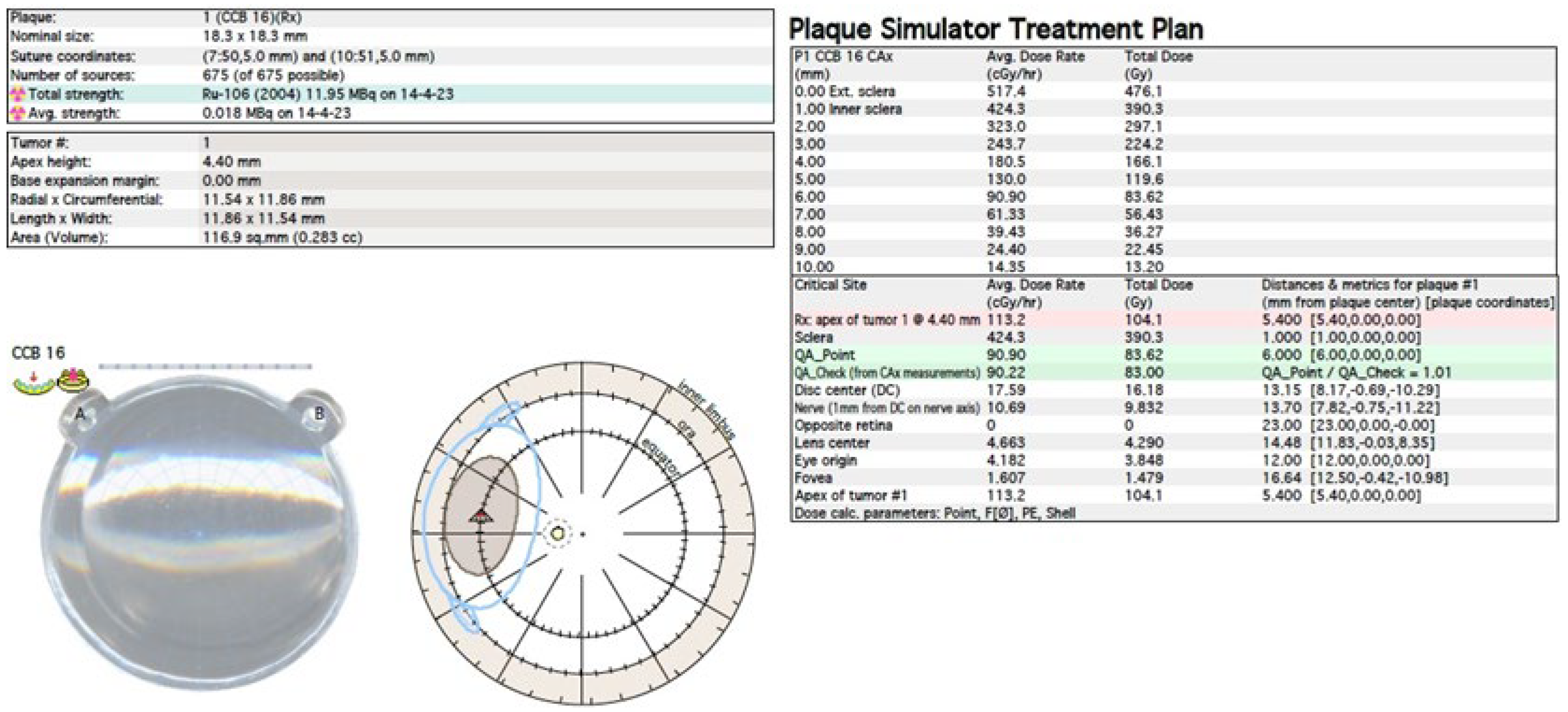
Figure 3. Ruthenium-106 brachytherapy planning system in Plaque Simulator 6.8.5—planning cards.
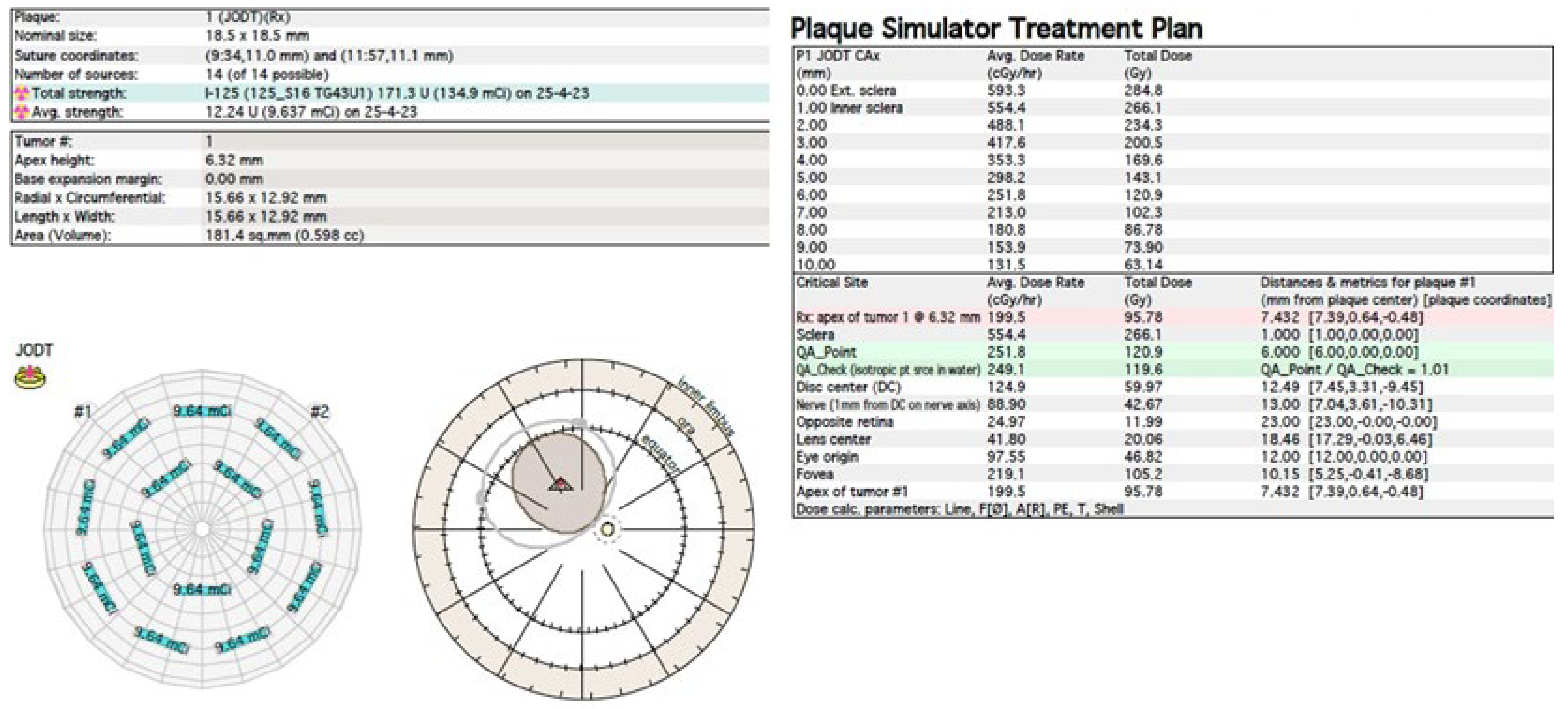
Figure 4. Iodine-125 brachytherapy planning system in Plaque Simulator 6.8.5—planning cards.
Another method of irradiation is radiation therapy from an external source. Among this group, proton therapy is the most common. It is also much less frequently used method than brachytherapy. It uses a beam of protons, which, owing to the Bragg peak phenomenon, allows the irradiation of the tumor mass to an equal extent while sparing the surrounding healthy tissues. It’s development gave hope for reduction in post-radiation complications and thus better eye function after treatment. Before using this method, the patient first undergoes a surgical procedure of the placement of a few tantalum markers by sutures to the outer sclera to localize the base of the tumor, which is visualized by transillumination. Measurements of the distance between the structures of the eyeball are also performed using ophthalmic imaging techniques, such as magnetic resonance (MRI), ultrasonography (US) and fundus photography. As in the case of brachytherapy, treatment planning is carried out using specialized computer systems (Figure 5) [1][18][19]. Proton therapy has excellent local tumor control of 95% [23].
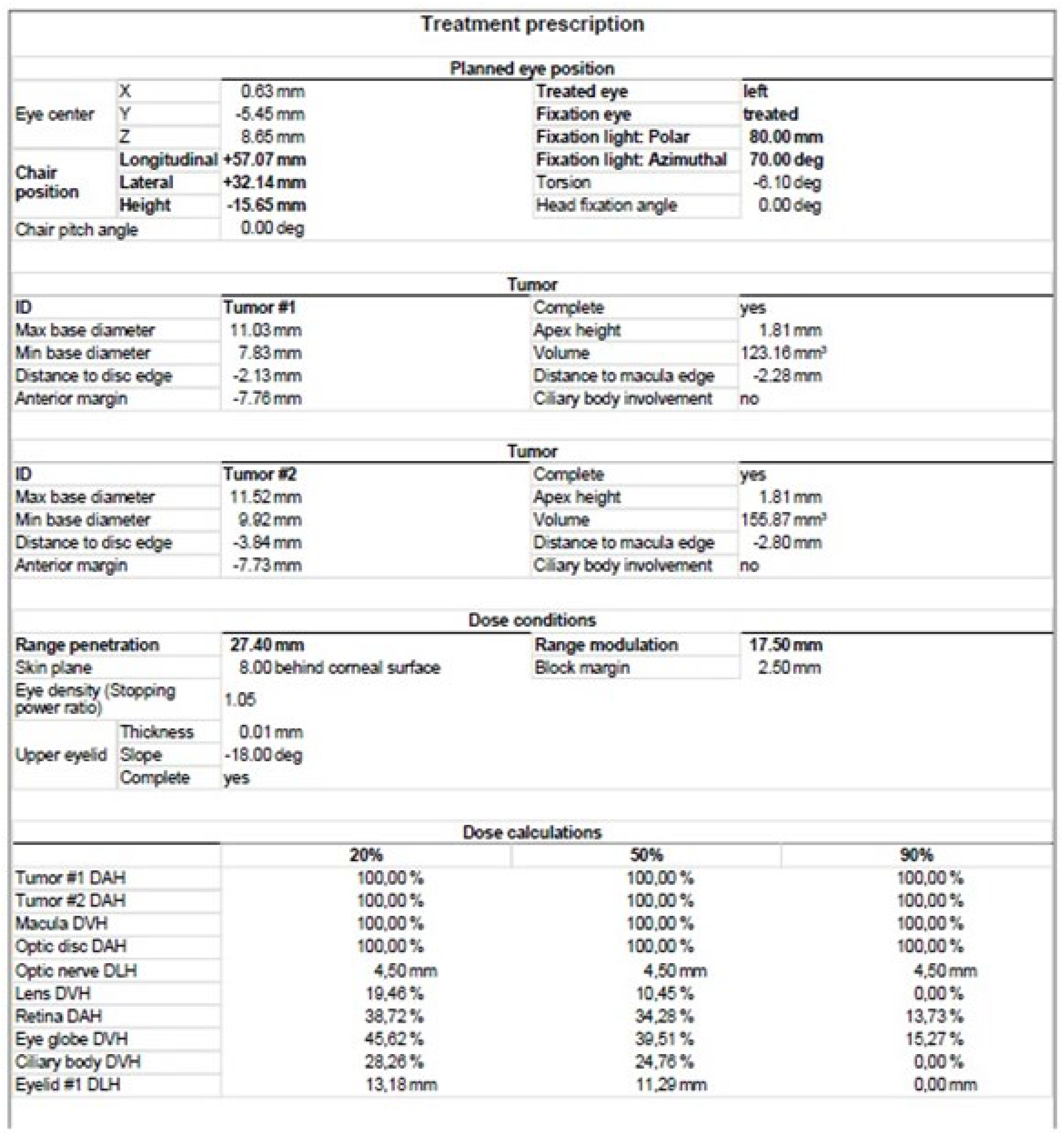
Figure 5. Proton therapy planning system in Eclipse Ocular Proton Planning version 13.5.01−planning card.
2. Ru-106 Brachytherapy
Ru-106 brachytherapy emits beta radiation and is used for tumors up to 5 mm in height due to the limited range of radiation penetration, but in many centers, it is successfully used to treat thicker tumors (Figure 6). Therefore, ruthenium is intended for the treatment of small and some medium-sized tumors [19][31].
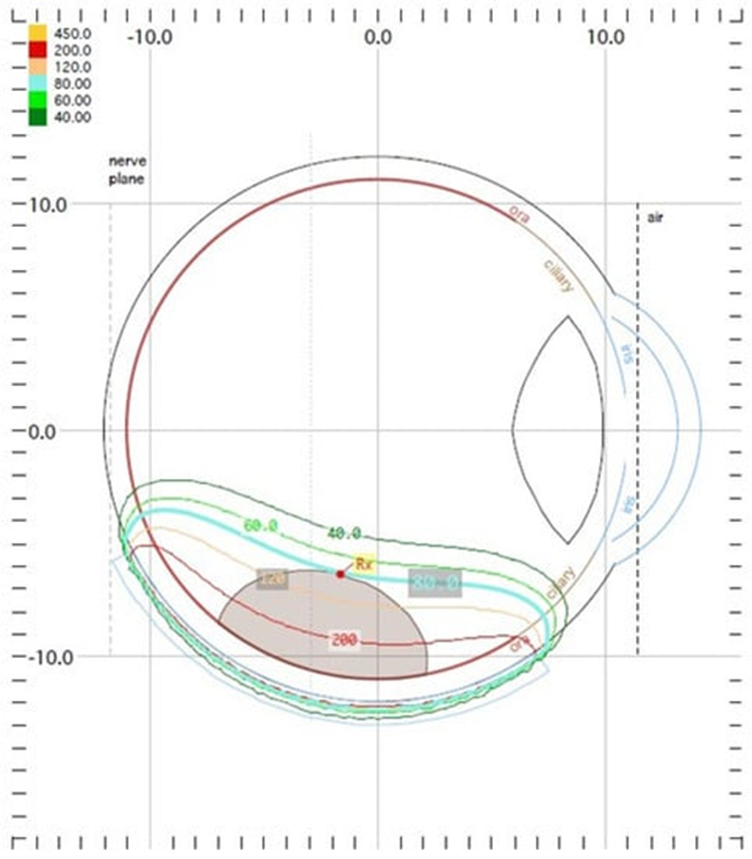
Figure 6. Ruthenium−106 brachytherapy planning system in Plaque Simulator 6.8.5−dose distribution presentation.
References
- Romanowska-Dixon, B.; Jakubowska, B.; Karska-Basta, I.; Kubicka-Trząska, A.; Damato, B. Guzy naczyniówki. In Onkologia Okulistyczna, 1st ed.; Romanowska-Dixon, B., Jager, M.J., Coupland, S.E., Eds.; PZWL: Warszawa, Poland, 2019; pp. 215–318.
- Romanowska-Dixon, B.; Jakubowska, B. Czerniak błony naczyniowej. Atlas. Diagnostyka Różnicowa Nowotworów Wewnątrzgałkowych, 1st ed.; Wydawnictwo Uniwersytetu Jagiellońskiego: Krakow, Poland, 2014; pp. 23–30.
- Margo, C.E. The Collaborative Ocular Melanoma Study: An overview. Cancer Control 2004, 11, 304–309.
- Jovanovic, P.; Mihajlovic, M.; Djordjevic-Jocic, J.; Vlajkovic, S.; Cekic, S.; Stefanovic, V. Ocular melanoma: An overview of the current status. Int. J. Clin. Exp. Pathol. 2013, 6, 1230–1244.
- Markiewicz, A.; Gerba-Górecka, K.; Jakubowska, B.; Dębicka-Kumela, M.; Kowal, J.; Karska-Basta, I.; Skórkiewicz, K.; Romanowska-Dixon, B. Brachytherapy or enucleation in ring melanoma patients: Which is better? Preliminary results of the authors’ own experiences. J. Contemp. Brachyther. 2021, 13, 433–440.
- Markiewicz, A.; Donizy, P.; Nowak, M.; Krzyziński, M.; Elas, M.; Płonka, P.M.; Orłowska-Heitzmann, J.; Biecek, P.; Hoang, M.P.; Romanowska-Dixon, B. Amelanotic Uveal Melanomas Evaluated by Indirect Ophthalmoscopy Reveal Better Long-Term Prognosis Than Pigmented Primary Tumours-A Single Centre Experience. Cancers 2022, 14, 2753.
- Kowal, J.; Markiewicz, A.; Dębicka-Kumela, M.; Bogdali, A.; Jakubowska, B.; Karska-Basta, I.; Romanowska-Dixon, B. Analysis of local recurrence causes in uveal melanoma patients treated with 125I brachytherapy—A single institution study. J. Contemp. Brachyther. 2019, 11, 554–562.
- Kowal, J.; Markiewicz, A.; Dębicka-Kumela, M.; Bogdali, A.; Romanowska-Dixon, B. Outcomes of I-125 brachytherapy for uveal melanomas depending on irradiation dose applied to the tumor apex—A single institution study. J. Contemp. Brachyther. 2018, 10, 532–541.
- Buonanno, F.; Conson, M.; de Almeida Ribeiro, C.; Oliviero, C.; Itta, F.; Liuzzi, R.; Pacelli, R.; Cella, L.; Clemente, S. Local tumor control and treatment related toxicity after plaque brachytherapy for uveal melanoma: A systematic review and a data pooled analysis. Radiother. Oncol. 2022, 166, 15–25.
- Collaborative Ocular Melanoma Study Group. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch. Ophthalmol. 2001, 119, 670–676.
- Zemba, M.; Dumitrescu, O.M.; Gheorghe, A.G.; Radu, M.; Ionescu, M.A.; Vatafu, A.; Dinu, V. Ocular Complications of Radiotherapy in Uveal Melanoma. Cancers 2023, 15, 333.
- Lane, A.M.; Kim, I.K.; Gragoudas, E.S. Survival Rates in Patients After Treatment for Metastasis from Uveal Melanoma. JAMA Ophthalmol. 2018, 136, 981–986.
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal melanoma. Nat. Rev. Dis. Prim. 2020, 6, 24.
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206.
- Dhillon, S. Tebentafusp: First Approval. Drugs 2022, 82, 703–710.
- Khzouz, J.; Coupland, S.E. Aspekty genetyczne nowotworów oka. In Onkologia Okulistyczna, 1st ed.; Romanowska-Dixon, B., Jager, M.J., Coupland, S.E., Eds.; PZWL: Warszawa, Poland, 2019; pp. 22–25.
- Beenakker, J.M.; Brouwer, N.J.; Chau, C.; Coupland, S.E.; Fiorentzis, M.; Heimann, H.; Heufelder, J.; Joussen, A.M.; Kiilgaard, J.F.; Kivelä, T.T.; et al. Outcome Measures of New Technologies in Uveal Melanoma: Review from the European Vision Institute Special Interest Focus Group Meeting. Ophthalmic Res. 2023, 66, 14–26.
- Messineo, D.; Barile, G.; Morrone, S.; La Torre, G.; Turchetti, P.; Accetta, L.; Trovato Battagliola, E.; Agostinelli, E.; Pacella, F. Meta-analysis on the utility of radiotherapy for the treatment of Ocular Melanoma. Clin. Ter. 2020, 170, e89–e98.
- Reichstein, D.A.; Brock, A.L. Radiation therapy for uveal melanoma: A review of treatment methods available in 2021. Curr. Opin. Ophthalmol. 2021, 32, 183–190.
- Karimi, S.; Arabi, A.; Siavashpour, Z.; Shahraki, T.; Ansari, I. Efficacy and complications of ruthenium-106 brachytherapy for uveal melanoma: A systematic review and meta-analysis. J. Contemp. Brachyther. 2021, 13, 358–364.
- Echegaray, J.J.; Bechrakis, N.E.; Singh, N.; Bellerive, C.; Singh, A.D. Iodine-125 Brachytherapy for Uveal Melanoma: A Systematic Review of Radiation Dose. Ocul. Oncol. Pathol. 2017, 3, 193–198.
- Stoffelns, B.M.; Kutzner, J.; Schöpfer, K.; Frising, M. Prospektive nicht randomisierte Analyse der “Sandwich-Therapie” beim malignen Melanom der Aderhaut . Klin. Monbl. Augenheilkd. 2002, 219, 211–215.
- Hérault, J.; Gérard, A.; Carnicer, A.; Aloi, D.; Peyrichon, M.L.; Barnel, C.; Vidal, M.; Angellier, G.; Fayaud, D.; Grini, J.C.; et al. 30 years of ocular proton therapy, the Nice view. Cancer Radiother. 2022, 26, 1016–1026.
- Yazici, G.; Kiratli, H.; Ozyigit, G.; Sari, S.Y.; Elmali, A.; Yilmaz, M.T.; Koc, I.; Deliktas, O.; Gumeler, E.; Cengiz, M.; et al. Every other day stereotactic radiation therapy for the treatment of uveal melanoma decreases toxicity. Radiother. Oncol. 2022, 176, 39–45.
- Rusňák, Š.; Hecová, L.; Kasl, Z.; Sobotová, M.; Hauer, L. Therapy of uveal melanoma A Review. Cesk. Slov. Oftalmol. 2020, 77, 1–13.
- Eibenberger, K.; Dunavoelgyi, R.; Gleiss, A.; Sedova, A.; Georg, D.; Poetter, R.; Dieckmann, K.; Zehetmayer, M. Hypofractionated stereotactic photon radiotherapy of choroidal melanoma: 20-year experience. Acta Oncol. 2021, 60, 207–214.
- Kosydar, S.; Robertson, J.C.; Woodfin, M.; Mayr, N.A.; Sahgal, A.; Timmerman, R.D.; Lo, S.S. Systematic Review and Meta-Analysis on the Use of Photon-based Stereotactic Radiosurgery Versus Fractionated Stereotactic Radiotherapy for the Treatment of Uveal Melanoma. Am. J. Clin. Oncol. 2021, 44, 32–42.
- van Beek, J.G.M.; van Rij, C.M.; Baart, S.J.; Yavuzyigitoglu, S.; Bergmann, M.J.; Paridaens, D.; Naus, N.C.; Kiliç, E. Fractionated stereotactic radiotherapy for uveal melanoma: Long-term outcome and control rates. Acta Ophthalmol. 2022, 100, 511–519.
- Guleser, U.Y.; Sarici, A.M.; Ucar, D.; Gonen, B.; Sengul Samanci, N.; Özgüroğlu, M. Comparison of iodine-125 plaque brachytherapy and gamma knife stereotactic radiosurgery treatment outcomes for uveal melanoma patients. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 1337–1343.
- Oare, C.; Sun, S.; Dusenbery, K.; Reynolds, M.; Koozekanani, D.; Gerbi, B.; Ferreira, C. Analysis of dose to the macula, optic disc, and lens in relation to vision toxicities—A retrospective study using COMS eye plaques. Phys. Med. 2022, 101, 71–78.
- Dogrusöz, M.; Jager, M.J.; Damato, B. Uveal Melanoma Treatment and Prognostication. Asia Pac. J. Ophthalmol. 2017, 6, 186–196.
- Cennamo, G.; Montorio, D.; D’ Andrea, L.; Farella, A.; Matano, E.; Giuliano, M.; Liuzzi, R.; Breve, M.A.; De Placido, S.; Cennamo, G. Long-Term Outcomes in Uveal Melanoma After Ruthenium-106 Brachytherapy. Front. Oncol. 2022, 11, 754108.
- Mirshahi, R.; Sedaghat, A.; Jaberi, R.; Azma, Z.; Mazloumi, M.; Naseripour, M. Ruthenium-106 plaque radiotherapy for uveal melanoma: Analysis of tumor dimension and location on anatomical and functional results. BMC Ophthalmol. 2022, 22, 309.
- O’Day, R.F.J.; Roelofs, K.A.; Negretti, G.S.; Hay, G.; Arora, A.K.; Stoker, I.; Damato, B.E.; Sagoo, M.S.; Cohen, V.M.L. Long-term visual outcomes after ruthenium plaque brachytherapy for posterior choroidal melanoma. Eye 2023, 37, 959–965.




