Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yu Jiang and Version 2 by Rita Xu.
Nature-abundant sodium metal is regarded as ideal anode material for advanced batteries due to its high specific capacity of 1166 mAh g−1 and low redox potential of −2.71 V. The uncontrollable dendritic Na formation and low coulombic efficiency remain major obstacles to its application. Notably, the unstable and inhomogeneous solid electrolyte interphase (SEI) is recognized to be the root cause.
- sodium metal
- artificial SEI
- dendrite formation
- batteries
1. Introduction
To date, sodium (Na) ion batteries have been commercialized as a supplemental technology for lithium (Li) ion batteries due to natural-abundant Na resources and low costs [1]. However, the energy density of Na ion batteries appears to be unsatisfactory as compared to updated Li ion batteries [2][3][2,3]. To meet the rapidly growing demands for the energy density of Na ion batteries, the development of advanced electrode materials with high capacity is highly desired [4].
Among various materials, the metal Na has been proposed as an ideal candidate due to its high specific capacity (1166 mAh g−1) and low redox potential (−2.71 V) [5][6][7][5,6,7]. In this regard, investigations regarding Na-based batteries, including Na-S, Na-O2 and Na-CO2 batteries, have been widely reported [5]. However, the cycling performances and safety issues of Na anodes remain unsatisfactory. It has been reported that growth of dendrites may be the root reason. The spontaneous reaction between Na and electrolytes can form a chemically/mechanically unstable solid electrolyte interphase (SEI), which cannot maintain long-term cycling of the Na anode [8][9][8,9]. During plating/stripping, the SEI would be thickened, broken and collapsed [7][9][7,9], inducing dendrite formation. Additionally, the thickness change during Na plating/stripping can lead to great local stress, making the SEI much more unstable and more easily cracked [10][11][10,11]. In particular, the dendritic Na can penetrate through the separator and detach from the matrix easily to form “dead” Na, leading to battery short circuits and a short cycle life [11][12][13][14][11,12,13,14]. Therefore, effective efforts to modify Na metal anodes are highly necessary.
Under this background, several approaches have been proposed to stabilize Na anodes: for instance, constructing a 3D host to resolve infinite volume expansion [6][14][15][6,14,15], coating the separator to block Na dendrites [16][17][16,17] and employing an Na alloy to build stable anodes [18][19][20][18,19,20]. Although these approaches have some positive effects in suppressing dendritic Na formation, the properties of SEI films remain unsatisfactory, and the irreversible side reactions cannot be totally suppressed. The electrolyte modification seems to be promising for increasing the stability of the SEI interphase. However, the additives, salts and solvents cannot hold for long-term cycling due to continuous consumption [11][21][11,21]. Accordingly, the ideal SEI for Na metal should possesses excellent chemical/electrochemical stability, good ionic conductivity, even Na+ flux/electric field distribution, sufficient Young’s module, good flexibility and robustness [22]. In this regard, artificial interphase engineering is of vital importance, since the protective layer can be precisely designed and easily adjusted. More importantly, the artificial SEI boasts most of the above-mentioned merits of an ideal SEI. So far, extensive research has been conducted on artificial interphase configuration to improve the stability of the SEI [23][24][23,24]. Therefore, it is necessary to summarize the research progress in artificial SEI design in recent years.
2. Challenges for Dendrite-Free Na Metal Anodes
Like other alkali metals, Na is thermodynamically unstable; this is the root cause of uncontrollable parasitic reactions and the formation of chemically/mechanically unstable SEIs [23][24][23,24]. Figure 1a shows the main challenges of Na metal anodes. As compared with Li metal, Na metal is more prone to deposits in dendritic morphology and suffers from severe volume expansion [25][26][25,26]. During plating/stripping, the SEI can be cracked and form “dead” and isolated Na. Meanwhile, the growth of dendrites can lead to battery short-circuiting.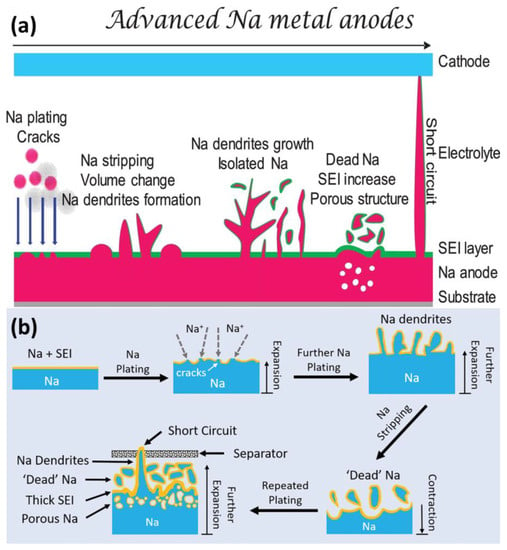
Figure 1. (a) Schematic illustration of challenges for Na metal anodes. (b) The growth of dendrites and formation of “dead” Na.
2.1. High Reactivity
The Na atom can lose electrons to form Na+ easily. In dry air, the Na metal can react with O2 and CO2. When contacting water or moist air, the Na metal can form flammable H2 to cause fire or even explosions. Due to high reactivity, the Na metal will induce unavoidable side reactions with liquid electrolytes, resulting in SEI formation, Na corrosion and poor cycling performance, as shown in Figure 1b. Even worse, the leakage or breakage of batteries can cause safety issues.2.2. Unstable SEIs
It is expected that the ideal SEI layer is dense and inert so as to effectively isolate electron transfer and prevent further parasitic reactions [24][27][24,27]. Nevertheless, the structure of the SEI layer formed in common electrolytes is demonstrated to be porous and fragile. As it is recognized, the properties of the SEI layer formed in common electrolytes depend on the solvents, additives and Na salts. Typically, the SEI layer is mainly composed of inorganic species (e.g., NaF, Na2O and Na2CO3) and organic species (e.g., RONa, ROCO2Na and RCOONa; where R is the alkyl group) [25]. The possible formation mechanism is summarized in the following equations [28][29][28,29].C3H4O3(EC) + 2Na+ + 2e− → Na2CO3↓ + C2H4↑
C3H6O3(PC) + 2Na+ + 2e− → Na2CO3↓ + C3H6↑
C3H3FO3(FEC) + Na+ + e− → NaF↓ + CO2↑ + CH2CHO↑
PF6− + 3Na+ + 2e− → 3NaF↓ + PF3
Na + 3Na+ + 2e− → 3NaF↓ + PF3
