Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shi, P.; Wang, X.; Cheng, X.; Jiang, Y. Dendrite-Free Sodium Metal Anodes. Encyclopedia. Available online: https://encyclopedia.pub/entry/46449 (accessed on 09 February 2026).
Shi P, Wang X, Cheng X, Jiang Y. Dendrite-Free Sodium Metal Anodes. Encyclopedia. Available at: https://encyclopedia.pub/entry/46449. Accessed February 09, 2026.
Shi, Pengcheng, Xu Wang, Xiaolong Cheng, Yu Jiang. "Dendrite-Free Sodium Metal Anodes" Encyclopedia, https://encyclopedia.pub/entry/46449 (accessed February 09, 2026).
Shi, P., Wang, X., Cheng, X., & Jiang, Y. (2023, July 05). Dendrite-Free Sodium Metal Anodes. In Encyclopedia. https://encyclopedia.pub/entry/46449
Shi, Pengcheng, et al. "Dendrite-Free Sodium Metal Anodes." Encyclopedia. Web. 05 July, 2023.
Copy Citation
Nature-abundant sodium metal is regarded as ideal anode material for advanced batteries due to its high specific capacity of 1166 mAh g−1 and low redox potential of −2.71 V. The uncontrollable dendritic Na formation and low coulombic efficiency remain major obstacles to its application. Notably, the unstable and inhomogeneous solid electrolyte interphase (SEI) is recognized to be the root cause.
sodium metal
artificial SEI
dendrite formation
batteries
1. Introduction
To date, sodium (Na) ion batteries have been commercialized as a supplemental technology for lithium (Li) ion batteries due to natural-abundant Na resources and low costs [1]. However, the energy density of Na ion batteries appears to be unsatisfactory as compared to updated Li ion batteries [2][3]. To meet the rapidly growing demands for the energy density of Na ion batteries, the development of advanced electrode materials with high capacity is highly desired [4].
Among various materials, the metal Na has been proposed as an ideal candidate due to its high specific capacity (1166 mAh g−1) and low redox potential (−2.71 V) [5][6][7]. In this regard, investigations regarding Na-based batteries, including Na-S, Na-O2 and Na-CO2 batteries, have been widely reported [5]. However, the cycling performances and safety issues of Na anodes remain unsatisfactory. It has been reported that growth of dendrites may be the root reason. The spontaneous reaction between Na and electrolytes can form a chemically/mechanically unstable solid electrolyte interphase (SEI), which cannot maintain long-term cycling of the Na anode [8][9]. During plating/stripping, the SEI would be thickened, broken and collapsed [7][9], inducing dendrite formation. Additionally, the thickness change during Na plating/stripping can lead to great local stress, making the SEI much more unstable and more easily cracked [10][11]. In particular, the dendritic Na can penetrate through the separator and detach from the matrix easily to form “dead” Na, leading to battery short circuits and a short cycle life [11][12][13][14]. Therefore, effective efforts to modify Na metal anodes are highly necessary.
Under this background, several approaches have been proposed to stabilize Na anodes: for instance, constructing a 3D host to resolve infinite volume expansion [6][14][15], coating the separator to block Na dendrites [16][17] and employing an Na alloy to build stable anodes [18][19][20]. Although these approaches have some positive effects in suppressing dendritic Na formation, the properties of SEI films remain unsatisfactory, and the irreversible side reactions cannot be totally suppressed. The electrolyte modification seems to be promising for increasing the stability of the SEI interphase. However, the additives, salts and solvents cannot hold for long-term cycling due to continuous consumption [11][21]. Accordingly, the ideal SEI for Na metal should possesses excellent chemical/electrochemical stability, good ionic conductivity, even Na+ flux/electric field distribution, sufficient Young’s module, good flexibility and robustness [22]. In this regard, artificial interphase engineering is of vital importance, since the protective layer can be precisely designed and easily adjusted. More importantly, the artificial SEI boasts most of the above-mentioned merits of an ideal SEI. So far, extensive research has been conducted on artificial interphase configuration to improve the stability of the SEI [23][24]. Therefore, it is necessary to summarize the research progress in artificial SEI design in recent years.
2. Challenges for Dendrite-Free Na Metal Anodes
Like other alkali metals, Na is thermodynamically unstable; this is the root cause of uncontrollable parasitic reactions and the formation of chemically/mechanically unstable SEIs [23][24]. Figure 1a shows the main challenges of Na metal anodes. As compared with Li metal, Na metal is more prone to deposits in dendritic morphology and suffers from severe volume expansion [25][26]. During plating/stripping, the SEI can be cracked and form “dead” and isolated Na. Meanwhile, the growth of dendrites can lead to battery short-circuiting.
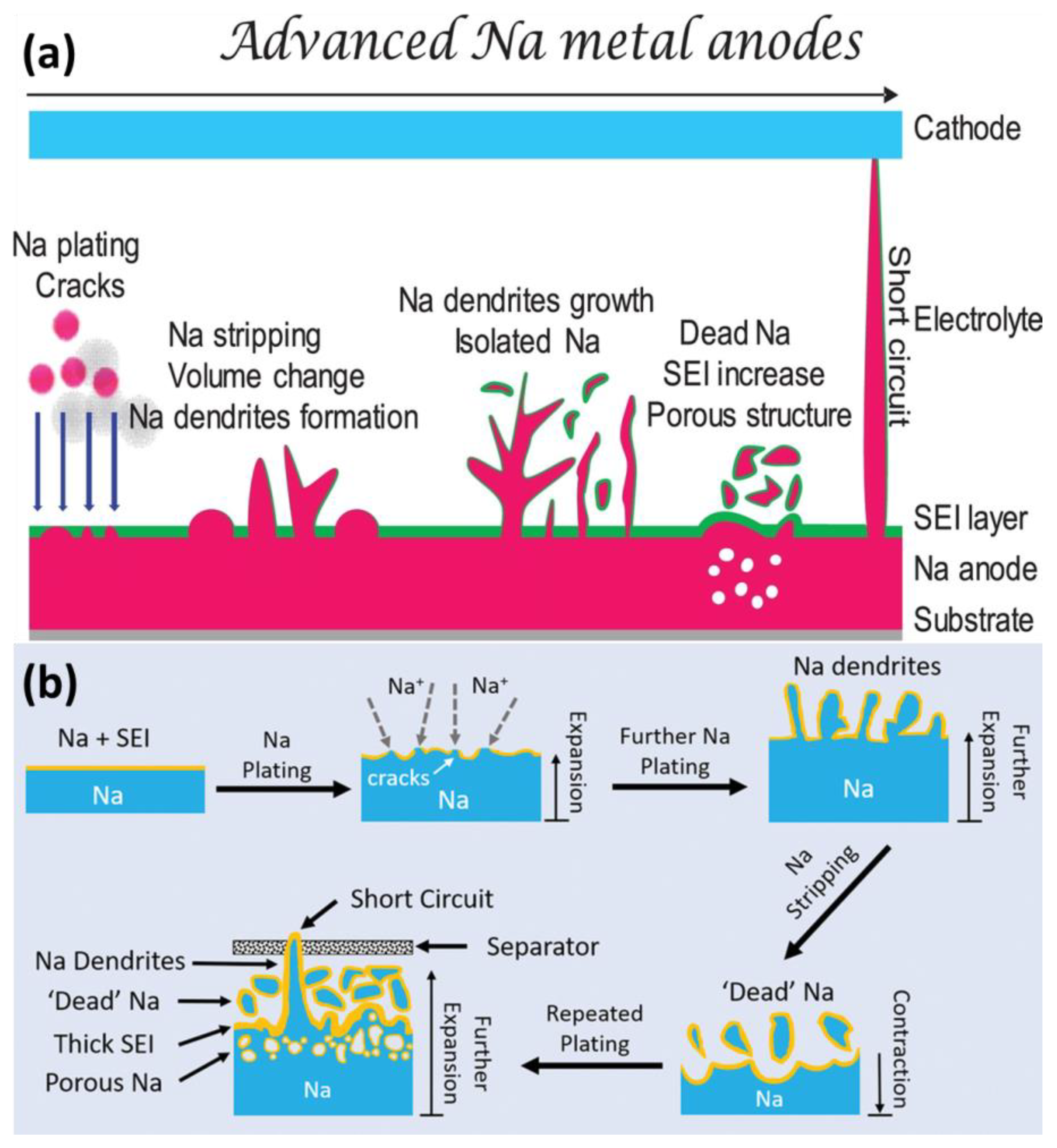
Figure 1. (a) Schematic illustration of challenges for Na metal anodes. (b) The growth of dendrites and formation of “dead” Na.
2.1. High Reactivity
The Na atom can lose electrons to form Na+ easily. In dry air, the Na metal can react with O2 and CO2. When contacting water or moist air, the Na metal can form flammable H2 to cause fire or even explosions. Due to high reactivity, the Na metal will induce unavoidable side reactions with liquid electrolytes, resulting in SEI formation, Na corrosion and poor cycling performance, as shown in Figure 1b. Even worse, the leakage or breakage of batteries can cause safety issues.
2.2. Unstable SEIs
It is expected that the ideal SEI layer is dense and inert so as to effectively isolate electron transfer and prevent further parasitic reactions [24][27]. Nevertheless, the structure of the SEI layer formed in common electrolytes is demonstrated to be porous and fragile.
As it is recognized, the properties of the SEI layer formed in common electrolytes depend on the solvents, additives and Na salts. Typically, the SEI layer is mainly composed of inorganic species (e.g., NaF, Na2O and Na2CO3) and organic species (e.g., RONa, ROCO2Na and RCOONa; where R is the alkyl group) [25]. The possible formation mechanism is summarized in the following equations [28][29].
C3H4O3(EC) + 2Na+ + 2e− → Na2CO3↓ + C2H4↑
C3H6O3(PC) + 2Na+ + 2e− → Na2CO3↓ + C3H6↑
C3H3FO3(FEC) + Na+ + e− → NaF↓ + CO2↑ + CH2CHO↑
PF6− + 3Na+ + 2e− → 3NaF↓ + PF3
Na + 3Na+ + 2e− → 3NaF↓ + PF3
Meanwhile, the reduction of solvents can supply a large amount of oxygen atoms, leading to the formation of Na2O. Owing to the lack of advanced characterization techniques, the formation mechanism and detailed composition of the SEI layer remain controversial. Further investigations are needed for understanding the mechanism. Additionally, the SEI layer formed on the Na metal is dissolved in electrolytes more easily than that of Li [30][31]. Due to the non-uniform distribution of compositions, the ionic conductivity of the SEI layer is spatial varying, resulting in uneven distribution of the Na+ flux. Meanwhile, due to the “host-less” nature of the Na matrix, the SEI layer cracks easily during repeated Na+ plating/stripping, which in turn accelerates dendrite growth due to increased Na+ flux and preferential Na+ plating around the cracks. Furthermore, the repeated breakage of the SEI layer also leads to uncontrollable electrolyte consumption, followed by low coulombic efficiency and high SEI impedance [32][33]. As a result, Na metal with unsatisfied SEI properties inevitably suffers from poor performance.
Based on previous research [34][35], further progress on building ideal SEI layers for dendrite-free Na metal should be centered around the following characteristics: firstly, high Na+ conductivity so as to facilitate uniform Na+ deposition and regulate preferential Na plating; secondly, electrochemical stability and electronic insulation to prevent further side reactions; thirdly, sufficiently robust to maintain long-term large volume expansion and dendrite propagation; finally, homogeneous in composition to decentralize the Na+ flux.
2.3. Uncontrollable Dendritic Na Formation
Dendrite growth is also a serious problem, as shown in Figure 1b. The dendrite growth can penetrate the separator and form “dead” Na, leading to battery short circuiting and poor cycling stability. The morphology of Na dendrites can be divided into needle-like, tree-like and mossy-like types; however, it is difficult to distinguish them clearly. In most case, these types of dendrites can co-exist in rechargeable batteries [36][37].
Based on previous research [38], it is widely accepted that the concentration of Na+ will decrease to zero near the surface in Sand’s time. Due to the spatial variation in ionic conductivity and the localized electric field, the rough surface will induce uneven Na+ plating/stripping, resulting in dendrite formation. Subsequently, the tips of dendrites become hot sites for further dendritic Na nucleation and growth due to their larger electric field and ionic concentration gradients. Once the dendrite is nucleated, the growth rate of dendrites is a key parameter to determine the lifetime of Na anode. According to Sand’s law, the speed of dendrite formation is inversely proportional to the square of the deposition current [32][37][39][40].
Dendrite growth can expose the fresh Na surface to depletion of electrolytes and active Na. Meanwhile, the unstable dendrites detach from the matrix to form “dead” Na. Through microscopy observation, it has been proven that the porous Na dendrites can break away from the bulk Na matrix easily, as compared with Li dendrites. The dendrites intrinsically exhibit much higher chemical reactivity and weaker mechanical stability [39].
2.4. Severe Volume Expansion
The severe volume expansion can be regarded as the root cause of the continuous side reactions. Theoretically, the thickness would increase by 8.86 µm with 1 mAh cm−2 Na. To satisfy industrial requirements, the deposited capacity would be above 3.5 mAh cm−2 [34][41]. Due to uneven deposition, the practical volume variation would be more evident than theoretically expected. In addition, due to the host-less nature, the volume expansion is considered to be relatively infinite [42]. Meanwhile, due to lack of flexibility, the SEI can be cracked easily during volume expansion, which accelerates the formation of “dead” Na and consumption of electrolytes, as shown in Figure 1b.
To alleviate the volume expansion and mitigate the inner strain, nanostructured hosts such as Cu foam [43][44][45], carbon matrix [42][46][47] and Mxene [48][49] are proposed to accommodate Na. Nevertheless, these hosts increase the total weight and volume of the Na anode at the expense of total energy density. The recent development of hosts for dendrite-free Na metal has been discussed in several reviews [26][50].
References
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614.
- Abraham, K.M. How Comparable are sodium-ion batteries to lithium-ion counterparts? ACS Energy Lett. 2020, 5, 3544–3547.
- Hirsh, H.S.; Li, Y.X.; Tan, D.H.S.; Zhang, M.H.; Zhao, E.Y.; Meng, Y.S. Sodium-ion batteries paving the way for grid energy storage. Adv. Energy Mater. 2020, 10, 2001274.
- Liu, T.; Zhang, Y.; Jiang, Z.; Zeng, X.; Ji, J.; Li, Z.; Gao, X.; Sun, M.; Lin, Z.; Ling, M.; et al. Exploring competitive features of stationary sodium ion batteries for electrochemical energy storage. Energy Environ. Sci. 2019, 12, 1512–1533.
- Zhao, Y.; Yang, X.F.; Kuo, L.Y.; Kaghazchi, P.; Sun, Q.; Liang, J.N.; Wang, B.Q.; Lushington, A.; Li, R.Y.; Zhang, H.M.; et al. High capacity, dendrite-free growth, and minimum volume change Na metal anode. Small 2018, 14, 1703717.
- Sun, B.; Xiong, P.; Maitra, U.; Langsdorf, D.; Yan, K.; Wang, C.Y.; Janek, J.; Schröder, D.; Wang, G.X. Design strategies to enable the efficient use of sodium metal anodes in high-energy batteries. Adv. Mater. 2019, 32, 1903891.
- Cao, R.G.; Mishra, K.; Li, X.L.; Qian, J.F.; Engelhard, M.H.; Bowden, M.E.; Han, S.H.; Mueller, K.T.; Henderson, W.A.; Zhang, J.-G. Enabling room temperature sodium metal batteries. Nano Energy 2016, 30, 825–830.
- Zheng, J.M.; Chen, S.R.; Zhao, W.G.; Song, J.H.; Mueller, K.T.; Zhang, J.-G. Extremely stable sodium metal batteries enabled by localized high-concentration electrolytes. ACS Energy Lett. 2018, 3, 315–321.
- Lee, Y.; Lee, J.; Lee, J.M.; Kim, K.; Cha, A.; Kang, S.J.; Wi, T.; Kang, S.J.; Lee, H.-W.; Choi, N.-S. Fluoroethylene carbonate-based electrolyte with 1 M sodium bis (fluorosulfonyl) imide enables high-performance sodium metal electrodes. ACS Appl. Mater. Interfaces 2018, 10, 15270–15280.
- Ma, B.; Bai, P. Fast charging limits of ideally stable metal anodes in liquid electrolytes. Adv. Energy Mater. 2022, 12, 2102967.
- Ji, Y.Y.; Li, J.B.; Li, J.L. Recent development of electrolyte engineering for sodium metal batteries. Batteries 2022, 8, 157.
- Zheng, X.Y.; Cao, Z.; Gu, Z.Y.; Huang, L.Q.; Sun, Z.H.; Zhao, T.; Yu, S.J.; Wu, X.L.; Huang, Y.H. Toward high temperature sodium metal batteries via regulating the electrolyte/electrode interfacial chemistries. ACS Energy Lett. 2022, 7, 2032–2042.
- Xia, X.M.; Lv, X.; Yao, Y.; Chen, D.; Tang, F.; Liu, N.; Feng, Y.Z.; Rui, X.H.; Yu, Y. A sodiophilic VN interlayer stabilizing a Na metal anode. Nanoscale Horiz. 2022, 7, 899–907.
- Jäckle, M.; Groß, A. Microscopic properties of lithium, sodium, and magnesium battery anode materials related to possible dendrite growth. J. Chem. Phys. 2014, 141, 174710.
- Wang, H.; Bai, W.L.; Wang, H.; Kong, D.Z.; Xu, T.Q.; Zhang, Z.F.; Zang, J.H.; Wang, X.C.; Zhang, S.; Tian, Y.T.; et al. 3D printed Au/rGO microlattice host for dendrite-free sodium metal anode. Energy Storage Mater. 2023, 55, 631–641.
- Kang, T.; Sun, C.; Li, Y.; Song, T.; Guan, Z.; Tong, Z.; Nan, J.; Lee, C.S. Dendrite-free sodium metal anodes via solid electrolyte interphase engineering with a covalent organic framework separator. Adv. Energy Mater. 2023, 13, 2204083.
- Li, M.H.; Lu, G.J.; Zheng, W.K.; Zhao, Q.N.; Li, Z.P.; Jiang, X.P.; Yang, Z.G.; Li, Z.Y.; Qu, B.H.; Xu, C.H. Multifunctionalized safe separator toward practical sodium-metal batteries with high-performance under high mass loading. Adv. Funct. Mater. 2023, 33, 2214759.
- Wang, Y.X.; Dong, H.; Katyal, N.; Hao, H.C.; Liu, P.C.; Celio, H.; Henkelman, G.; Watt, J.; Mitlin, D. Sodium-antimony-telluride intermetallic allows sodium metal cycling at 100% depth of discharge and as anode-free metal battery. Adv. Mater. 2022, 34, 2106005.
- Liu, H.; Cheng, X.B.; Huang, J.-Q.; Kaskel, S.; Chou, S.L.; Park, H.S.; Zhang, Q. Alloy anodes for rechargeable alkali-metal batteries: Progress and challenge. ACS Mater. Lett. 2019, 1, 217–229.
- Tang, S.; Zhang, Y.Y.; Zhang, X.G.; Li, J.T.; Wang, X.Y.; Yan, J.W.; Wu, D.Y.; Zheng, M.S.; Dong, Q.F.; Mao, B.W. Stable Na plating and stripping electrochemistry promoted by in situ construction of an alloy-based sodiophilic interphase. Adv. Mater. 2019, 31, 1807495.
- Wang, H.; Wang, C.L.; Matios, E.; Luo, J.M.; Lu, X.; Zhang, Y.W.; Hu, X.F.; Li, W.Y. Enabling ultrahigh rate and capacity sodium metal anodes with lightweight solid additives. Energy Storage Mater. 2020, 32, 244–252.
- Bao, C.Y.; Wang, B.; Liu, P.; Wu, H.; Zhou, Y.; Wang, D.L.; Liu, H.K.; Dou, S.X. Solid electrolyte interphases on sodium metal anodes. Adv. Funct. Mater. 2020, 30, 2004891.
- Gao, L.; Chen, J.; Chen, Q.L.; Kong, X.Q. The chemical evolution of solid electrolyte interface in sodium metal batteries. Sci. Adv. 2022, 8, eabm4606.
- Wang, T.; Hua, Y.B.; Xu, Z.W.; Yu, J.S. Recent advanced development of artificial interphase engineering for stable sodium metal anodes. Small 2022, 18, 2102250.
- Zhao, C.L.; Lu, Y.X.; Yue, J.M.; Pan, D.; Qi, Y.R.; Hu, Y.S.; Chen, L.Q. Advanced Na metal anodes. J. Energy Chem. 2018, 27, 1584–1596.
- Li, Z.P.; Zhu, K.J.; Liu, P.; Jiao, L.F. 3D confinement strategy for dendrite-free sodium metal batteries. Adv. Energy Mater. 2022, 12, 2100359.
- Chen, X.; Bai, Y.K.; Shen, X.; Peng, H.-J.; Zhang, Q. Sodiophilicity/potassiophilicity chemistry in sodium/potassium metal anodes. J. Energy Chem. 2020, 51, 1–6.
- Lu, Z.Y.; Yang, H.J.; Guo, Y.; He, P.; Wu, S.C.; Yang, Q.H.; Zhou, H.S. Electrolyte sieving chemistry in suppressing gas evolution of sodium-metal batteries. Angew Chem. Int. Ed. 2022, 61, e202206340.
- Wang, E.H.; Wan, J.; Guo, Y.-J.; Zhang, Q.Y.; He, W.H.; Zhang, C.H.; Chen, W.-P.; Yan, H.-J.; Xue, D.-J.; Fang, T.T.; et al. Mitigating electron leakage of solid electrolyte interface for stable sodium-ion batteries. Angew Chem. Int. Ed. 2023, 62, e202216354.
- Mandl, M.; Becherer, J.; Kramer, D.; Mönig, R.; Diemant, T.; Diemant, T.; Behm, J.; Hahn, M.; Böse, O.; Danzer, M.A. Sodium metal anodes: Deposition and dissolution behaviour and SEI formation. Electrochim. Acta 2020, 354, 136698.
- Lee, B.; Paek, E.; Mitlin, D.; Lee, S.W. Sodium metal anodes: Emerging solutions to dendrite growth. Chem. Rev. 2019, 119, 5416–5460.
- Zhao, Y.; Adair, K.R.; Sun, X.L. Recent developments and insights into the understanding of Na metal anodes for Na-metal batteries. Energy Environ. Sci. 2018, 11, 2673–2695.
- Lei, D.N.; He, Y.B.; Huang, H.J.; Yuan, Y.F.; Zhong, G.M.; Zhao, Q.; Hao, X.G.; Zhang, D.F.; Lai, C.; Zhang, S.W.; et al. Cross-linked beta alumina nanowires with compact gel polymer electrolyte coating for ultra-stable sodium metal battery. Nat. Commun. 2019, 10, 4244.
- Liu, T.F.; Yang, X.K.; Nai, J.W.; Wang, Y.; Liu, Y.J.; Liu, C.T.; Tao, X.Y. Recent development of Na metal anodes: Interphase engineering chemistries determine the electrochemical performance. Chem. Eng. J. 2021, 409, 127943.
- Ji, Y.Y.; Sun, H.C.; Li, Z.B.; Ma, L.; Zhang, W.G.; Liu, Y.M.; Pan, L.K. Salt engineering toward stable cation migration of Na metal anodes. J. Mater. Chem. A 2022, 10, 25539–25545.
- Liu, W.; Liu, P.C.; Mitlin, D. Review of emerging concepts in SEI analysis and artificial SEI membranes for lithium, sodium, and potassium metal battery anodes. Adv. Energy Mater. 2020, 10, 2002297.
- Matios, E.; Wang, H.; Wang, C.L.; Li, W.Y. Enabling safe sodium metal batteries by solid electrolyte interphase engineering: A review. Ind. Eng. Chem. Res. 2019, 58, 9758–9780.
- Xia, X.M.; Du, C.F.; Zhong, S.E.; Jiang, Y.; Yu, H.; Sun, W.P.; Pan, H.G.; Rui, X.H.; Yu, Y. Homogeneous Na deposition enabling high-energy Na-metal batteries. Adv. Funct. Mater. 2022, 32, 2110280.
- Lee, J.; Kim, J.; Kim, S.; Jo, C.S.; Lee, J. A review on recent approaches for designing the SEI layer on sodium metal anodes. Mater. Adv. 2020, 1, 3143–3166.
- Liu, W.; Liu, P.C.; Mitlin, D. Tutorial review on structure-dendrite growth relations in metal battery anode supports. Chem. Soc. Rev. 2020, 49, 7284–7300.
- Lin, Z.; Liu, T.F.; Ai, X.P.; Liang, C.D. Aligning academia and industry for unified battery performance metrics. Nat. Commun. 2018, 9, 5262.
- Lee, K.; Lee, Y.J.; Lee, M.J.; Han, J.H.; Lim, J.; Ryu, K.; Yoon, H.; Kim, B.H.; Kim, B.J.; Lee, S.W. A 3D hierarchical host with enhanced sodiophilicity enabling anode-free sodium-metal batteries. Adv. Mater. 2022, 34, 2109767.
- Yang, W.; Yang, W.; Dong, L.B.; Shao, G.J.; Wang, G.X.; Peng, X.W. Hierarchical ZnO nanorod arrays grown on copper foam as an advanced three-dimensional skeleton for dendrite-free sodium metal anodes. Nano Energy 2021, 80, 105563.
- Wang, C.L.; Wang, H.; Matios, E.; Hu, X.F.; Li, W.Y. A chemically engineered porous copper matrix with cylindrical core-shell skeleton as a stable host for metallic sodium anodes. Adv. Funct. Mater. 2018, 28, 1802282.
- Ma, Y.; Gu, Y.T.; Yao, Y.Z.; Jin, H.D.; Zhao, X.H.; Yuan, X.T.; Lian, Y.L.; Qi, P.W.; Shah, R.; Peng, Y.; et al. Alkaliphilic Cu2O nanowires on copper foam for hosting Li/Na as ultrastable alkali-metal anodes. J. Mater. Chem. A 2019, 7, 20926–20935.
- Mubarak, N.; Ihsan-Ul-Haq, M.; Huang, H.; Cui, J.; Yao, S.S.; Susca, A.; Wu, J.X.; Wang, M.Y.; Zhang, X.H.; Huang, B.L.; et al. Metal organic framework-induced mesoporous carbon nanofibers as ultrastable Na metal anode host. J. Mater. Chem. A 2020, 8, 10269–10282.
- Yue, L.; Qi, Y.R.; Niu, Y.B.; Bao, S.J.; Xu, M.W. Low-barrier, dendrite-free, and stable Na plating/stripping enabled by gradient sodiophilic carbon skeleton. Adv. Energy Mater. 2021, 11, 2102497.
- Wang, Z.X.; Huang, Z.X.; Wang, H.; Li, W.D.; Wang, B.Y.; Xu, J.M.; Xu, T.T.; Zang, J.H.; Kong, D.Z.; Li, X.J.; et al. 3D-printed sodiophilic V2CTx/rGO-CNT MXene microgrid aerogel for stable Na metal anode with high areal capacity. ACS Nano 2022, 16, 9105–9116.
- Shi, H.D.; Yue, M.; Zhang, C.F.; Dong, Y.F.; Liu, P.F.; Zheng, S.H.; Huang, H.J.; Chen, J.; Wen, P.C.; Xu, Z.C.; et al. 3D flexible, conductive, and recyclable Ti3C2Tx MXene-melamine foam for high-areal-capacity and long-lifetime alkali-metal anode. ACS Nano 2020, 14, 8678–8688.
- Chu, C.X.; Li, R.; Cai, F.P.; Bai, Z.C.; Wang, Y.X.; Xu, X.; Wang, N.; Yang, J.; Dou, S.X. Recent advanced skeletons in sodium metal anodes. Energy Environ. Sci. 2021, 14, 4318–4340.
More
Information
Subjects:
Electrochemistry
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
06 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




